We evaluate the results and complications of our intraventricular fibrinolysis protocol.
Material and methodsA retrospective analysis was made of the cases of intraventricular hemorrhage with 13-bed Intensive Care Unit. Graeb score 6 or above subjected to intraventricular fibrinolysis. We gathered demographic parameters, clinical risk scores, tomography data and case histories showing neurological status and complications related to intraventricular treatment. The results between those who died and the survivors were compared.
ResultsIntraventricular fibrinolysis was performed in 42 patients (69% males) with intraventricular hemorrhage. The average age was 58.36 years (SD 16.67), with a median APACHE II score of 17.5 (r 3–29). A total of 16.7% were receiving acenocoumarol, and 7.1% were on antiplatelet drugs. The median Glasgow Coma Score at the start of treatment was 8 (r 3–13). The median Graeb score was 9 (r 6–12), and was severe (Graeb 9–12) in almost 62%. In turn, 26.2% of the patients developed ventriculitis, and there was further bleeding in 7.1%. Death occurred in 50% of the cases. None of the analyzed variables were significantly related to increased mortality. In the 21 survivors, the Glasgow Outcome Score at 3 months was 2 in 23.8% of the cases, 3 in 28.57%, 4 in 23.8% and 5 in 28.57% of the patients.
ConclusionsIntraventricular fibrinolysis does not appear to involve a high rate of complications, and may result in lesser mortality, with a better functional outcome after three months than that estimated and published in the literature in reference to intraventricular hemorrhage.
Evaluar los resultados y complicaciones de un protocolo de fibrinólisis intraventricular empleado durante 10 años.
Ámbito de aplicación y métodosServicio de Medicina Intensiva de 13 camas. Análisis retrospectivo de nuestra base prospectiva de pacientes con hemorragia intraventricular con Graeb mayor de 5 tratados con fibrinólisis intraventricular. Registramos datos demográficos, escalas de gravedad, datos tomográficos y evolutivos neurológicos, y complicaciones relacionadas con la fibrinólisis. Comparamos los resultados entre fallecidos y supervivientes.
ResultadosRecibieron fibrinolíticos intraventriculares 42 pacientes (69% varones) con hemorragia intraventricular. La edad media fue 58,36 años (DE 16,67), con una mediana de APACHE II de 17,5 (rango 3-29). El 16,7% tomaban acenocumarol y el 7,1% estaban en tratamiento antiagregante. La mediana del Glasgow Coma Score en el momento de inicio de la fibrinólisis fue de 8 (rango 3-13), y la mediana de Graeb fue 9 (rango 6-12). Más del 62% de las hemorragias fueron clasificadas como graves (Graeb 9-12). Se complicaron con ventriculitis el 26,2% y con sangrado el 7,1%. Falleció el 50% de la serie. Ninguna de las variables analizadas se relacionó de modo significativo con la mortalidad. De los 21 supervivientes, el Glasgow Outcome Score a los 3 meses fue de 2 en el 23,8%, de 3 en el 28,57%, de 4 en el 23,8% y de 5 en el 28,57%.
ConclusionesLa fibrinólisis intraventricular no parece asociar una alta tasa de complicaciones, y puede contribuir a una menor mortalidad con mejor resultado funcional a los 3 meses que la estimada y publicada en la hemorragia intraventricular.
Intraventricular haemorrhage in adults (IVH), both in the primary and the more frequent secondary form, is an entity with a poor prognosis1–12 with mortality rates estimated at 50–80%3,5–9 and a considerable associated morbidity.1–11 By itself it can lead to a communicating hydrocephalus or an obstructive hydrocephalus.1,3,10–12 It frequently requires treatment by the insertion of a ventricular drain to drain away blood or cerebro-spinal fluid. The insertion of drainage alone has not been shown to reduce morbimortality1 and furthermore is not without risks that of ventriculitis being the most important.13–18
Studies have been published that indicate the efficacy of treatment with intraventricular fibrinolysis (IVF) in the increased clearance of the blood, lysis of clots, the reduction of hydrocephalus and even of mortality.1,3–7,11,12,20–22 Such results have been questioned due to methodological problems in the design and performance of the studies.1
Secondary IVH, whose main causes are intraparenchymal haemorrhage (40%) and subarachnoid haemorrhage (15%), has a worse prognosis than the primary.3,10 It has been linked with a greater mortality in advanced age, prior coagulopathy, a score of 8 or less on the Glasgow Coma Scale and the presence of secondary hydrocephalus on admission.10 There is some controversy over whether the extent is correlated with a worse prognosis.10
The purpose of our study was to evaluate from the prognosis standpoint the effectiveness and safety of an IVF protocol in the treatment of IVH.
