To determine the predictive value of the shock index and modified shock index in patients with massive bleeding due to severe trauma.
DesignRetrospective cohort.
SettingSevere trauma patient's initial attention at the intensive care unit of a tertiary hospital.
SubjectsPatients older than 14 years that were admitted to the hospital with severe trauma (injury severity score >15) form January 2014 to December 2015.
VariablesWe studied the sensitivity (Se), specificity (Sp), positive and negative predictive value (PV+ and PV−), positive and negative likelihood ratio (LR+ and LR−), ROC curves (receiver operating characteristics) and the area under the same (AUROC) for prediction of massive hemorrhage.
Results287 patients were included, 76.31% (219) were male, mean age was 43.36 (±17.71) years and ISS was 26 (interquartile range [IQR]: 21–34). The overall frequency of massive bleeding was 8.71% (25). For shock index: AUROC was 0.89 (95% confidence intervals [CI] 0.84–0.94), with an optimal cutoff at 1.11, Se was 91.3% (95% CI: 73.2–97.58) and Sp was 79.69% (95% CI: 74.34–84.16). For the modified shock index: AUROC was 0.90 (95% CI: 0.86–0.95), with an optimal cutoff at 1.46, Se was 95.65% (95% CI: 79.01–99.23) and Sp was 75.78% (95% CI: 70.18–80.62).
ConclusionShock index and modified shock index are good predictors of massive bleeding and could be easily incorporated to the initial workup of patients with severe trauma.
Determinar la capacidad de predicción del índice de shock y del índice de shock modificado para hemorragia masiva tras sufrir un trauma grave.
DiseñoCohorte retrospectiva.
ÁmbitoAtención inicial hospitalaria al paciente con enfermedad traumática grave en una unidad de cuidados intensivos de trauma de un hospital terciario.
SujetosPacientes mayores de 14 años con trauma grave (injury severity score [ISS]>15), admitidos de forma consecutiva desde enero de 2014 hasta diciembre de 2015.
VariablesSe estudiaron sensibilidad (Se), especificidad (Sp), valores predictivos positivo y negativo (VP+ y VP−), razones de verosimilitud positiva y negativa (RV+ y RV−), curvas ROC (receiver operating characteristics) y el área bajo las mismas (AUROC) para predicción de hemorragia masiva.
ResultadosSe incluyeron 287 pacientes, el 76,31% (219) fueron varones, con una edad media de 43,36 (±17,71) e ISS de 26 (rango intercuartil [RIC]: 21-34). La frecuencia global de hemorragia masiva fue de 8,71% (25). Para el índice de shock se obtuvo: AUROC de 0,89 (intervalo de confianza [IC] 95%: 0,84-0,94), con un punto de corte óptimo en 1,11, Se del 91,3% (IC 95%: 73,2-97,58) y Sp del 79,69% (IC 95%: 74,34-84,16). Para el índice de shock modificado se obtuvo: AUROC de 0,90 (IC 95%: 0,86-0,95), con un punto de corte óptimo en 1,46, Se del 95,65% (IC 95%: 79,01-99,23) y Sp del 75,78% (IC 95%: 70,18-80,62).
ConclusionesEl índice de shock y el índice de shock modificado son buenos predictores de hemorragia masiva y de fácil aplicación durante la atención inicial del trauma grave.
Hemorrhagic shock is the leading cause of avoidable mortality following severe trauma. Its early identification remains a challenge both out and in hospital–a fact that results in deficient diagnosis and inappropriate patient transfer.1,2
The early diagnosis of hemorrhagic shock is essential to improve the outcomes after trauma and control bleeding. Early and precise prediction of hemorrhagic shock makes it possible to adequately prepare the initial patient management team, with prompt activation of the massive bleeding protocol (MBP).3–5
Retrospective studies analyze different predictive indices, assessing their capacity to diagnose massive bleeding (MB). These indices are difficult to implement, however: their determination requires laboratory test results and imaging data that are very time consuming and difficult to perform at out-hospital level.6
On the other hand, simple clinical parameters such as heart rate or arterial pressure have been shown to be inexact in predicting MB.7–9
The shock index (SI), defined as heart rate divided by systolic blood pressure, and the modified shock index (MSI), defined as heart rate divided by mean blood pressure, are simple and easy to apply, and have therefore been studied by a number of authors.
Different studies have shown that a high SI after severe trauma is associated to high mortality10–12 and to the severity of injury.13 The SI has also been used as a predictor of days on mechanical ventilation and of hospital stay,14 as well as of the probability of admission to critical care.15 Lastly, some authors have related SI to the need for blood transfusion and hemostatic intervention.16 Less is known of its capacity to predict MB, however.
