On 26 September 2020, this journal published an important article that reported on the existence of a series of statistically significant associations (P < .05) between different genetic polymorphisms of the human leukocyte antigen (HLA) system and mortality due to COVID-19 in 72 patients,1 one of which included the HLA-A*11 gene, based on measurement of the odds ratio (OR = 7.693).
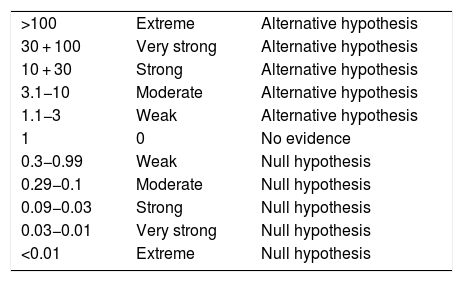
The replication of clinical investigations based on significance tests is advised. This can be done through Bayesian inference, since it allows us to reanalyze the significant finding reported by Lorente et al.,1 where the Bayes factor (BF) method is referred to as the probability of the data under one hypothesis in relation to the other hypothesis (null hypothesis versus alternative hypothesis).2,3 In other words, the BF estimates quantification of the strength of proof on which the data support both hypotheses in contrasting them, beyond the dichotomic interpretation or rejection or acceptance of the null hypothesis.2,3 The statistical replication of significant findings with the BF allows us to strengthen the practical credibility of articles in the field of Intensive Care Medicine (experimental research, clinical trials, interventions and treatments). This is required when Bayesian inference reports conclusive or greater level evidence (BF10 > 10), based on Jeffrey’s classification scale4 for BF: weak, moderate, strong, very strong and extreme (Table 1).
Bayes factor quantifiable interpretation values.
| >100 | Extreme | Alternative hypothesis |
| 30 + 100 | Very strong | Alternative hypothesis |
| 10 + 30 | Strong | Alternative hypothesis |
| 3.1−10 | Moderate | Alternative hypothesis |
| 1.1−3 | Weak | Alternative hypothesis |
| 1 | 0 | No evidence |
| 0.3−0.99 | Weak | Null hypothesis |
| 0.29−0.1 | Moderate | Null hypothesis |
| 0.09−0.03 | Strong | Null hypothesis |
| 0.03−0.01 | Very strong | Null hypothesis |
| <0.01 | Extreme | Null hypothesis |
Note: Proprietary table based on Jeffrey’s classification scale.4
The aim of the present letter is to report an example of Bayesian reanalysis to define the level of evidence of statistical hypotheses. Accordingly, consideration was made of the sample size and conversion of OR to correlation effect size (r) through an online calculator,5 where the conversion value was r = 0.49. This method considers two interpretations: BF10 (in favor of the alternative hypothesis) and BF01 (in favor of the null hypothesis), with a credibility interval of 95%. The results obtained for BF are: BF10 = 1.690 and BF01 = 0.0006, with 95%CI: 0.284−0.64, which warrants the significant finding reported by Lorente et al.,1 with extreme evidence in favor of the alternative hypothesis (correlation). This allows us to infer that the rest of statistical associations of greater magnitude between the HLA genes and mortality also exhibit extreme evidence – thus contributing greater credibility to the clinical conclusions drawn in the mentioned study.
In conclusion, the inclusive use of effect size conversion and the BF is a great methodological contribution that has practical implications for medical decision making, based on the confirmation of results that are effectively conclusive and of greater relevance in the context of COVID-19.
Financial supportThis study has received no specific funding from the public, commercial or non-profit sectors.
Please cite this article as: Ramos-Vera C. Uso inclusivo de la conversión del tamaño de efecto y del factor Bayes en la investigación de medicina intensiva. Med Intensiva. 2022;46:171–172.