To determine the relative effectiveness of Helmet-CPAP (H_CPAP) with respect to high-flow nasal cannula oxygen therapy (HFNO) in avoiding greater need for intubation or mortality in a medium complexity hospital in Chile during the year 2021.
DesignCohort analytical study, single center.
SettingUnits other than intensive care units.
PatientsRecords of adults with mild to moderate hypoxemia due to coronavirus type 2.
InterventionsNone.
Main variables of interestNeed for intubation or mortality.
Results159 patients were included in the study, with a ratio by support of 2:10 (H_CPAP:HFNO). The 46.5% were women, with no significant differences by sex according to support (p = 0.99, Fisher test). The APACHE II score, for HFNO, had a median of 10.5, 3.5 units higher than H_CPAP (p < 0.01, Wilcoxon rank sum). The risk of intubation in HFNO was 42.1% and in H_CPAP 3.8%, with a significant risk reduction of 91% (95% CI: 36.9%–98.7%; p < 0.01). APACHE II does not modify or confound the support and intubation relationship (p > 0.2, binomial regression); however, it does confound the support and mortality relationship (p = 0.82, RR homogeneity test). Despite a 79.1% reduction in mortality risk with H_CPAP, this reduction was not statistically significant (p = 0.11, binomial regression).
ConclusionsThe use of Helmet CPAP, when compared to HFNO, was an effective therapeutic ventilatory support strategy to reduce the risk of intubation in patients with mild to moderate hypoxemia caused by coronavirus type 2 in inpatient units other than intensive care. The limitations associated with the difference in size, age and severity between the arms could generate bias.
Determinar la efectividad relativa del Helmet-CPAP (H_CPAP) respecto a la oxigenoterapia con cánula nasal de alto flujo (CNAF) para evitar mayor necesidad de intubación o mortalidad en un hospital de mediana complejidad en Chile durante el año 2021.
DiseñoEstudio analítico de cohorte, en centro único.
ÁmbitoUnidades clínicas que no sean de cuidados intensivos.
PacientesRegistros de adultos con hipoxemia leve a moderada debida a coronavirus tipo 2.
IntervencionesNinguna.
Principales variables de interésNecesidad de intubación o mortalidad.
ResultadosSe incluyeron 159 pacientes en el estudio, con una razón por soporte de 2:10 (H_CPAP:HFNO). El 46,5% fueron mujeres, sin diferencias significativas por sexo según soporte (p = 0,99, test de Fisher). La puntuación APACHE II, para CNAF, tuvo una mediana de 10,5, 3,5 unidades mayor que H_CPAP (p < 0,01, Wilcoxon rank sum). El riesgo de intubación en CNAF fue del 42,1% y en H_CPAP del 3,8%, con una reducción significativa del riesgo del 91% (IC 95%: 36,9% a 98,7%; p < 0,01). APACHE II no modifica ni confunde la relación soporte e intubación (p > 0,2, regresión binomial); sin embargo, sí confunde la relación soporte y mortalidad (p = 0,82, test de homogeneidad RR). A pesar de que se observa un 79,1% de reducción del riesgo de mortalidad con H_CPAP, esta reducción no fue estadísticamente significativa (p = 0,11, regresión binomial).
ConclusionesEl uso de Helmet CPAP, al compararse con CNAF, fue una estrategia de soporte ventilatorio terapéutico eficaz para reducir el riesgo de intubación en pacientes con hipoxemia leve a moderada causada por coronavirus tipo 2 en unidades de hospitalización distintas de cuidados intensivos. Las limitaciones asociadas a la diferencia de tamaño, edad y gravedad entre los brazos de estudio podrían generar sesgo.
During the peak of the coronavirus type 2 pandemic, in addition to the use of high-flow nasal cannula oxygen, continuous positive airway pressure was also used in hospitalization units other than intensive care units, due to the saturation of these services due to the overdemand of patients. In Chile, until the end of December 2021, 2,156,268 cases of COVID occurred, with a cumulative incidence rate of 11,081.5 per 1,000,000 inhabitants. According to official data delivered by the EPIVIGILA epidemiological surveillance system of the MINSAL Department of Epidemiology, by the end of 2021 the total number of deaths reported was 133,735 which represented 19.26% of the total number of deaths in the country; of the total number of critical beds available (1967), 1729 were occupied, of which 83.91% were used for invasive mechanical ventilation and 16.09% without mechanical ventilation. There is no official report on the use of noninvasive mechanical ventilation in inpatient units other than intensive care.
The use of high-flow oxygen therapy (HFO) through nasal cannula (HFNO) was the most widely used therapeutic tool for the treatment of hypoxemia caused by coronavirus type 2.1,2 However, the use of continuous positive airway pressure (CPAP) is also a useful tool for the treatment of hypoxemia caused by other respiratory pathologies, showing a decrease in mortality.3 In general, values greater than 10 cmH2O of continuous positive airway pressure are required to correct mild to moderate hypoxemia.