Material and methodsWe carried out descriptive retrospective analysis of all the patients admitted to our General Intensive Care Unit (ICU) with a diagnosis of IVH and treated with IVF between the years 2000 and 2009. The unit is located in a tertiary hospital that has a reference population of 400,000 for neurosurgical procedures. The patients were identified through an in depth review of our prospective database of admissions and discharges.
Graeb Score23 was used to stratify the severity of the IVH. For each lateral ventricle the scoring could be from 1 (with traces of blood) to 4 (full of blood and dilated). Scoring from 1 (with blood) to 2 (full of blood and dilated) was used to measure blood volume in the third and fourth ventricles. Maximum score is 12. IVH was classified in three levels, following recommendations: mild (Graeb score 0–5), moderate (Graeb score 6–8) and severe (Graeb score 9–12). Graeb score over 5 was the beginning criteria for IVF. All consecutive patients with a moderate or severe IVH (with or without hydrocephalus) were treated with IVF and included in the study. For the IVF, 10,000IU of intraventricular urokinase were administered every 12h under aseptic conditions. External ventricular drain was only clamped for 1h after urokinase administration. The external ventricular drains were placed by neurosurgeons in operating room or intensive care unit, and connected to cerebrospinal fluid (CSF) collection system (external ventricular drain system EDS 3; Codman®) and an intracranial pressure transductor (Camino® laboratories; NeuroCare San Diego, USA). CSF samples were routinely sent for culture and biochemical test each 24–48h. The IVF time was decided jointly between the intensivist and the neurosurgeon according to the clearance of intraventricular blood (Graeb Score 0–5).
Carrying out a review of the clinical histories, we gathered demographic data (age, gender), Glasgow Coma Score (GCS)24 at admission, clinical risk scores (Acute Physiology Score -APACHE II-,25 Simplified Acute Physiology Score -SAPS II-26), tomography scores (Graeb Score23) and risk factors for developing a cerebral haemorrhage (history of prior treatment with anti-platelet drugs or anticoagulants, or of hypertension). We recorded the cause of the IVH (primary or secondary), the performance and result of arteriography, the time of ventricular drainage and of IVF and the complications that arose in relation to the use of ventricular drains and their manipulation: ventriculitis, colonisation (defined according to the criteria proposed by previously published studies13–16), haemorrhage and obstruction. The prognostic outcome was determined using the Glasgow Outcome Score (GOS)27 at three months, and we reviewed the cause of death in those patients who had died.
For the statistical analysis, we used the SPSS 11.0 grad pack for Windows (SPSS® Inc., Chicago, IL). The continuous variables are expressed as the mean and standard deviation (SD) or median and range (r) according to their distribution. The categorical variables are presented as frequencies. We compared the deceased patients with the survivors through the relative risk (RR), the Student's t-test, the Chi-square test and Fisher's Exact Test as necessary. A value of p<0.05 was considered to be statistically significant.
ResultsWe carried out IVF on 42 patients (69% males), 16 of whom had a moderate IVH 38%) and 26 (62%) with a severe IVH according to Graeb's grading system. Almost 19% were primary IVH and the remainder secondary. The aetiology was traumatic in 4.8%, secondary to an SAH in 7.1%, and 88.1% were secondary to other haemorrhagic CVAs. The median Graeb score was 9 (range 6–12). The cerebral arteriography was performed in 21 patients; we found 10 with arteriovenous malformations, 6 with aneurysms and 5 with normal test.