The present study was carried out to determine the capacity of SI and MSI to define the optimum cutoff point in patients who have suffered severe trauma.
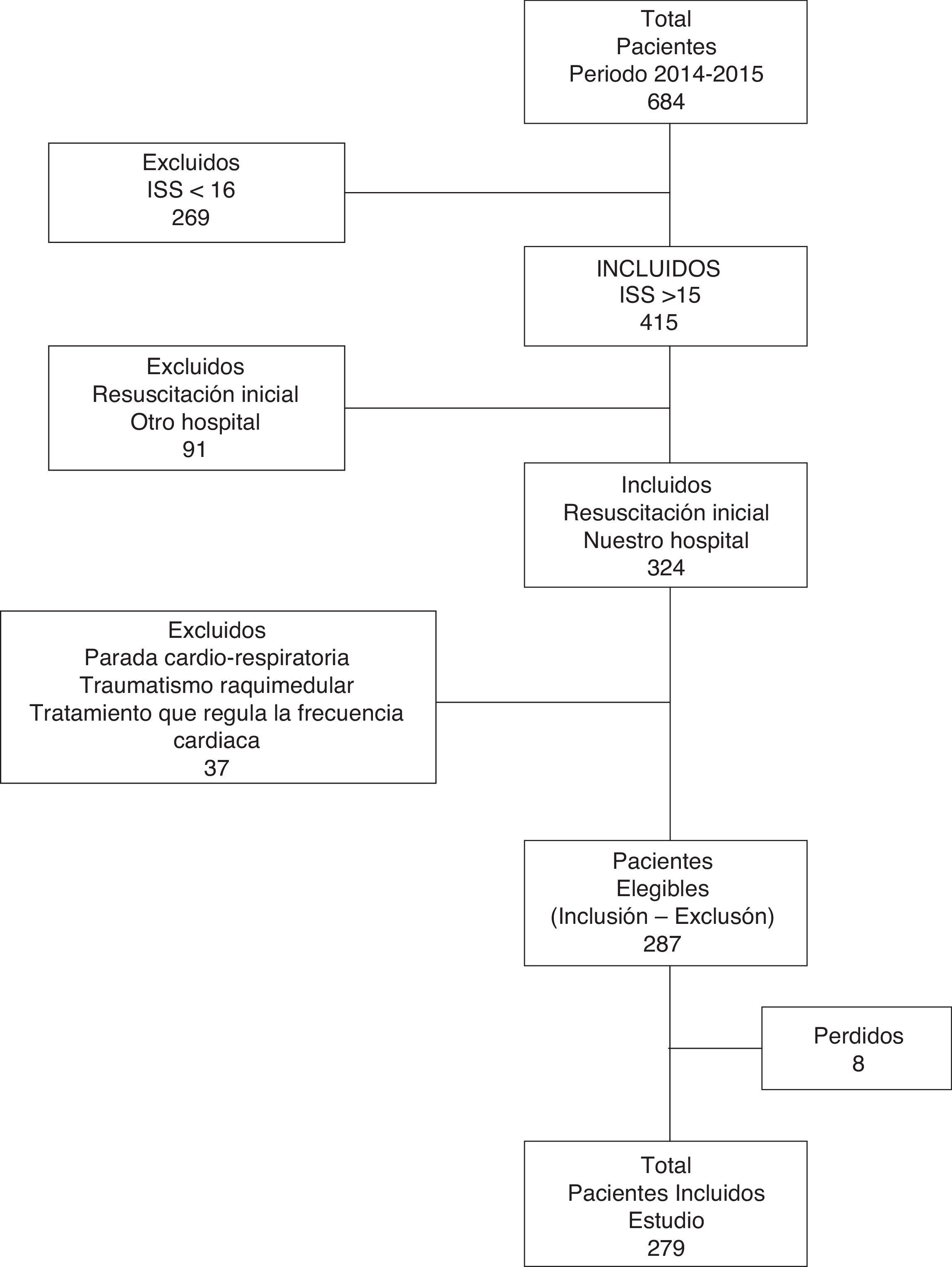
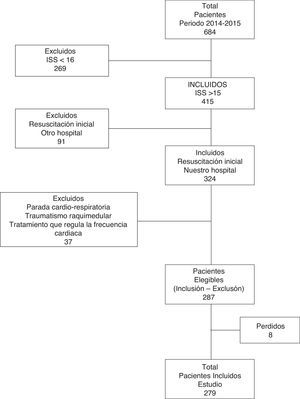
Design and methodsA retrospective cohort study involving the consecutive inclusion of all patients was carried out in the trauma and emergencies intensive care unit (ICU) of a tertiary hospital. We included patients over 14 years of age with severe trauma (injury severity score [ISS] >15) in which initial management took place upon arrival in hospital. The study patients were admitted between January 2014 and December 2015 (excluding individuals with cardiorespiratory arrest in out-hospital care, spinal cord injury, the use of heart rate-regulating drugs, and initial resuscitation performed in another center). Initial trauma care is provided by a specialized team composed of two intensivists (staff physician and resident), two nurses, a clinical assistant and two hospital attendants. The team can also consult different specialists related to trauma care. Management is carried out following the Advanced Trauma Life Support (ATLS) guidelines. The hospital has a MBP that has been approved by the transfusions commission and by hospital management.
