Traumatic disease is the leading cause of mortality among individuals under 40 years of age, as well as the main cause of disabilities and sequelae–many of which are avoidable, while some are related to shortcomings in the organization and provision of medical care.1 For years there have been reports of important deficiencies in the management of severe trauma patients (STPs), reflected through differences in mortality among countries with similar economical levels and among different hospitals, as a result of unwarranted delays or care provided by professionals with insufficient preparation.1–4 Survival and functional recovery are directly dependent upon promptness in recognizing and repairing the anatomical and physiological alterations caused by trauma. In this regard, the initial patient care provided is of capital importance.1 Such an initial management fundamentally aims to identify and secure immediate, systematic and prioritized solutions to serious problems affecting the airway, respiration and circulation, since these are globally responsible for an important proportion of avoidable deaths. In this respect, the most widely accepted clinical protocol in the world today is the Advanced Trauma Life Support (ATLS®)5 instrument, which constitutes the management standard for STPs. As a last priority in the first evaluation of the patient we must assess possible neurological damage. Cervical immobilization must be ensured until computed tomography (CT) can confirm the absence of injuries. In this first phase we only obtain X-rays of the chest and pelvis, and perform focused assessment with sonography in trauma (FAST).6 Posteriorly, in the course of secondary evaluation, we conduct a patient study “from head to foot”, using the opportune imaging diagnostic techniques.
Physical examination of STPs can allow the immediate suspicion of risk injuries such as tension pneumothorax, pelvic fractures or internal bleeding. However, the reliability of physical examination is limited, particularly when the patient level of consciousness is altered or other important injuries are also present.7 There is also a risk of “unnoticed injuries”, particularly in seriously injured elderly patients, with traumatic brain injury or visible vascular damage8 (Fig. 1). The information collected on the trauma scene can provide clues as to the possible mechanism and pattern of injuries, but is subjective and has low sensitivity as an indicator of serious injury.9
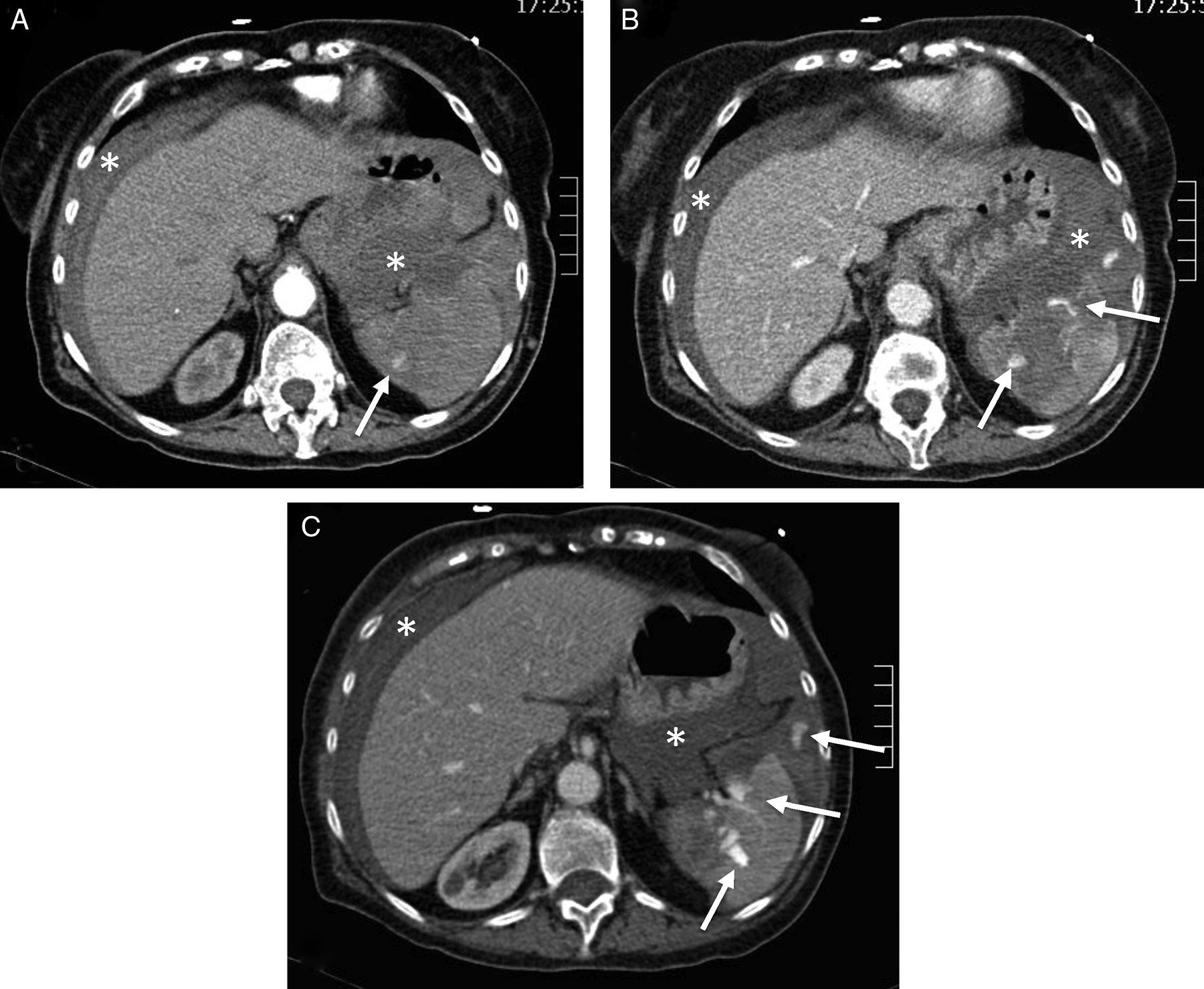
Splenic damage. A 77-year-old woman subjected to anticoagulation, who suffered a fall at home as a result of “dizziness”. In the emergency room she presented hypotension and abdominal distension. (A) WBCT with contrast, axial acquisitions of the upper abdomen in arterial phase. (B and C) Imaging in venous phase. Hemoperitoneum (*). Intravenous contrast leakage into splenic parenchyma and peritoneal cavity (arrow), increasing in size and density in the portal phase (b and c) due to splenic laceration with active bleeding.