Studies in the context of hypoxemia of non-infectious etiology have shown that the use of CPAP reduces the need for reintubation and also corrects hypoxemia.4 These studies support the benefit of CPAP in mild-moderate hypoxemia in children, in immunocompromised adults, in abdominal surgery and anesthesia, and mainly in hypoxemia of bacterial infectious etiology. In addition, there is little evidence regarding the use of CPAP with Helmet interface (H_CPAP) in hospital wards other than intensive care.
The main objective of this study is to analyze the potential effectiveness of the supports (H_CPAP and HFNO) in relation to response variables (intubation requirement and mortality).
Materials and methodsThis is an observational, analytical, single cohort study of hospital records from March to December 2021 of all patients at Hospital de Quilpué, Viña del Mar, Chile. The variables were collected in an anonymized electronic database. The primary objective was to analyze the effectiveness of H_CPAP in reducing the requirement for intubation or mortality, with respect to standard therapy with HFNO. The design of this study complied with the rigorous standards for design (STROBE).
Ventilatory supportHigh-flow oxygen therapy was provided by means of AIRVO2 (Fisher&Paykel®, New Zealand) brand nasal cannula, connected to the wall oxygen inlet and was regulated by the kinesiologist as indicated by the treating physician (specialist and non-specialist), based on the institutional protocol.
CPAP support was achieved by means of a flow generator system through a Venturi tube (DIMAR®, Italy) connected to oxygen mixing lines from two wall intakes, through high-flow flowmeters. Helmets were of two brands: Dimar® and Eolite® (Chile). Achievement of PEEP (CPAP level) within the Helmet was achieved through the use of an adjustable PEEP valve connected to the exhalation port downstream of the antibacterial/antiviral filter. Helmet CPAP was managed by the attending physician (specialist) and kinesiologist in charge, based on the institutional protocol.
Study populationThis study includes all available medical records of patients meeting the inclusion/exclusion criteria. Inclusion criteria were: patients with mild to moderate hypoxemia defined by PaO2/FiO2 (P/F) or SpO2/FiO2 (S/F) ratio and according to the Berlin criteria,5 with coronavirus 2 viral pneumonia confirmed by PCR test for SARS-CoV-2 and who were hospitalized in units other than intensive care units (ICU). Exclusion criteria were: patients with ventilatory exhaustion at the time of hospitalization, hypoxemia due to other causes, severe hypoxemia, terminal pathology, patients with neurological alteration, patients hospitalized in ICU.
The study group was composed of 159 patient records, with a 2:10 H_CPAP:HFNO ratio, for a confidence level of 95%.
The variables were collected sequentially at three times (before, during and after support), both ventilatory effort (clinical) and oxygen therapy (flow in L/min), laboratory tests, and arterial blood gases. In addition, the number of days of use until the outcome variable: intubation or death, was recorded.
This study has the approval of the Scientific Ethical Committee of the Viña del MarQuillota Health Service and the support of the authorities of the Quilpué Hospital. Given the characteristics of the design, the same Committee exceptionally granted an exemption from informed consent.
Statistical analysisSTATA v.18 (StatsCorp, College Station, TX, USA) was used for data analysis. A descriptive analysis of the data according to type of support was performed. Continuous variables are reported as means and standard deviations, and categorical variables are reported as percentages. Initial comparison of the groups was performed using the T-test for independent samples. Comparison between two nominal categorical variables was performed using Fisher’s exact test. This study contemplates the analysis of gas exchange variables, ventilatory work (P/F, S/F, FiO2, SpO2, flow, PEEP, respiratory rate, Cabrini/iROX score, PaO2, PCO2) according to the support. These variables were taken at three different observation times, so the statistical analysis used was linear regression with repeated measures.
The outcomes were evaluated dichotomously by establishing the presence of support failure, which induced intubation, patient death or patient recovery. In this scheme, the statistical model used was the binomial regression model to estimate the difference or risk ratio of exposed and unexposed, and in its absence the logistic regression model to estimate the Odds Ratio (OR) when convergence was not achieved. The construction of these models (multivariate) involves the identification of variables that modify or confound the relationship between exposure and outcome.
Additionally, the Cox regression model was used to estimate the Hazard according to treatment, in the presence of control variables.
The identification of the control variables for the models (multivariate) was done based on the clinical experience present in the theoretical framework and using Bayesian meta-logic for the calculation of the posterior inclusion probability (PIP) by using BMA (Bayes Model Average) with MCMC (Markov Chain Monte Carlo) strategy.
To evaluate the significance of the effect modifier variables, a significance level of 20% was used, and for the rest of the tests a significance level of 5% was used.