The data were collected with masking of the observer: demographic variables (age and gender), physiological parameters (first recorded heart rate, systolic and diastolic blood pressure after arrival in hospital–initial resuscitation area of the ICU); laboratory test parameters (arterial blood gases–pH, base excess [BE], lactic acid) and prognostic variables (revised trauma score [RTS], ISS). The transfusions registry of our unit was consulted to document the number of packed red cell units transfused in the first 24h in each patient. Massive bleeding was defined as the administration of 10 or more packed red cell units in the first 24h of admission after trauma. Mathematical calculation of SI and MSI was subsequently made. As commented above, SI was defined as heart rate divided by systolic blood pressure, and MSI was defined as heart rate divided by mean blood pressure. No determination of MSI was made in patients without diastolic blood pressure. Lastly, we consulted the discharge reports to assess hospital stay and mortality.
Qualitative variables were reported as frequency and proportion, while quantitative variables were reported as the mean (±standard deviation) and median (interquartile range [IQR]). The χ2 test and Fisher exact test were used to estimate the association between two categorical variables, while the Student t-test was used for the comparison of two means (after checking normal distribution of the data with the Kolmogorov–Smirnov test, and homogeneity of variances with the Levene test). The Wilcoxon test was used in the case of a non-normal distribution. Statistical significance was considered for p≤0.05.
For each index we calculated sensitivity, specificity, positive predictive value (PV+), negative predictive value (PV−), positive likelihood ratio (LR+) and negative likelihood ratio (LR−). The receiver operating characteristic (ROC) curves were obtained, with calculation of the area under the curve (AUROC) and the corresponding 95% confidence interval (95% CI). The SPSS® version 19.0 statistical package for MS Windows was used throughout.