Worldwide diffusion of the ATLS protocol has been an important step forward in the systematic management of STPs, though in relation to imaging diagnosis it has some shortcomings–particularly referred to the use of CT.10–12 ATLS adopts a “step by step” clinical approach, performing resuscitation maneuvers while the patient is quickly evaluated to identify and “manage” possible risk injuries. At the same time, definitive treatment is considered. However, application of the ATLS protocol is inevitably not the same when applied by a rescue team at the trauma site–where little more than clinical skill is available–as when applied by a polytrauma team belonging to a reference hospital, in a critical care ward with adequate equipment, and with a nearby CT exploration room.
Radiology and imaging in severe traumaThe British Royal College of Radiologists (RCR), through its severe trauma radiology guide, has established a series of specifications referred to the design, location and technological equipment of imaging diagnostic facilities destined for use in STPs13:
- –
Quick action is essential.
- –
Both the number and distance of patient transfers are to be minimized.
- –
In general, imaging is more precise than clinical exploration.
- –
The technique of choice should be that offering conclusive findings–usually whole-body CT (WBCT).
- –
A conclusive imaging technique should not be postponed in order to perform other less precise explorations.
- –
The radiology facilities are to be equipped with the same life support resources available in the critical care ward.
- –
Trauma centers in which the multidetector CT (MDCT) room is not located within or near the critical care area should rehearse patient transfer according to pre-established protocols, with the adoption of plans to modify the location of the MDCT room in future.
The ideal imaging procedure in STPs should be rapid, exhaustive, and capable of immediately and systematically identifying all life-threatening injuries, following the same ABCD priorities.14
The classical approach: digital radiography, focused assessment with sonography in trauma (FAST), and guided computed tomographyX-rays of the cervical spine, pelvis and thoraxAccording to the ATLS protocol, the chest and pelvic X-rays and FAST remain the reference imaging diagnostic techniques in the primary evaluation of STPs.5 These explorations can be performed quickly, are accessible, and afford useful information despite important technical limitations and often suboptimal image quality. Their use decreases as CT systems become more widely accessible from the emergency care area. In this regard, the ATLS protocol acknowledges the limitations of X-rays of the cervical spine and suggests the use of CT if indicated for brain imaging or if so protocolized by the center. The pooled sensitivity of cervical X-rays is 52% versus 98% in the case of CT. Even in patients with diminished consciousness, normal CT is able to discard relevant injuries without the need for magnetic resonance imaging (MRI), allowing early removal of the neck brace.15
Up to one out of every four trauma deaths are related to the presence of chest injuries.16 Tension pneumothorax or hemothorax can be identified clinically, by radiography or ultrasound, but the visualization of small pneumothorax or lung contusion foci requires CT.
The specificity of chest X-rays exceeds 90%, though the sensitivity of the technique is low (20%),16 and worsens in critical patients to the point where over one-half of the cases with normal chest X-ray findings are seen to yield additional findings when CT is used.17 Up to 11% of all aortic traumatisms go undetected by conventional radiography.17,18 Chest X-rays are not needed in conscious patients with closed traumatisms and respiratory and hemodynamic stability–particularly if CT exploration is carried out.16,17 At the other extreme, normal findings on chest X-rays can avoid the performance of thoracic CT in young patients with mild trauma and no evidence of injury.19
Between 4% and 9% of all STPs suffer pelvic injuries, with a high probability of other serious damage and bleeding,14,20 which is of venous/bone origin in 85% of the cases, and of arterial origin in the rest.12 In this context, MDCT identifies more fractures than pelvic X-rays and is better able to establish stability and the approach to treatment. Recent publications disadvise routine pelvic X-rays.21 In order to prevent bleeding, external pelvic fixation should be maintained until CT evaluation has discarded fractures.13
Radiographs of the extremities should be obtained during secondary evaluation once all the risk injuries have been identified and treated, though in some concrete cases the exploration can be extended with initial CT.13
UltrasoundThe identification of hemoperitoneum in STPs, with signs of shock, classically has been regarded as an indication of laparotomy.22 In this context, ultrasound is noninvasive, accessible in the case of critical patients and inexpensive, and the fact that it uses no ionizing radiation means that the technique can be used repeatedly. In this regard, the use of focused assessment with sonography in trauma (FAST) is limited to the identification of intraperitoneal fluid, which is interpreted as representing hemoperitoneum. Its widespread clinical diffusion has displaced other invasive procedures such as peritoneal puncture-lavage.22
Despite its great precision in the detection of intraperitoneal fluid, there are important limitations in the routine use of ultrasound in STPs. Thirty-four percent of all internal organ injuries, confirmed by CT or surgery, are not associated to hemoperitoneum,23 i.e., a negative FAST result does not rule out possible relevant abdominal injury (Fig. 2). Because of this low sensitivity and the impossibility of providing specific visceral information, the technique is not advised in hemodynamically stable patients, in which the presence of hemoperitoneum does not necessarily alter the decision to provide conservative management.24,25 In unstable patients, the negative predictive value of ultrasound is 50–63%,26 and the positive predictive value is also low. In the series published by Charbit et al.,27 hemoperitoneum and active bleeding were only found to be of peritoneal origin in 63% of the cases–this figure decreasing to 52% in the presence of hypotension. Therefore, in patients with traumatic shock and hemoperitoneum, the authors recommended the exclusion of other possible sources of bleeding in the thorax, pelvis or retroperitoneum.