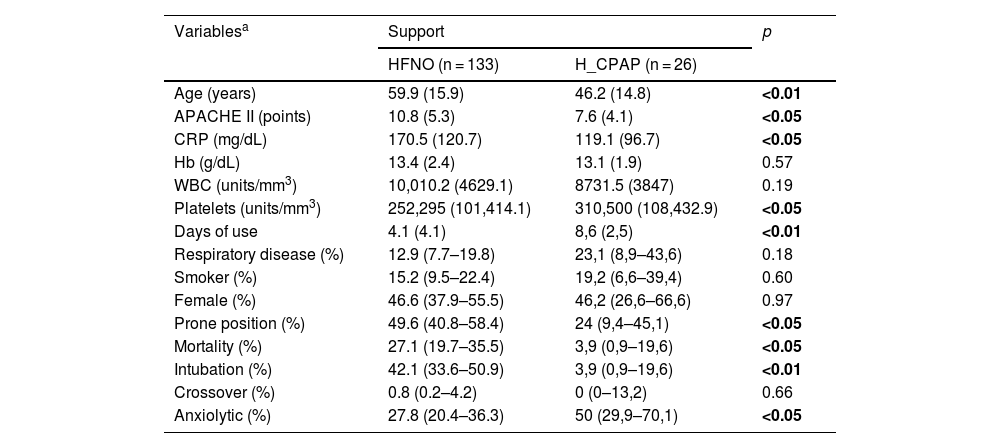
ResultsA total of 159 patients were included (HFNO = 133, H_CPAP = 27). The mean age was 59.9 years (SD: 15.9 years) for HFNO and 46.2 years (SD: 14.8 years) for H_CPAP; while female sex corresponded to 46.6% in HFNO and 46.2% for H_CPAP. The mean days of use until the event in the outcome variables were 4.1 days (SD: 4.1 days) for HFNO and 8.6 days (SD: 2.5 days) for H_CPAP (p < 0.01, T-test).
In HFNO, 42.1% (CI: 33.6%–50.9%) of intubations due to failure were observed, and in H_CPAP 3.9% (CI: 0.9%–19.6%). Regarding mortality, 27.1% (CI: 19.7%–35.5%) and 3.9% (CI: 0.9%–19.6%) were found for HFNO and H_CPAP, respectively. The rest of the baseline variables are described in Table 1.
Description and distribution of variables according to type of support (baseline).
| Variablesa | Support | p | |
|---|---|---|---|
| HFNO (n = 133) | H_CPAP (n = 26) | ||
| Age (years) | 59.9 (15.9) | 46.2 (14.8) | <0.01 |
| APACHE II (points) | 10.8 (5.3) | 7.6 (4.1) | <0.05 |
| CRP (mg/dL) | 170.5 (120.7) | 119.1 (96.7) | <0.05 |
| Hb (g/dL) | 13.4 (2.4) | 13.1 (1.9) | 0.57 |
| WBC (units/mm3) | 10,010.2 (4629.1) | 8731.5 (3847) | 0.19 |
| Platelets (units/mm3) | 252,295 (101,414.1) | 310,500 (108,432.9) | <0.05 |
| Days of use | 4.1 (4.1) | 8,6 (2,5) | <0.01 |
| Respiratory disease (%) | 12.9 (7.7–19.8) | 23,1 (8,9–43,6) | 0.18 |
| Smoker (%) | 15.2 (9.5–22.4) | 19,2 (6,6–39,4) | 0.60 |
| Female (%) | 46.6 (37.9–55.5) | 46,2 (26,6–66,6) | 0.97 |
| Prone position (%) | 49.6 (40.8–58.4) | 24 (9,4–45,1) | <0.05 |
| Mortality (%) | 27.1 (19.7–35.5) | 3,9 (0,9–19,6) | <0.05 |
| Intubation (%) | 42.1 (33.6–50.9) | 3,9 (0,9–19,6) | <0.01 |
| Crossover (%) | 0.8 (0.2–4.2) | 0 (0–13,2) | 0.66 |
| Anxiolytic (%) | 27.8 (20.4–36.3) | 50 (29,9–70,1) | <0.05 |
APACHE II, (Acute Physiology and Chronic Health Evaluation) prognostic classification scale according to disease severity; CRP, C-reactive protein; Hb, hemoglobin; WBC, white blood cell count; Prone, prone position in spontaneous ventilation; Crossover, passage from one support to another.
Bold values signifies values are statistically significant.