ResultsA total of 287 patients were studied (Fig. 1). Of these, 76.31% (n=219) were men. The mean age was 43.36 (±17.71) years and the median ISS score was 26 (IQR: 21–34). Closed trauma represented 91.6% of the cases (falls from a height being the most frequent cause). The overall MB rate was 8.71% (n=25); the median duration of stay in the ICU was 3.74 days (IQR: 1.81–11.55); and the mortality rate was 15.33% (n=44).
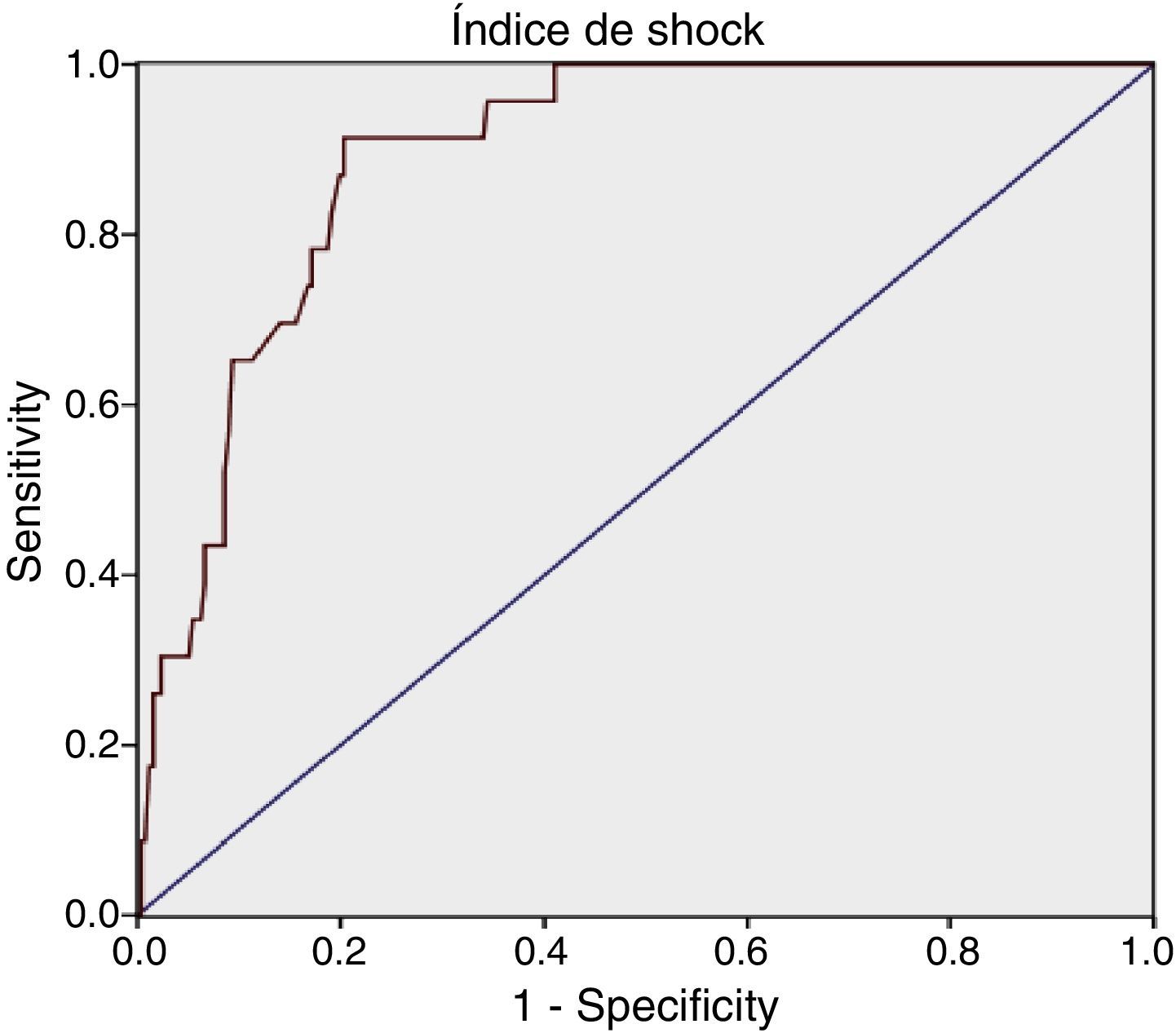
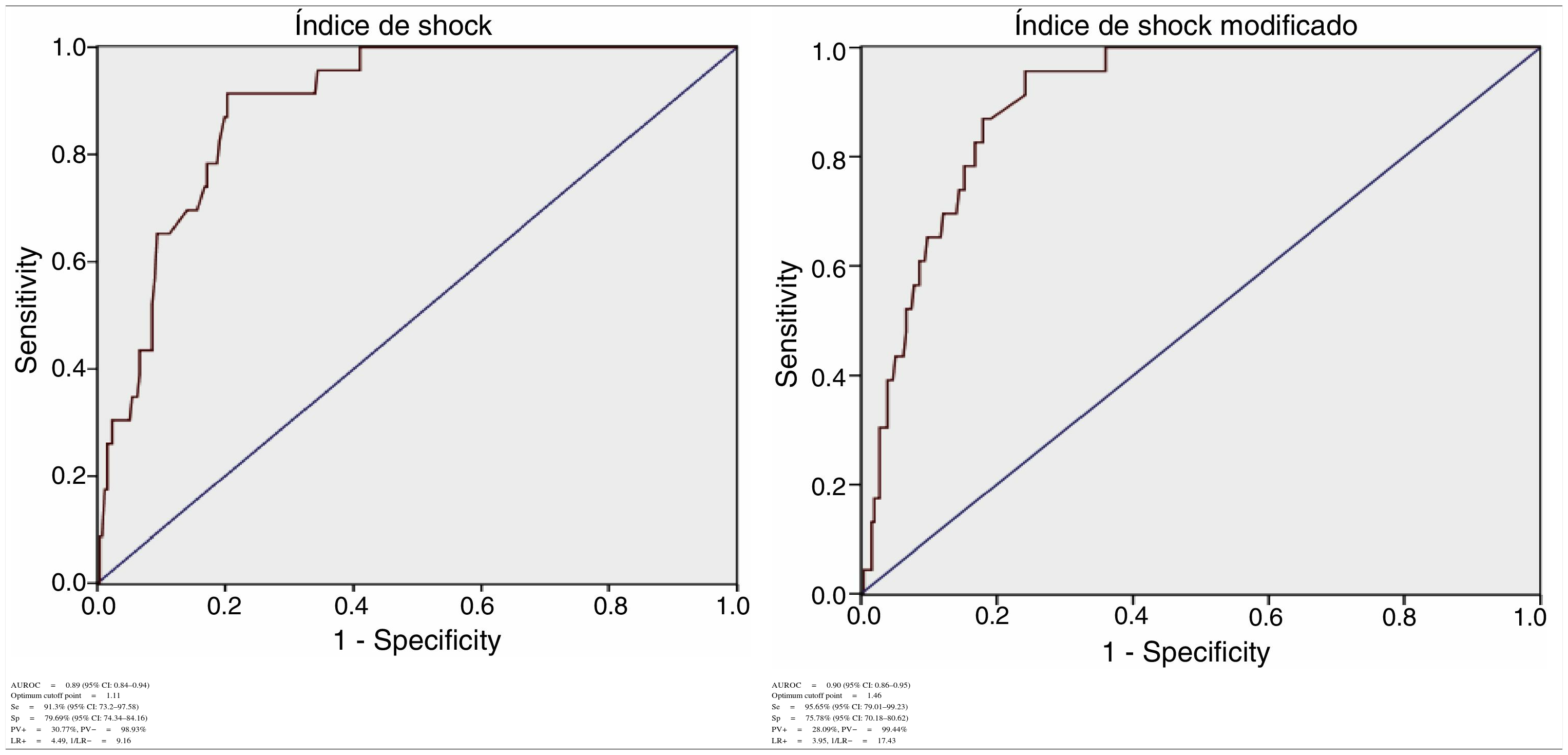
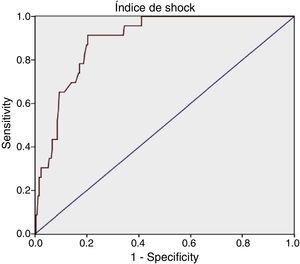
For SI we obtained: AUROC 0.89 (95% CI: 0.84–0.94), with an optimum cutoff point of 1.11, sensitivity 91.3% (95% CI: 73.2–97.58) and specificity 79.69% (95% CI: 74.34–84.16).
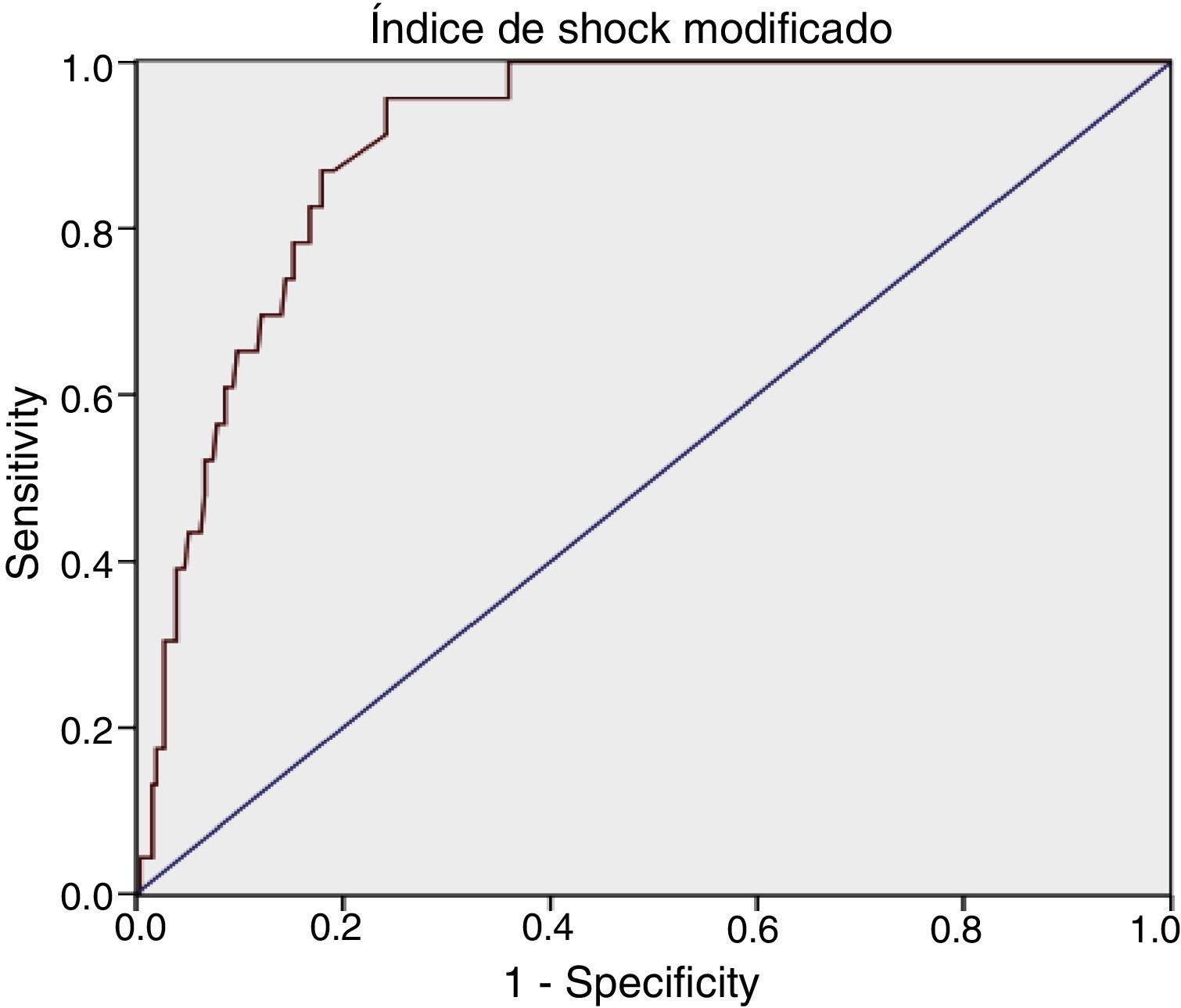
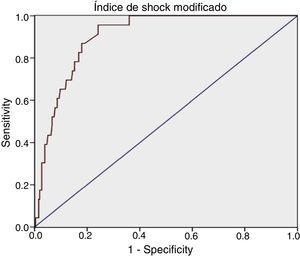
For MSI we obtained: AUROC 0.90 (95% CI: 0.86–0.95), with an optimum cutoff point of 1.46, sensitivity 95.65% (95% CI: 79.01–99.23) and specificity 75.78% (95% CI: 70.18–80.62) (Table 1).