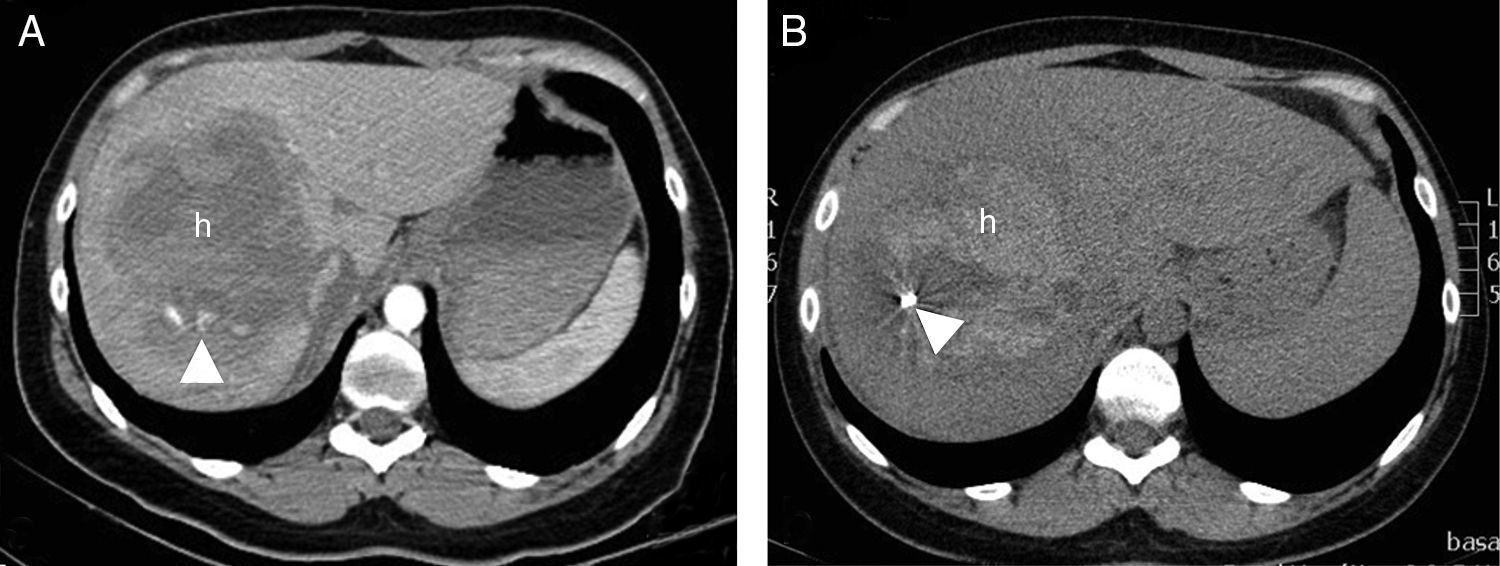
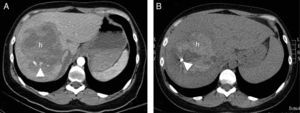
A 30-year-old woman who collided with the handlebars of her bicycle. Conscious. Pain in response to deep palpation. No peritoneal irritation. Sustained blood pressure, normal blood count. Negative FAST (no hemoperitoneum). (A) Axial CT with intravenous contrast injection, showing a focal lesion with decreased uptake (h) affecting much of the right liver lobe, with active bleeding foci (arrow head). Percutaneous embolization was indicated. (B) The CT control without contrast one week after embolization showed a hyperdense, focal liver lesion (h), corresponding to the hematoma, with a reduction in size and metal image attributable to the embolization material (coil).
The sensitivity of ultrasound in detecting intra- or retroperitoneal organ damage is very low (50%), even in experienced hands.28 In this respect, the ultrasound appearance of the kidneys is often normal even in the presence of acute injury.6 The use of ultrasound contrast can improve the sensitivity of the technique, but at the cost of losing its main advantage: immediateness.