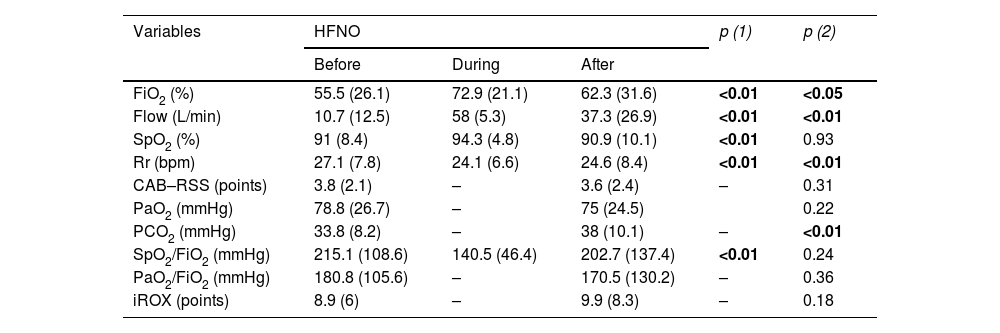
In the primary analysis, prior to support, mean FiO2 was 55.5% (SD: 26.1%) for HFNO and 45.1% (SD: 11.5%) for H_CPAP, and 62.3% (SD: 31.6%) and 27.4% (SD: 8.6%) post-support, for HFNO (p < 0.05) and H_CPAP (p < 0.01) respectively. The mean pre-support respiratory rate (Rr) was 27.1 bpm (SD: 7.8 bpm) for HFNO and 31.4 bpm (SD: 4 bpm) for H_CPAP. In HFNO, the Cabrini score (CAB-RSS) prior to the start of support was unchanged from post support [3.8 pts (SD: 2.1 pts) and 3.6 pts (SD: 2.4 pts) respectively, (p = 0.31)]; while for H_CPAP the same score (CAB-RSS) did show significant difference [4.5 pts (SD: 0.5 pts) and 0.5 pts (SD: 0.7 pts) respectively, (p < 0.01)]. PCO2 levels, prior to supports, were 33.8 mmHg (SD: 8.2 mmHg) and 34.2 mmHg (SD: 4.2 mmHg) for HFNO and H_CPAP. Mean pre-support SF was 215.1 mmHg (SD: 108.6 mmHg) for HFNO and 218.6 mmHg (SD: 47.1 mmHg) for H_CPAP, and post-support was 202.7 mmHg (SD: 137.4 mmHg) and 345.4 mmHg (SD: 95.7 mmHg) for HFNO (p = 0.24) and H_CPAP (p < 0.01) respectively. The rest of the variables are described in Table 2.
Sequential description of ventilatory and gas exchange variables according to type of support.
| Variables | HFNO | p (1) | p (2) | ||
|---|---|---|---|---|---|
| Before | During | After | |||
| FiO2 (%) | 55.5 (26.1) | 72.9 (21.1) | 62.3 (31.6) | <0.01 | <0.05 |
| Flow (L/min) | 10.7 (12.5) | 58 (5.3) | 37.3 (26.9) | <0.01 | <0.01 |
| SpO2 (%) | 91 (8.4) | 94.3 (4.8) | 90.9 (10.1) | <0.01 | 0.93 |
| Rr (bpm) | 27.1 (7.8) | 24.1 (6.6) | 24.6 (8.4) | <0.01 | <0.01 |
| CAB–RSS (points) | 3.8 (2.1) | – | 3.6 (2.4) | – | 0.31 |
| PaO2 (mmHg) | 78.8 (26.7) | – | 75 (24.5) | 0.22 | |
| PCO2 (mmHg) | 33.8 (8.2) | – | 38 (10.1) | – | <0.01 |
| SpO2/FiO2 (mmHg) | 215.1 (108.6) | 140.5 (46.4) | 202.7 (137.4) | <0.01 | 0.24 |
| PaO2/FiO2 (mmHg) | 180.8 (105.6) | – | 170.5 (130.2) | – | 0.36 |
| iROX (points) | 8.9 (6) | – | 9.9 (8.3) | – | 0.18 |
| Variablesa | HELMET CPAP | p (1) | p (2) | ||
|---|---|---|---|---|---|
| Before | During | After | |||
| FiO2 (%) | 45.1 (11.5) | 63.7 (6.8) | 27.4 (8.6) | <0.01 | <0.01 |
| Flow (L/min) | 33 (27.9) | 56.2 (13.6) | 3.8 (3.9) | <0.01 | <0.01 |
| SpO2 (%) | 94 (2.9) | 98.6 (1.6) | 95.8 (2.2) | <0.01 | <0.01 |
| Rr (bpm) | 31.4 (4) | 22.5 (4.9) | 19.6 (4.1) | <0.01 | <0.01 |
| CAB-RSS (points) | 4.5 (0.5) | – | 0.5 (0.7) | – | <0.01 |
| PaO2 (mmHg) | 86.5 (36.9) | – | 91.4 (20.7) | – | 0.59 |
| PCO2 (mmHg) | 34.2 (4.2) | – | 39.6 (4.6) | – | <0.01 |
| SpO2/FiO2 (mmHg) | 218.6 (47.1) | 161 (21.2) | 345.4 (95.7) | <0.01 | <0.01 |
| PaO2/FiO2 (mmHg) | 193.9 (60.5) | – | 331.2 (123.4) | – | <0.01 |
| PEEP (cmH2O) | – | 10.7 (1) | – | – | – |
p (1) Statistical test for comparison before and during support, p (2) comparison before and after the use of support (Wald Test).