Diagnostic determination of the shock index and modified shock index.
| AUROC=0.89 (95% CI: 0.84–0.94) Optimum cutoff point=1.11 | AUROC=0.90 (95% CI: 0.86–0.95) Optimum cutoff point=1.46 |
| Se=91.3% (95% CI: 73.2–97.58) | Se=95.65% (95% CI: 79.01–99.23) |
| Sp=79.69% (95% CI: 74.34–84.16) | Sp=75.78% (95% CI: 70.18–80.62) |
| PV+=30.77%, PV−=98.93% | PV+=28.09%, PV−=99.44% |
| LR+=4.49, 1/LR−=9.16 | LR+=3.95, 1/LR−=17.43 |
AUROC: area under the ROC curve; CI: confidence interval; LR+: positive likelihood ratio; LR−: negative likelihood ratio; Se: sensitivity; Sp: specificity; PV+: positive predictive value; PV−: negative predictive value.
An a posteriori analysis was made with the defined cutoff points, contrasting different variables to obtain significant differences for MB, the severity of injury (ISS), coagulopathy, ICU stay and mortality (Table 2).
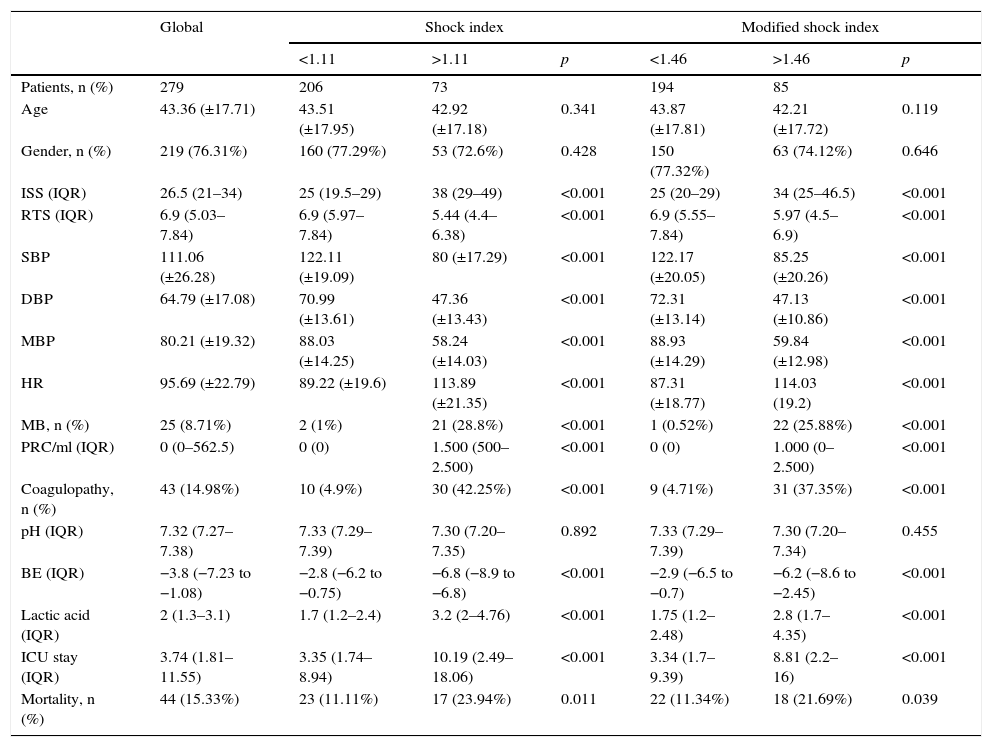
Characteristics of the patients according to the shock index and modified shock index.