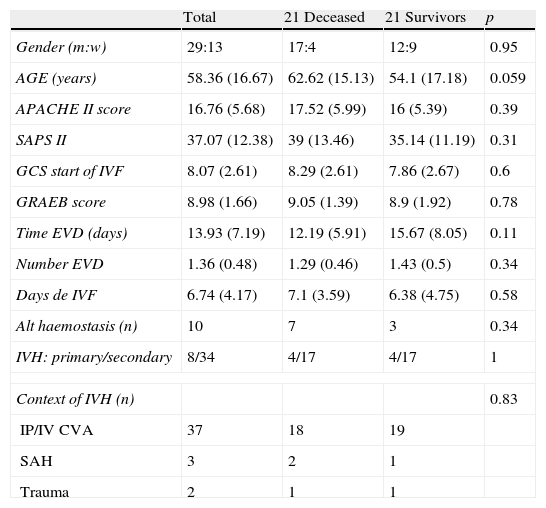
Characteristics of the group studiedWe have summarised the general characteristics of the group and the differences between those who died and the survivors in Table 1. The average age was 58.36 years (SD 16.67), with a median of APACHE II of 17.5 (range 3–29), and median SAPS of 39 (range 7–68). We found 16.7% were taking acenocoumarol and 7.1% anti-platelet agents. There was a history of hypertension in 38.1%.
Characteristics of the group studied.
| Total | 21 Deceased | 21 Survivors | p | |
| Gender (m:w) | 29:13 | 17:4 | 12:9 | 0.95 |
| AGE (years) | 58.36 (16.67) | 62.62 (15.13) | 54.1 (17.18) | 0.059 |
| APACHE II score | 16.76 (5.68) | 17.52 (5.99) | 16 (5.39) | 0.39 |
| SAPS II | 37.07 (12.38) | 39 (13.46) | 35.14 (11.19) | 0.31 |
| GCS start of IVF | 8.07 (2.61) | 8.29 (2.61) | 7.86 (2.67) | 0.6 |
| GRAEB score | 8.98 (1.66) | 9.05 (1.39) | 8.9 (1.92) | 0.78 |
| Time EVD (days) | 13.93 (7.19) | 12.19 (5.91) | 15.67 (8.05) | 0.11 |
| Number EVD | 1.36 (0.48) | 1.29 (0.46) | 1.43 (0.5) | 0.34 |
| Days de IVF | 6.74 (4.17) | 7.1 (3.59) | 6.38 (4.75) | 0.58 |
| Alt haemostasis (n) | 10 | 7 | 3 | 0.34 |
| IVH: primary/secondary | 8/34 | 4/17 | 4/17 | 1 |
| Context of IVH (n) | 0.83 | |||
| IP/IV CVA | 37 | 18 | 19 | |
| SAH | 3 | 2 | 1 | |
| Trauma | 2 | 1 | 1 | |
m: men; w: women. GCS: Glasgow Coma Score; IVF: intraventricular fibrinolysis; Time EVD: days with external ventricular drainage; Alt haemostasis: treatment with acenocoumarol or with anti-platelet drugs; IVH: intraventricular haemorrhage; Context of IVH: with relation to IP/IV CVA: intraparenchymal/intraventricular cerebrovascular accident, SAH: subarachnoid haemorrhage.
Comparison tests (Student's t-test, Pearson’ Chi-square test, and Fisher's Exact Test according to the type of variable) of averages or frequencies with a significance level of p<0.05.
Clinically, at the beginning of IVF, patient's median GCS was 8 (range of 3–13) and received intraventricular fibrinolysis for a median time of 6 days (range 1–21). We placed 57 external ventricular drains (two in 15 patients), with a median shunt time of 12.5 days (range 1–33).
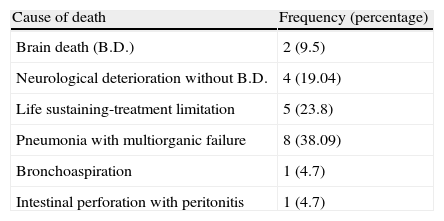
Progress and risk factorsDuring the first three months, 21 patients died (50%): 17 in the general ICU (9 of them from neurological causes and the rest due to septic or respiratory complications), 3 in the neurosurgical ward (1 from neurological deterioration, another from bronchoaspiration and the third from pneumonia with multiorganic failure), and lastly, another patient died following neurological deterioration in a centre for chronic patients in the second month after the ICU admission (Table 2).