FiO2, fraction inspired oxygen; SpO2, peripheral oxygen saturation; Rr, respiratory rate (bpm); CAB-RSS, modified Cabrini scale; PaO2, arterial oxygen pressure; PCO2, arterial carbon dioxide pressure; SpO2/FiO2, resultant ratio of peripheral oxygen saturation to inspired oxygen fraction; PaO2/FiO2, resultant ratio of arterial oxygen pressure to inspired oxygen fraction; iROX, resultant ROX ratio of SpO2/FiO2 over Rr; PEEP, positive end-expiratory continuous positive end-expiratory pressure.
Bold values signifies values are statistically significant.
In the bivariate analysis of HFNO, for the mortality outcome, the pre-support variables: age (OR = 1.1; 95% CI: 1–1.1; p < 0.01), APACHE II (OR = 1.2; 95% CI: 1.1–1.3; p < 0.01) and female sex (OR = 3.1; 95% CI: 1.4–6.9; p < 0.05) were statistically significant. For the same outcome, after support, the significant variables were: SF (OR = 0.9; 95% CI: 0.9–1; p < 0.01), PaO2/FiO2 (OR = 0.9; 95% CI: 0.9–1; p < 0.01), CAB-RSS (OR = 1.3; 95% CI: 1.1–1.5; p < 0.05). For the intubation outcome, in HFNO, the significant variables prior to support were: age (OR = 0.9; 95% CI: 0.9–1; p < 0.05), Rr (OR = 0.9; 95% CI: 1–1.1; p < 0.05). For the same outcome, during HFNO support, the significant variables were: Rr (OR = 1.1; 95% CI: 1–1.2; p < 0.01), SF (OR = 0.9; 95% CI: 0.9–1; p < 0.01), while after support the significant variables were: SF (OR = 0.9; 95% CI: 0.9–1; p < 0.01), PaO2/FiO2 (OR = 0.9; 95% CI: 0.9–1; p < 0.01), Rr (OR = 1.1; 95% CI: 1–1.1; p < 0.01), CAB-RSS (OR = 1.5; 95% CI: 1.2–1.8; p < 0.01). The rest of the analysis can be found in Supplementary material [Supp 1].
In H_CPAP, for both outcomes, the variables that showed perfect prediction were PaO2/FiO2 (≤123.4 mmHg) prior to support; Rr (>30 bpm) during support; and FiO2 (0.21), SF (≤131.9 mmHg), PaO2/FiO2 (≤93 mmHg), CAB-RSS (>1 point) post-support. The rest of the analysis can be found in Supp 1.
In the multivariate analysis, H_CPAP showed a lower Relative Risk (RR) with respect to HFNO, both for mortality (RR = 0.14; 95% CI: 0.02–0.99; p < 0.05) and intubation (RR = 0.09; 95% CI: 0.01–0.6; p < 0.05) [Supp 2].
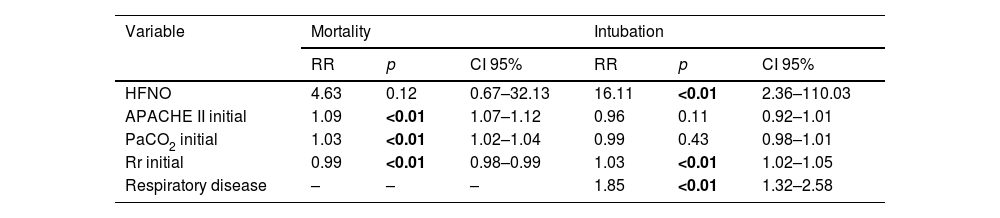
In the multivariate model analysis, when control variables were included, HFNO showed a non-significant association with the mortality outcome with respect to H_CPAP (RR = 4.63; 95% CI: 0.67–32.13; p = 0.12). For the same outcome, APACHE II was the variable that showed the greatest association (RR = 1.09; 95% CI: 1.07–1.12; p < 0.01). The remaining variables that were independently associated with the same outcome are shown in Table 3.
Multivariate analysis for the outcomes.
| Variable | Mortality | Intubation | ||||
|---|---|---|---|---|---|---|
| RR | p | CI 95% | RR | p | CI 95% | |
| HFNO | 4.63 | 0.12 | 0.67–32.13 | 16.11 | <0.01 | 2.36–110.03 |
| APACHE II initial | 1.09 | <0.01 | 1.07–1.12 | 0.96 | 0.11 | 0.92–1.01 |
| PaCO2 initial | 1.03 | <0.01 | 1.02–1.04 | 0.99 | 0.43 | 0.98–1.01 |
| Rr initial | 0.99 | <0.01 | 0.98–0.99 | 1.03 | <0.01 | 1.02–1.05 |
| Respiratory disease | – | – | – | 1.85 | <0.01 | 1.32–2.58 |
Results of the multivariate binomial regression model for the estimation of the Relative Risk (RR) of HFNO with respect to H_CPAP. None of the control variables were shown to be effect modifiers.
HFNO, high-flow nasal cannula oxygen; APACHE II, (Acute Physiology and Chronic Health Evaluation) prognostic rating scale according to severity of illness; PCO2, arterial carbon dioxide pressure; Rr, respiratory rate measured in breaths per minute (bpm).