| Global | Shock index | Modified shock index | |||||
|---|---|---|---|---|---|---|---|
| <1.11 | >1.11 | p | <1.46 | >1.46 | p | ||
| Patients, n (%) | 279 | 206 | 73 | 194 | 85 | ||
| Age | 43.36 (±17.71) | 43.51 (±17.95) | 42.92 (±17.18) | 0.341 | 43.87 (±17.81) | 42.21 (±17.72) | 0.119 |
| Gender, n (%) | 219 (76.31%) | 160 (77.29%) | 53 (72.6%) | 0.428 | 150 (77.32%) | 63 (74.12%) | 0.646 |
| ISS (IQR) | 26.5 (21–34) | 25 (19.5–29) | 38 (29–49) | <0.001 | 25 (20–29) | 34 (25–46.5) | <0.001 |
| RTS (IQR) | 6.9 (5.03–7.84) | 6.9 (5.97–7.84) | 5.44 (4.4–6.38) | <0.001 | 6.9 (5.55–7.84) | 5.97 (4.5–6.9) | <0.001 |
| SBP | 111.06 (±26.28) | 122.11 (±19.09) | 80 (±17.29) | <0.001 | 122.17 (±20.05) | 85.25 (±20.26) | <0.001 |
| DBP | 64.79 (±17.08) | 70.99 (±13.61) | 47.36 (±13.43) | <0.001 | 72.31 (±13.14) | 47.13 (±10.86) | <0.001 |
| MBP | 80.21 (±19.32) | 88.03 (±14.25) | 58.24 (±14.03) | <0.001 | 88.93 (±14.29) | 59.84 (±12.98) | <0.001 |
| HR | 95.69 (±22.79) | 89.22 (±19.6) | 113.89 (±21.35) | <0.001 | 87.31 (±18.77) | 114.03 (19.2) | <0.001 |
| MB, n (%) | 25 (8.71%) | 2 (1%) | 21 (28.8%) | <0.001 | 1 (0.52%) | 22 (25.88%) | <0.001 |
| PRC/ml (IQR) | 0 (0–562.5) | 0 (0) | 1.500 (500–2.500) | <0.001 | 0 (0) | 1.000 (0–2.500) | <0.001 |
| Coagulopathy, n (%) | 43 (14.98%) | 10 (4.9%) | 30 (42.25%) | <0.001 | 9 (4.71%) | 31 (37.35%) | <0.001 |
| pH (IQR) | 7.32 (7.27–7.38) | 7.33 (7.29–7.39) | 7.30 (7.20–7.35) | 0.892 | 7.33 (7.29–7.39) | 7.30 (7.20–7.34) | 0.455 |
| BE (IQR) | −3.8 (−7.23 to −1.08) | −2.8 (−6.2 to −0.75) | −6.8 (−8.9 to −6.8) | <0.001 | −2.9 (−6.5 to −0.7) | −6.2 (−8.6 to −2.45) | <0.001 |
| Lactic acid (IQR) | 2 (1.3–3.1) | 1.7 (1.2–2.4) | 3.2 (2–4.76) | <0.001 | 1.75 (1.2–2.48) | 2.8 (1.7–4.35) | <0.001 |
| ICU stay (IQR) | 3.74 (1.81–11.55) | 3.35 (1.74–8.94) | 10.19 (2.49–18.06) | <0.001 | 3.34 (1.7–9.39) | 8.81 (2.2–16) | <0.001 |
| Mortality, n (%) | 44 (15.33%) | 23 (11.11%) | 17 (23.94%) | 0.011 | 22 (11.34%) | 18 (21.69%) | 0.039 |
PRC: packed red cell units; BE: base excess; HR: heart rate; MB: massive bleeding; ISS: injury severity score; DBP: diastolic blood pressure; MBP: mean blood pressure; SBP: systolic blood pressure; IQR: interquartile range; RTS: revised trauma score; ICU: intensive care unit.
Hemorrhagic shock is the type of shock most often seen in severe trauma patients, and its early identification is crucial to patient prognosis.
Our study shows both shock indices to offer good MB predicting capacity, and the results obtained are comparable to those of other studies that describe SI and MSI as potentially useful for the identification of hemorrhagic shock both in and out of hospital.