In penetrating traumatisms, ultrasound can be useful when it offers positive findings: hemothorax or hemoperitoneum in a hypotensive patient, as a prior step to surgery. When the findings are negative, CT is required–a fact that questions the usefulness of the technique.6,24
Extension of FAST exploration to the pericardial and pleural spaces is referred to as Extended FAST (EFAST), and allows us to identify hemopericardium and hemo- or pneumothorax of traumatic origin.29 The incidence of occult pneumothorax (visible with CT but not chest X-rays) is close to 5%, but reaches 55% when the Injury Severity Score (ISS) exceeds 12.24 In detecting traumatic pneumothorax, the sensitivity of ultrasound is greater than that of clinical evaluation and conventional radiography,29 coming close to 100% in the detection of hemothorax.24 Ultrasound is also useful for detecting pericardial fluid and for assessing preload and cardiovascular response to volume replacement, measuring the diameter of the inferior vena cava.
Apart from the fact that ultrasound is operator-dependent, with problems of access in the case of skin wounds, burns, obesity or subcutaneous emphysema, the technique does not offer information additional to that afforded by CT, and its use should not cause delays in performing CT.13 Negative findings do not rule out serious abdominal injury, and positive findings do not identify the origin of bleeding or offer information in deciding conservative management. Despite the large number of publications, there is no evidence of a genuine impact upon patient survival.25 It has been suggested that ultrasound is useful in extremely serious cases that cannot be moved to the CT room, no matter how nearby, and in cases of minor trauma in young patients, with a view to avoiding CT. The technique is used for the follow-up of already diagnosed injuries19 and in patient classification in scenarios involving numerous victims.13
Magnetic resonance imagingProlonged exploration times and a particularly “hostile” environment limit the use of MRI in STPs. The great negative predictive value of MDCT in application to vertebral traumatisms15 reduces the use of MRI to the few cases with neurological manifestations in the presence of normal CT findings.
The application of MRI to explore STPs requires the removal of any ferromagnetic elements from the patient, including foreign bodies, and the use of compatible life support material. The high incidence of multiple vertebral injuries means that a whole-spine scan should be performed.5,12
Clinical and imaging guided computed tomographyThe ATLS protocol recognizes that CT offers specific information on internal organ damage and its extent, and is able to identify retroperitoneal and pelvic injuries that are difficult to assess through physical examination, FAST or peritoneal lavage. However, it also underscores that the technique is slow, and must only be performed in patients without hemodynamic alterations in which there is no apparent indication of emergency laparotomy. In other words, it should not be used with unstable patients.
Clinical observation, with the support of plain X-rays and ultrasound, and completed with the CT exploration of some concrete body region oriented by the findings of the previous techniques, constitutes a diagnostic management option in patients with a low suspicion of injury–particularly young individuals. This approach reduces the number of explorations, the radiation doses and the costs. Up to 10% of all injuries may go undetected at primary evaluation, though the clinical relevance of the delay in diagnosis does not appear to be significant.30 It is important to avoid successive additional explorations of several anatomical zones, which require further patient transfer and increase the delivered dosage due to overlapping of the irradiated fields.
Serious trauma patients: integrated whole-body computed tomography at primary evaluationThe old single-slice CT systems needed 30min to obtain a study of low quality and poor diagnostic yield. A number of myths were created at the time and persist to this day regarding the scant usefulness of radiology in STPs, the subjectiveness of the analysis of the radiological findings, or the “coil of death” in which no hemodynamically unstable patient should be placed.