Bold values signifies values are statistically significant.
For the intubation outcome, HFNO showed a strong risk association (RR = 16.11; 95% CI: 2.36–110.03; p < 0.01) with respect to H_CPAP. In addition, for the same outcome, the variable respiratory diseases showed an independent and significant association (RR = 1.85; 95% CI: 1.32–2.58; p < 0.01). [Table 3]. The analysis of the sub-associations can be found in Supp 3.
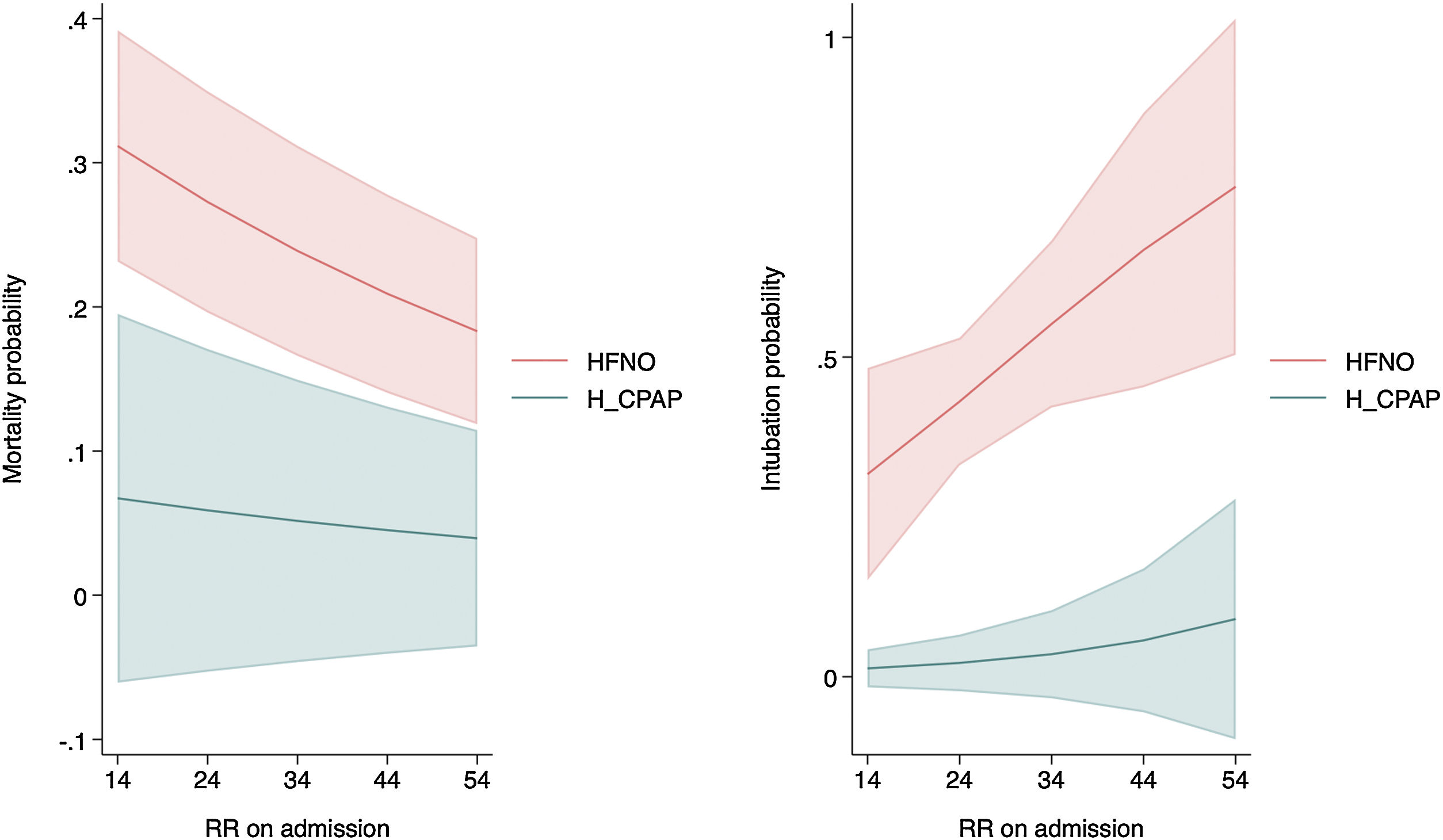
For both mortality and intubation, admission respiratory rate was statistically significant and showed greater strength of risk association with HFNO (Table 3, Fig. 1).