In the out-hospital setting, Mitra et al. used an SI cutoff point ≥1 for predicting MB (the latter being defined as at least 5 packed red cell units in 4h), resulting in a sensitivity of 47.9% and a specificity of 90.5%.17 Vandromme et al., in patients assisted on an out-hospital basis, recorded a significant increase in the risk of MB (the latter being defined as at least 10 packed red cell units in 24h) among patients with SI >0.9.18
In the in-hospital setting, Mutschler et al. found that with SI between 1–1.4 and >1.4, a total of 31% and 57% of the patients require at least 10 packed red cell units in the first 24h, respectively.19 Hagiwara et al. in turn found that in patients with MB (the latter being defined as at least 4 packed red cell units in 24h), the mean SI was 1.05 (±0.58), versus 0.64 (±0.21) in patients without MB.20
Both SI and MSI can be modified by a number of factors. For example, at out-hospital level, the presence of pain and anxiety can cause tachycardia, and this in turn increases the mentioned indices. Another conditioning factor is the use of manual or automatic systems for recording blood pressure. In this regard, it is known that measuring arterial pressure with automatic systems results in increased pressure values, and this in turn lowers the index scores. The influence of treatment in the out-hospital setting also must be taken into account. Hagiwara et al. found that patients with bleeding and who received at least 1l of fluid therapy in the out-hospital setting presented higher SI scores (1.1±0.6 versus 1.05±0.58).20 On the other hand, differences are observed according to whether the indices are estimated on a point basis or in the form of repeated measurements. In this regard, Chen et al. concluded that the determination of SI over a period of time is more predictive of MH.21
Both SI and MSI have been studied in application to parameters other than the prediction of MB. In effect, high index scores have been associated to increased mortality, severity of injury (ISS) and days of stay in the ICU. Cannon et al. found that patients with an SI score of 0.9 and who have suffered severe trauma present higher mortality rates.10 McNab et al. in turn examined the trends in SI scores during patient transfer to hospital and found an increase of ≥0.3 points to be associated to increased mortality (27.6% versus 5.8%) as well as to longer hospital stay.14 Our study likewise recorded differences in the severity of injury (ISS), coagulopathy, ICU stay and mortality.
The present study has many limitations, including the fact that the variables were compiled on a retrospective basis. Nevertheless, most studies published to date also involve a retrospective design, as demonstrated by Olaussen et al. in a review in which only 5 of the 351 studies identified in the literature search met their inclusion criteria for assessing SI as a predictor of critical bleeding.22
Another fundamental limitation is the lack of a universal definition of MB. The definition of 10packed red cell units in 24h introduces bias in that it excludes early deaths as well as patients with active bleeding and posterior bleeding control (arteriography and/or operating room) that did not receive 10 units, and the inclusion of patients not requiring acute transfusion.23 Our group conducted a subanalysis of the patients that received between 4 and 10 packed red cell units in the first 24h: 39 patients had a probable active bleeding point (need for 4–10 packed red cell units in 24h), and of these, 84.7% (n=33) suffered hemodynamic instability, 53.8% (n=21) required surgery and/or arteriography for bleeding control, and 7 died from refractory hemorrhagic shock without receiving 10 packed red cell units. Because of this, and in coincidence with Hagiwara et al.20 and Olaussen et al.,22 we consider it necessary to revise the diagnostic criteria referred to MB and to seek a definition offering greater sensitivity in identifying the condition (at least 4 packed red cell units in 24h or at least 5 packed red cell units in 4h).23,24
ConclusionsBoth SI and MSI are good predictors of MB and are easy to apply during initial management of severe trauma. Our cutoff point for SI is 1.11, versus 1.46 for MSI.
AuthorshipLuis Juan Terceros-Almanza: literature search, database, data collection, statistical analysis, drafting of the manuscript. Carlos García-Fuentes: literature search, database, data collection, statistical analysis, drafting of the manuscript. Susana Bermejo-Aznárez: drafting of the manuscript. Isidro Javier Prieto-del Portillo: data collection. Carolina Mudarra-Reche: data collection. Ignacio Sáez-de la Fuente: data collection. Mario Chico-Fernández: drafting of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the Trauma and Emergencies Unit of the Department of Intensive Care Medicine of Hospital Universitario 12 of Octubre (Madrid, Spain).
Please cite this article as: Terceros-Almanza LJ, García-Fuentes C, Bermejo-Aznárez S, Prieto-del Portillo IJ, Mudarra-Reche C, Sáez-de la Fuente I, et al. Predicción de hemorragia masiva. Índice de shock e índice de shock modificado. Med Intensiva. 2017;41:532–538.