Although the positioning and connection of lines and tubes may take several minutes, the current CT systems allow us to explore the patient from “head to foot” in seconds, with simultaneous inclusion of the head, cervical spine, thorax, abdomen and pelvis, i.e., so-called “whole-body CT” (WBCT) exploration in trauma. Their great imaging resolution entails greater sensitivity and specificity than clinical examination, radiography and ultrasound in identifying and classifying injuries,16,17 with greater between- and within-observer concordance than any other imaging technique.31 In effect, WBCT performed during initial patient evaluation allows us to establish a quick and detailed global balance of the injuries, identifying those that are life-threatening, including possible active bleeding sites and (in many cases) unsuspected injuries1,11,32–35 (Fig. 3). The main advantages of WBCT in primary evaluation are the availability of a definitive diagnosis before treatment decisions are made, and rapidity.13 Compared with the conventional approach, WBCT takes only a quarter as much time and avoids one-half of the patient transfers.36 Patients studied with this technique require a shorter stay in the Intensive Care Unit (ICU), with fewer days on ventilation, and suffer a lesser percentage of organ failure.37 During secondary or tertiary patient evaluation, the technique accelerates definitive treatment and, eventually, hospital discharge.38 Likewise, WBCT minimizes unnoticed injuries16,39 and drastically reduces all the time indicators,40 with beneficial effects upon mortality, particularly in critical patients.41
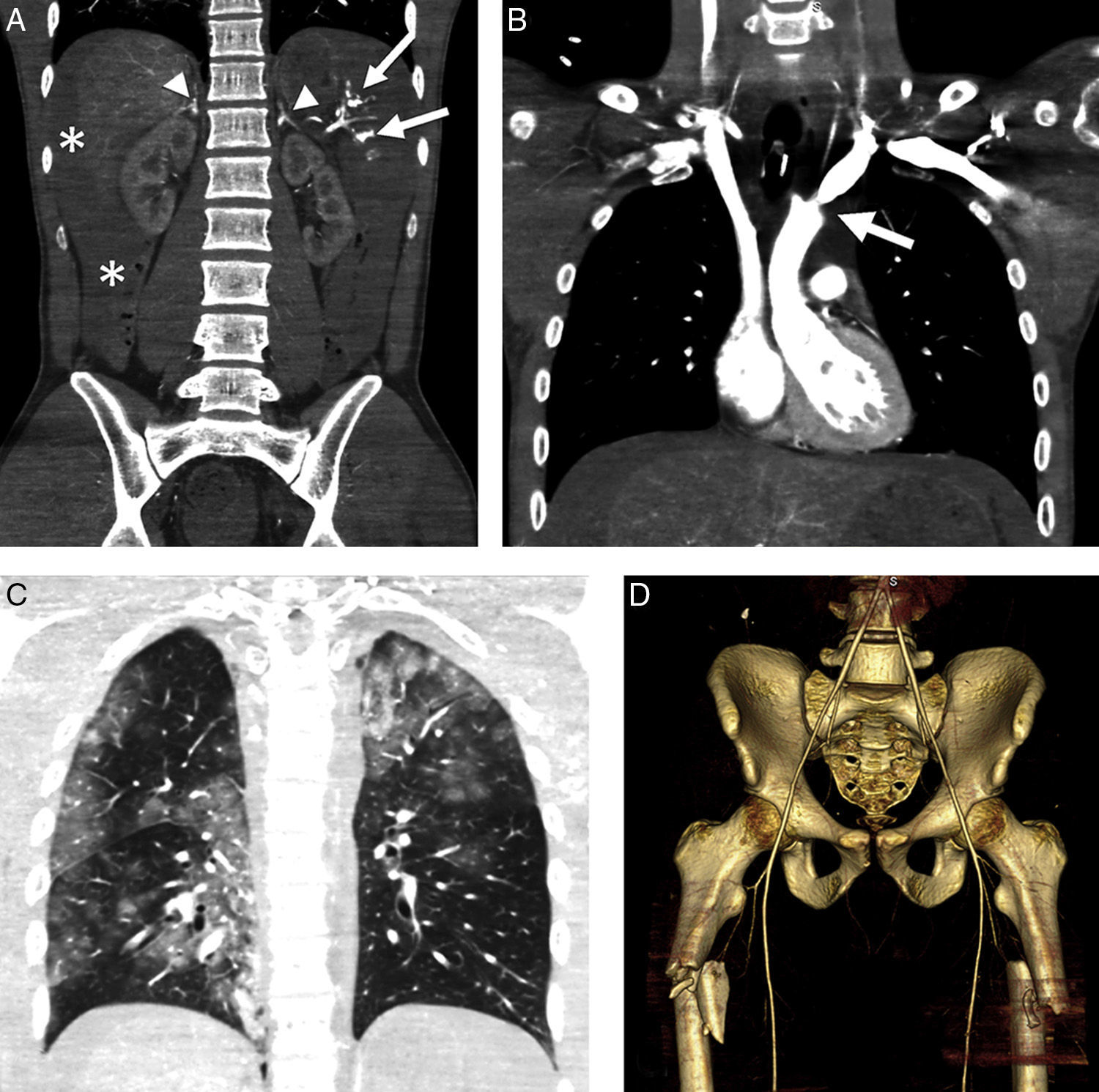
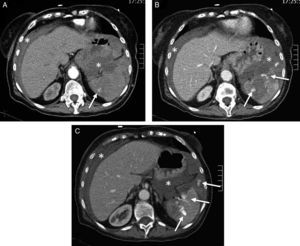
Hypoperfusion-shock complex in a 24-year-old male secondary to automobile accident. The patient was not wearing the seatbelt and was projected outside the vehicle. Signs of hypovolemic shock. WBCT with coronal multiplanar reformatting (MPR) of the abdomen. (A) Abdomen with hemoperitoneum (*) due to splenic rupture (arrows) and enhanced uptake of both adrenal glands. (B) Coronal MPR of thorax/mediastinum with ascending aortic trauma; no evidence of mediastinal hematoma (arrow). (C) Coronal MPR of thorax/parenchyma: multiple lung contusion foci. (D) Three-dimensional reformatting of the pelvis: proximal fracture of both femurs (note diminished caliber of aorta and iliac arteries).
Multidetector CT (MDCT) is currently a fundamental imaging technique in STPs. From the imaging perspective, and when used under adequate conditions, it questions the update on the diagnostic approach of the ATLS protocol. Among other aspects, the classical idea that unstable patients should not be moved to the CT room, although widespread, is not based on scientific evidence1 and has been questioned in recent years by the technological development of CT and the opinions of many authors, based on growing though still low-level evidence,10,33–35 in the same way as the ATLS protocol itself. Computed tomography is presently the only imaging technique capable of providing reliable information on possible intrathoracic or vascular injuries with or without associated active bleeding. The number of non-therapeutic laparotomies is greater in unstable patients because of the impossibility of gaining surgical access to the bleeding vessel,42 and CT can help reduce this number.43 A prospective study is currently comparing the diagnostic performance of WBCT in primary patient evaluation and the ATLS protocol (radiography and FAST with CT according to demand).43
When active bleeding is suspected, we perform a first angioCT scan with intraarterial contrast injection, followed by another scan in the venous phase. The diagnostic performance of angioCT is comparable to that of conventional angiography,44 allowing the detection of bleeding and characterizing the latter as either arterial or venous. complete arterial wall trauma causes active bleeding, with occasional formation of an arteriovenous fistula or pseudoaneurysm–with the associated risk of rupture and late bleeding. Such lesions are therefore usually subjected to embolization. In the CT scan, these injuries are seen as a dilatation of the arterial lumen in the arterial phase that disappears in the venous phase–in contrast to active bleeding, which is characterized by persist extravasation of the contrast medium (Figs. 1 and 4).