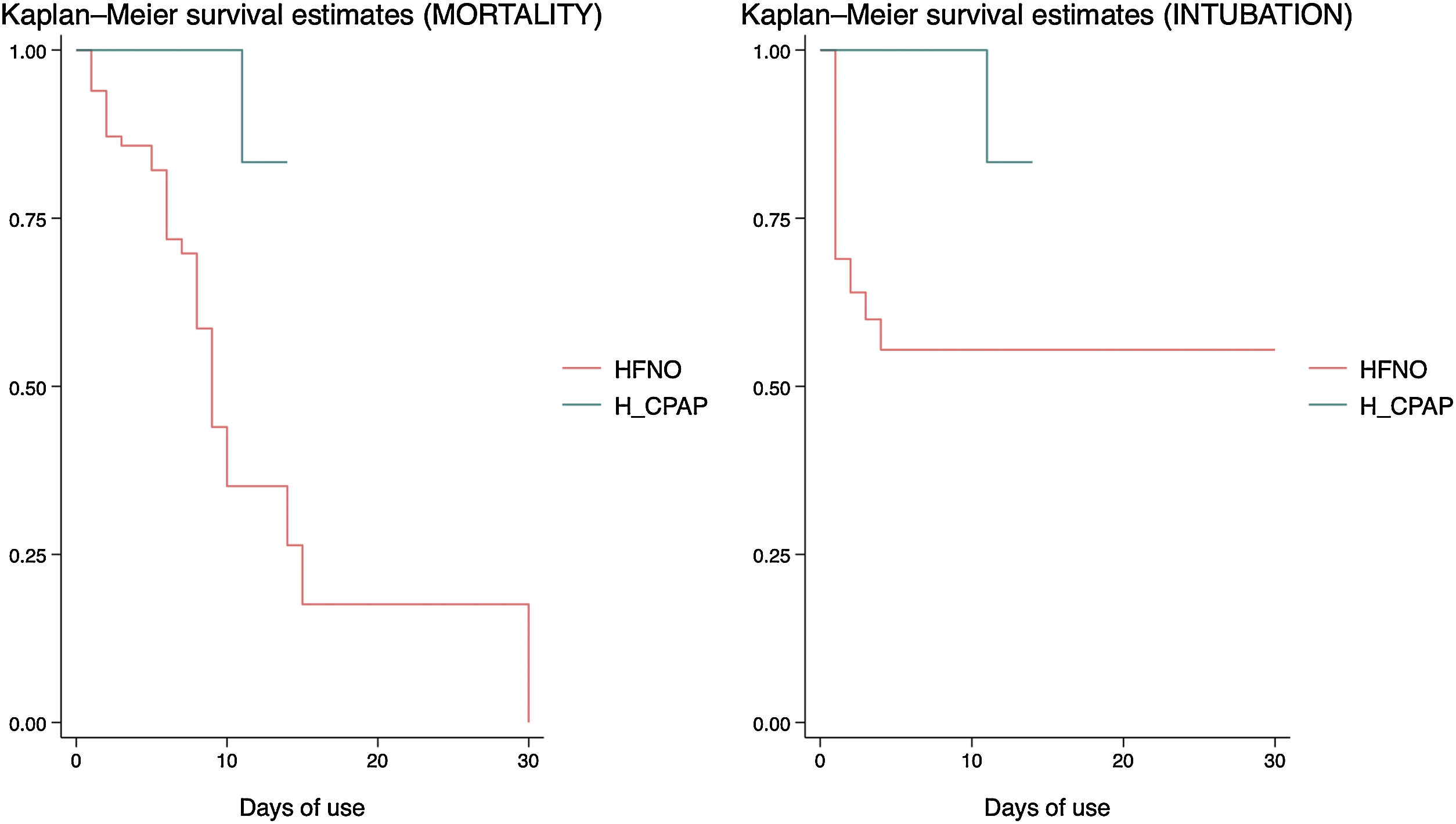
In the survival analysis, it was found that HFNO was associated with a higher risk of mortality (HR = 14.28; 95% CI: 1.85–110.45; p < 0.01 Long Rank Test), but also with a higher risk of intubation (HR = 20.79; 95% CI: 2.73–158.29; p < 0.01 Long Rank Test), when compared to H_CPAP (Fig. 2), and controlled for the rest of the variables in the model.
DiscussionThis observational study suggests that H_CPAP, in units other than intensive care, was associated with a lower risk of requiring intubation when compared to HFNO in adult patients with mild to moderate hypoxemia caused by coronavirus type 2 viral pneumonia.
The associations described in favor of H_CPAP support its feasibility as a useful and potentially replicable therapy in populations with similar characteristics. Moreover, for H_CPAP this study provides perfect predictor variables for both outcomes, so they could be used as clinical response monitoring variables during the applicability of the support.
Our study presents some strengths. First, the high quality of the data, the rigorous statistical analysis and the strict control of covariables allowed us to demonstrate an effective association between ventilatory support and outcomes. Second, to our knowledge, this is the first Chilean study that analyzes and compares both ventilatory supports in adult patients outside critical units. Although it is true that there are several studies that evaluate the effectiveness of both supports, they do so separately and practically all of them within an intensive care unit. Our study uses the comparative argument to evaluate the effectiveness between both supports, in adult patients and in hospitalization units other than intensive care units, proposing a viable and plausible strategy even in hospitals of medium complexity. Third, it provides an attractive hypothesis where H_CPAP decreases the risk of requiring intubation, and is also associated with a lower risk of mortality, when compared with HFNO.