We did not find statistically significant differences between those who died and the survivors in any of the variables studied, although the greater age of those in the first group was close to the significance level (62.62 compared to 54.1 with p=0.059).
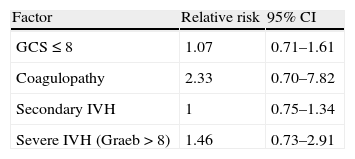
Secondary IVH, previous changes in coagulation, or coma on admission did not lead to an increase in mortality compared to the rest (Table 3). We found a mortality 1.46 times greater in those patients with a severe IVH compared to those with a moderate IVH, with a tendency to statistical significance (36.84% compared to 53.84%, 95% CI: 0.73–2.91).
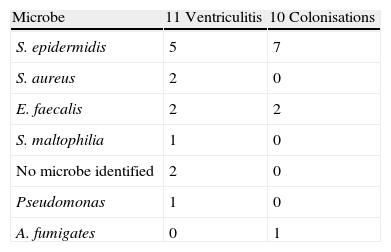
ComplicationsThree patients had a second bleed (one of these died) and 26.2% suffered from ventriculitis (11 patients) predominantly secondary to Gram positive bacteria (63%); two of these had polymicrobial aetiology. The percentage of colonisation of the ventricular shunt systems, and the microbiological agents involved, were similar to that of ventriculitis (Table 4). The development of this complication did not lead to an increase in risk of mortality (RR 0.83, 95% CI: 0.3–2.31).
DiscussionAn intraventricular haemorrhage may occur spontaneously or be secondary to an intracerebral haemorrhage or a subarachnoid haemorrhage. Its prognosis depends on the extent of the haemorrhage and the intensity of the initial symptoms,3 with estimated mortality rates of 50–80%3,5–9 and a significant associated morbidity.1–10 When secondary to an intraparenchymal haematoma it is an independent mortality factor.3,28
Occasionally, it leads to a communicating hydrocephalus or an obstructive hydrocephalus1,3,10 that may require drainage of the cerebrospinal fluid or blood through an external ventricular drain. There has been much debate on the usefulness of this measure, as there has been no recorded decrease in morbimortality1 and, on the other hand, it has been associated with certain complications, the most important of which is ventriculitis.3,13–18 There are studies that suggest the effectiveness of intraventricular fibrinolytic treatment in the increase of blood clearance, lysis of clots,5,11,12,19–22 the reduction of hydrocephalus and even of mortality.3–7,11,12,19–22,29 However, Lapointe and Haine1 recently analysed the safety and efficacy of intraventricular thrombolysis for the Cochrane review, concluding that no random trials exist of sufficient quality or size to allow evaluation of the safety and therapeutic usefulness of the use of intraventricular fibrinolytic therapy in adults with IVH. In their review, they document improvements in intraventricular blood clearance and prognosis, published in series of cases, random trials and one quasi-random. The in-depth review that Nyquist5 published a year before concludes that the fibrinolytic therapy may improve survival rates, reduce the number of patients requiring prolonged EVD and reduce length of stay in medical intensive care units. Following the improved prognosis described in the recent results of the CLEAR IVH trial30 (prospective but non randomized) and while waiting for the final results of the next – CLEAR III31 – the use of intraventricular thrombolytics seems justified in the treatment of IVH.
We present a retrospective analysis of 42 patients with IVF used in the treatment of IVH. The majority of articles published on this subject refer to smaller sample sizes. Our protocol for fibrinolysis, also used by other authors,3,5,6,11 has not undergone modifications in its application since use of this therapy was begun in our centre. For reasons of economy and formulation of the preparations, we chose urokinase rather than r-TPA for intraventricular administration. Both therapies have given good results in clearance and clot lysis with a similar rate of complications.3–5,9
Prognosis and associated complicationsIn our study, the severity of the patient's state was assessed by specific quantitative scales, enabling an analysis to be made of the prognosis and the risk factors associated with mortality. Advanced age, secondary IVH, comatose state and hydrocephalus on admission and previous coagulopathy seem to worsen intraventricular haemorrhage's prognosis.10 The possible implication of a greater extent of the haemorrhage in increased mortality has not been clarified.3,8,10 In our series, none of the variables showed statistically significant differences between the patients who died and those who did not, although the data suggest that advanced age, Graeb's scale over 8 and a change in haemostasis due to acenocoumarol or antiaggregants, worsen prognosis. It is possible that through analysis of wider series, the statistic potential may be improved. The design of the study we present assessed hydrocephalus according to the Graeb scoring, and we were not able to obtain data on its implication in isolation in the IVH prognosis.