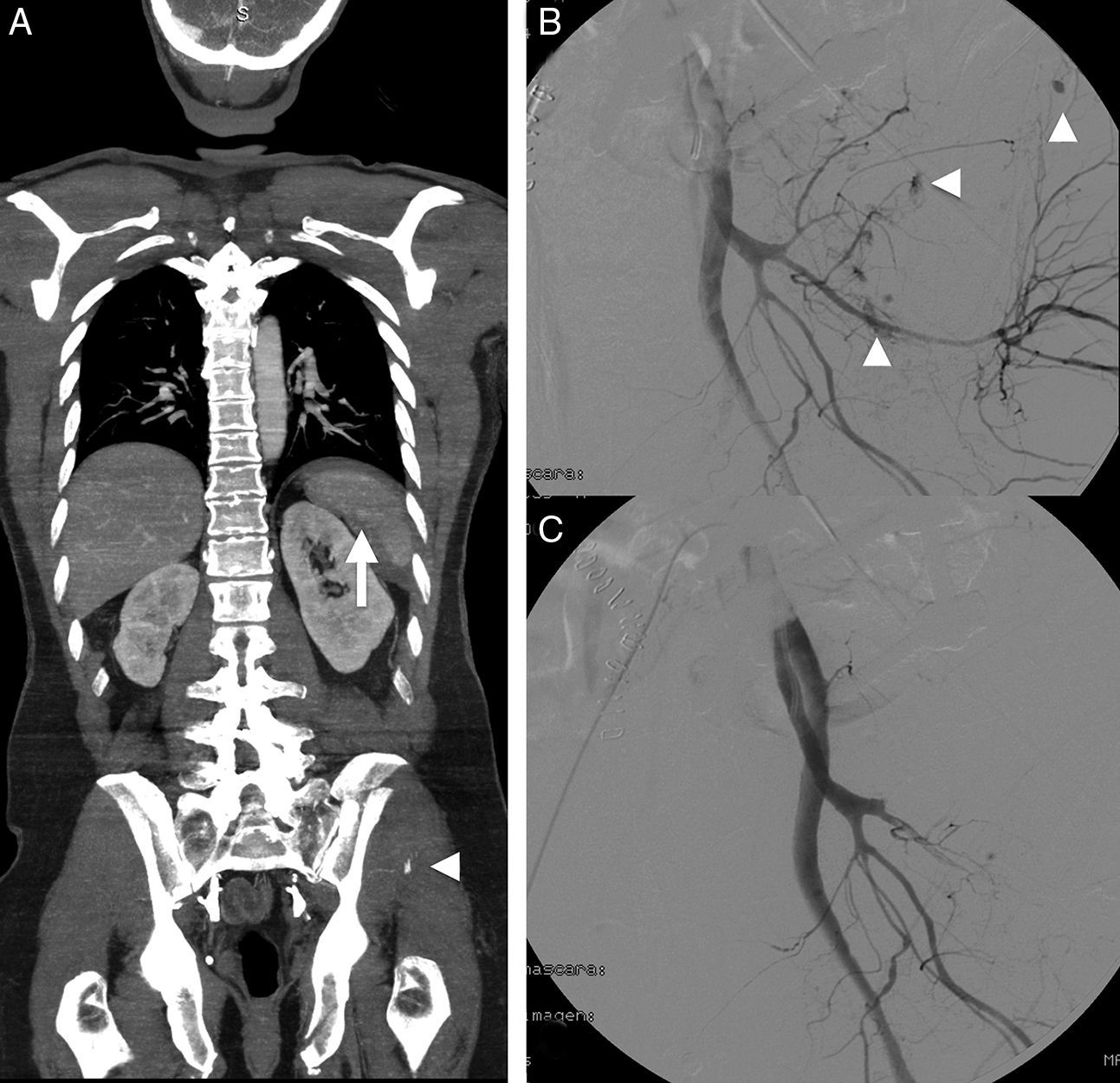
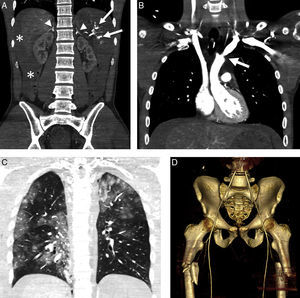
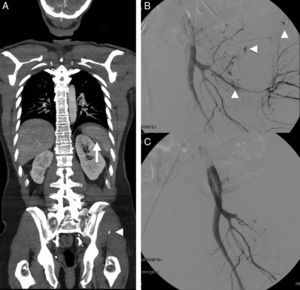
A 54-year-old male with multiple trauma secondary to automobile accident. Preserved vital signs. (A) WBCT in portal phase; coronal MPR showing splenic laceration with small perisplenic hematoma and pseudoaneurysm (arrow). Complex left pelvic fracture with active bleeding foci (arrow head). (B) Angiography confirmed the multiple active bleeding foci (arrow heads). (C) Bleeding points resolved by embolization, in the same way as the splenic pseudoaneurysm (not shown).