Our results coincide with those published in the RECOVERY-RS,6 in which the need for intubation or mortality was significantly lower with CPAP (36.3%) compared to conventional oxygen therapy (Absolute Difference in risk, −8 pp; 95% CI: −15 to −1; p = 0.03); although the difference between HFNO (44.3%) and conventional oxygen therapy (Absolute Difference in risk, −1 pp; 95% CI: −8 to 6; p = 0.83) did not reach statistical significance. González-Castro et al.,7 performed a post hoc analysis of the RECOVERY-RS for a direct comparison between CPAP and HFNO, and by means of a Bayesian analysis with a beta-binomial model using a priori non-informative distribution concluded that the probability that CPAP is superior to HFNO is 0.988; with a NNT of 12.4 patients (Int. Cred 95%: 6–52).
The study by Coppadoro et al.,8 who also used a Venturi system to generate positive pressure, reported a mean age of 67 years, while in our study it was 59.9 years for HFNO and 46.2 years for H_CPAP. The same study reports a mean CPAP of 10 cmH2O, similar to the 10.7 cmH2O in our analysis. The mean number of days of use was 4.5 days for that study, while ours showed a mean of 8.6 days of H_CPAP use. Likewise, the authors reported 11% mortality and 18% intubation; while in our study it corresponds to 3.9% for both mortality and intubation. This percentage is much lower than that also reported by other authors.9,10
Contrary to the findings of our study, Santus et al.,11 prior to the use of CPAP, averaged a PaO2/FiO2 ratio of 75 mmHg similar to other studies;12–14 however, in our study we reported an average of 193.9 mmHg for the same support.
Regarding the use of predictors of failure/success of the supports, Artacho et al.15 agree with what was found in our analysis. The authors mention that a respiratory rate < 29 bpm, a FiO2 < 59% and an iROX >5.98 points during support were the best predictors of success and were associated with a lower risk of intubation. In our study, although we observed that iROX in HFNO presented a 10% relative risk reduction as a predictor of risk for intubation, it was not significant; and in the multivariate model it behaved as a non-confoounding variable. Furthermore, on admission, a respiratory rate > 41 bpm (RR = 1.88; 95% CI: 1.19–3.17; p < 0.05) was significantly associated with an increased risk of requiring intubation. Likewise, a respiratory rate#x202F;> 30 bpm during H_CPAP support behaved as a perfect predictor variable for both mortality and intubation.
In our hospital, the application of H_CPAP outside the intensive care unit coincides with the results obtained by Coppadoro et al.8 who report its use even before the pandemic, in hospitalization units other than intensive care. This team also used H_CPAP using a Venturi tube system16 and fresh gas mixing, instead of the classic pressure generator (mechanical ventilator). This allowed an adequate distribution of mechanical ventilators for those patients in a more severe state. No H_CPAP user in our hospital presented mechanical complications or pressure skin lesions, similar to that described in the study by Coppadoro et al.8
One of the advantages of using H_CPAP is that the use of active humidification was not mandatory, mainly due to the spontaneous ventilation preserved in the patients and the mixing of fresh medical gases with the ambient air (Venturi), contrary to what is required with HFNO, which obliges active humidification. Finally, the mandatory use of a HEPA filter in the expiratory port of the Helmet ensured adequate sanitization of the air exhaled by the patient, minimizing the risk of environmental contamination.
LimitationsOur study has limitations inherent to the observational design with standardized data recording, as well as having been performed in a single center. In addition, it presents an unexposed exposed ratio of 2:10 due to the greater generalization of the use of HFNO in units other than intensive care. The limitations associated with the difference in size between the arms of our analysis could generate important bias, as well as the difference in age and the potential severity, which is reflected in the heterogeneity of the baseline data of the study. It is necessary to generate studies with more robust designs to confirm our hypotheses.
ConclusionsThe results of our analysis support the hypothesis that the use of Helmet CPAP outside intensive care units is an effective therapeutic ventilatory support strategy to reduce the risk of requiring intubation in patients with mild to moderate hypoxemia caused by coronavirus type 2, which implies a decrease in the requirement of ICU beds.
The use of HFNO, a high APACHE II score, hypocapnea, and a high respiratory rate were associated with increased mortality. HFNO support, a high respiratory rate and a history of respiratory disease were associated with an increased risk of requiring intubation. This attractive hypothesis should be tested by clinical trials and studies with a more robust design.
Authors’ contributionAF-C performed the study design, formal statistical analysis, wrote and edited the present manuscript; JJO-C supervised and formally corrected the manuscript; VF, FP, KR, PR performed the collection of data and variables. All authors read and approved the final manuscript.
FundingThis work did not receive any funding.
Conflict of InterestAll authors declare no conflict of interest.