The mortality we have found at 3 months was 50% and is in agreement with the data published for IVH.3.5–9 Vereecken6 reports studies with a mortality close to 100%; some authors relate this to the extent of occupation of the ventricle by the bleed.3 In our series, almost 62% of the patients had a severe IVH (Graeb greater than 8); the estimated mortality for this grading is much higher than we have found in our patients. Having a protocol for neurocritical patient care, the use fibrinolytic therapy and the fact that our review has not analysed the prognosis beyond the first three months may explain this difference. The prognosis we have observed is encouraging; in the survivors, the neurological situation at three months is favourable in over 50%, 28.57% suffered a severe disability and 23.8% were in a persistent vegetative state. Huttner et al.,4 with a smaller series, do not report statistically significant better prognoses with the use of intraventricular rt-PA. Their comparative analysis with non-fibrinolysed patients includes intraventricular bleeds measured by Graeb as being less serious than those we have analysed. It is worth noting the disproportionate number of changes of the ventricular drainages carried out in their patients because of clotting in the system (59% in the fibrinolysed patients against 32% in the rest, with p=0.08).
The need for external ventricular drains to control intracranial pressure, for removal of blood and the administration of local treatments has been questioned because of the risk of complications, ventriculitis being the most important.13–18,29 Its incidence varies according to the diagnostic criteria,17 with figures published of around 20%.13–18 In this series, 26.2% of the patients developed ventriculitis; however, this did not imply a worse prognosis (RR 0.83, CI 95%: 0.3–2.31). According to our data on infection associated with ventriculostomy,32 intraventricular haemorrhage and intraventricular fibrinolysis carry with them a significant risk of infection associated with ventriculostomy, with an RR compared to the rest of patients with ventricular drainage of 1.44 (CI 95%: 0.91–2.28) for IVH and of 2.33 (CI 95%: 1.33–4.2) for IVF. The predominant infectious agents were the Gram positive cocci, already described by other authors.13–18 Fountas et al.9 published an incidence of ventriculitis in patients with IVF of 14.3%, but with different diagnostic criteria.
Published haemorrhagic complications (recurrent IVH or intracerebral haemorrhage) with IVF vary from 8 to 20%,10 although some often exclude IVH resulting from arteriovenous malformations or aneurysms. In our review, 7.1% of the patients bled after beginning IVF although we treated sixteen patients with vascular anomalies; the cerebral arteriography was only made in 50% of the patients, so it was not possible to establish a direct relationship between the two factors, and they did not entail an increase in mortality risk.
Limitations of the studyWe identified various limitations in our study; its retrospective character is associated with possible errors in the collection and interpretation of the data from the clinical history. We did not record the time taken to clear the ventricles of blood content as the time of IVF was decided jointly with the neurosurgeon depending on the clinical progress and the clearance of intraventricular blood and was suspended in those patients with a bad neurological outcome, life sustaining-treatment limitation or improvement shown by tomography in the ventricular contamination with blood with a Graeb Score under 5. We cannot present data of the patients with mild IVH (Graeb under 5) as they were not an objective of our study.
ConclusionsIn our patients, IVH is an entity with a high morbimortality. Intraventricular fibrinolysis is associated with the development of certain complications that do not worsen the prognosis. While we await the results of the CLEAR III study, care protocols for the neurocritical patient that include the appropriate management of ventricular drainage and the administration of IVF in moderate or serious IVH, would appear to contribute to lower mortality rates and better functional results at three months than those described in the bibliographic references.
Conflict of interestThe authors declare no conflict of financial interest.
Please cite this article as: Castaño Ávila S., et al. Hemorragia intraventricular tratada con fibrinólisis local: experiencia de 10 años. Med Intensiva. 2011. http://dx.doi.org/10.1016/j.medine.2012.02.012\.