Application of the WBCT protocol has two basic requirements: accessibility of the CT room and adequate patient selection. The CT room must be located within the emergency care area, and must be equipped with monitoring and life support systems. The radiation dose administered to a predominantly young population is an important problem. Any exposure to ionizing radiation must be justified by the expectable clinical benefit, and should follow the ALARA criterion (“as low as reasonably achievable”). Although approximately 30% of the WBCT studies reveal no injuries,32 it is clear that in order to develop complications due to radiation exposure, the patient first must survive trauma.
Whole-body CT appears to involve a lesser radiation dose than segmented CT, due to the overlapping of zones.43,45 However, the latter technique is used in less serious cases, limiting the extent of exposure to the suspect anatomical region,31 while WBCT is reserved for STPs in which time is a critical factor.19 Radiology departments must keep their systems technologically up to date and adjust the exploration protocols to patient age and weight, and the diagnostic objective.19 Coordination with the rest of the implicated departments should be made within the context of a multidisciplinary team, with consensus regarding the intervention protocols–including mobilization–in accordance to each concrete patient profile.
The use of MDCT in emergency care is more cost-effective than any other technique both for deciding initial management (including admission or early discharge) and at a later stage, due to the costs associated to unnoticed lesions.32
Whole-body computed tomography patient selection criteriaThe RCR suggests restricting the use of WBCT to patients with ISS>15,13 but the initially occult nature of some injuries and retrospective calculation complicate its utilization. The advantages of WBCT in STPs are evident, but the associated radiation doses and costs are justified only in this concrete group of patients. Alterations in vital signs combined with the physical examination findings are the most sensitive markers in severe trauma, but their sensitivity in predicting ISS>15 is only 56%. The mechanism of injury is therefore added7,32 (Table 1).
Selection criteria for the use of whole-body computed tomography (WBCT) in severe trauma patients.
| 1. High-risk injury mechanism |
| • Traffic accidents |
| – Pedestrian/cyclist/motorcyclist hit by a vehicle |
| – Prolonged patient extrication (>15min) |
| – Death of another passenger |
| – Ejection from the vehicle |
| – High-speed automobile collision |
| – Motorcycle accident |
| • Fall from >3m, unknown height, stairs |
| • Building collapse |
| • Suspected to be within range of an explosion |
| 2. Evidence of anatomical injuries |
| • Visible injuries in two anatomical regions (head/neck/thorax/abdomen/pelvis/long bones) |
| • Signs of vascular damage (expansive hematoma, deep wound in arterial trajectory) |
| • Signs of spinal cord damage |
| • Unstable pelvic fracture |
| • Fractures of more than one long bone |
| 3. Vital signs |
| • Glasgow score <12, intubated |
| • Systolic blood pressure <100mmHg |
| • Respiratory frequency: <10 or >30rpm |
| • Pulse >120bpm |
| • SatO2 <90% |
| • Age >65 years |
| • Anticoagulation |
Following the musculoskeletal system, the abdomen is the most frequent location of unnoticed injuries, and the possibility of error is greater in some anatomical structures.39 In this regard, mesenteric-intestinal damage occurs in 5% of all patients with abdominal trauma, and is characteristically insidious and of a serious nature. As a result, diagnostic delays of only 8–12h increase morbidity-mortality due to peritonitis and sepsis.46 The visualization of extraluminal gas is considered to be diagnostic of perforation, though both radiography and ultrasound identify it in less than one-third of the cases. Computed tomography not only detects small amounts of extraluminal gas, but also zones of hypoperfusion, wall thickening, mesenteric edema or vascular damage, which can precede perforation. A negative CT scan with the suspicion of perforation would be one of the few indications of peritoneal puncture-lavage.
Pancreatic damage initially may yield no clinical findings. The appearance of diffuse abdominal pain and vomiting are alarm signs that lead to a second CT scan that detects the lesion. Endoscopic pancreatography identifies possible duct involvement, which implies a poorer prognosis.46
Bladder rupture is usually associated to pelvic fracture, and is confirmed by CT-cystography, which moreover allows us to distinguish between intra- and extraperitoneal rupture, which have different management approaches (surgical in the former and conservative in the latter)47 (Fig. 5). Injury of the diaphragm is usually associated to a sharp increase in abdominal pressure and implies a risk of intrathoracic herniation (and ischemia) of the abdominal organs. The diagnosis is facilitated by careful evaluation of the sagittal, coronal and axial CT planes, maintaining a high level of suspicion and seeking specific signs.46
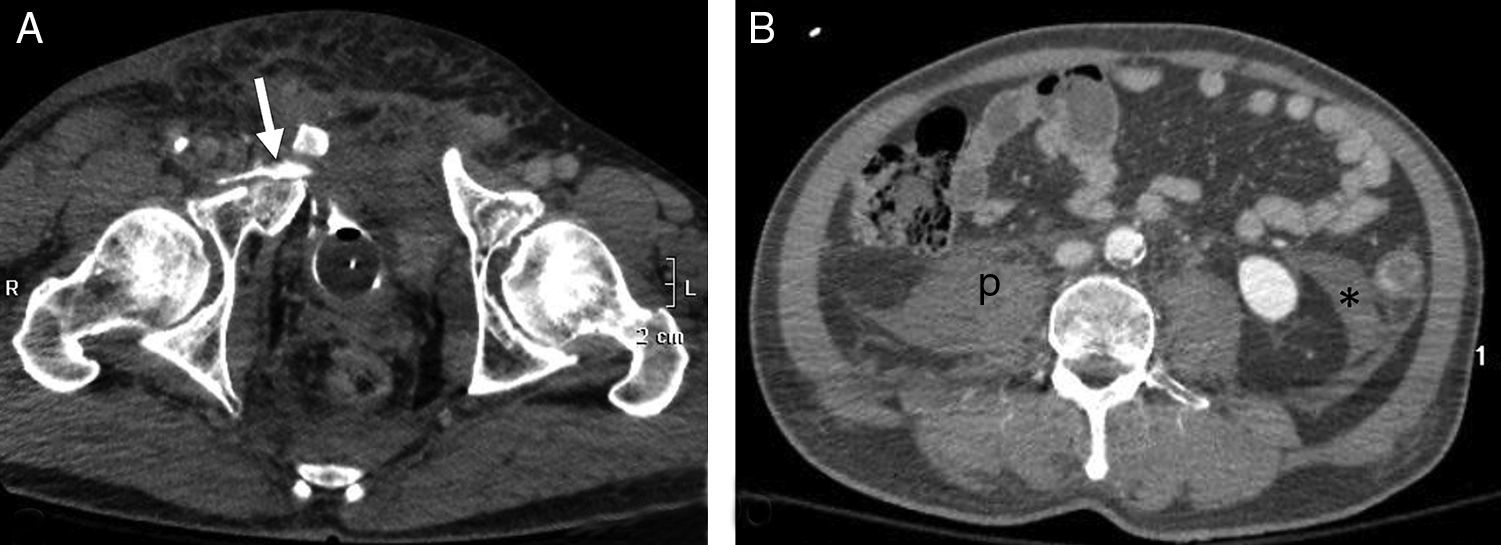
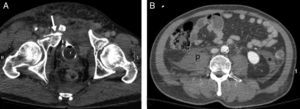
A 40-year-old male. Motorcycle accident. (A) WBCT, axial plane of the pelvis showing complex pelvic ring fracture with intravesical (Foley catheter) contrast leakage into soft tissues (arrow). (B) Axial plane of the abdomen showing absence of hemoperitoneum. Retroperitoneal hematoma occupying right psoas muscle (p) and retromesenteric fascia (*).
The retroperitoneum can be the site of blood collections not detectable with FAST. Pelvic fracture is the most frequent cause.48 The treatment options depend on the hemodynamic condition of the patient, the growth of the hematoma, and the presence of active bleeding as assessed by CT.46
Imaging in hypoperfusion-shock complexThe CT findings can provide clues as to the hemodynamic condition of the severe trauma patient and the persistence of hypovolemia.46 The global findings, referred to as the “hypoperfusion-shock complex”, are characterized by a decrease in the caliber of the aorta and inferior vena cava, diffuse thickening and enhanced uptake of the intestinal wall, diminished splenic uptake and an increase in renal and adrenal gland uptake, an increase in pancreatic size, and signs of adrenal gland edema49 (Fig. 3).
Ideal location, equipment and structure of the computed tomography room in trauma centersThe limiting factor for the use of CT in critical patients is not so much the rapidity or quickness of the equipment used but its distance from the critical care area. In order to maximize performance, it must be possible to study the most critically ill patients,41 with three concrete requirements: a technologically appropriate CT system located close to where the STPs are received, and trained personnel with availability 24h a day, 7 days a week (24/7). Ideally, the CT room should be integrated within the resuscitation area of the Emergency Care Department, as is already the case in many developed countries.50 Whenever possible, “the closer the better” is applicable, and all new facilities must follow this principle.1,13 In relation to design, patient safety is the main concern, and the contributions of the entire trauma team are needed in relation to monitoring equipment, gases, expendable materials and drugs. The location of the system in the room must allow continuous patient observation and monitoring. The services must be available uninterruptedly on a 24/7 basis, as commented above, with adequate personnel training.
Following reception of the patient, and after placement on a polyvalent table compatible with or integrated within the CT system of the resuscitation room, with due monitoring, we must check the airway and ventilation, and solve possible problems–including the securing of a definitive airway. Once this has been done, and using an adequate venous access for eventual volume replacement, WBCT is performed even in unstable patients. The radiologist checks the initial images1,13,32 in order to identify possible life-threatening injuries requiring immediate attention, and investigates the presence of active bleeding and its origin. With the information of the clinical exploration, causal mechanism of the injury, laboratory test or other findings, a first balance is made of the lesions assessable by CT, following the ABCD protocol. Based on these objective data, we then plan management: observation, admission to the ICU, endovascular intervention or surgery.11,50
Interventional radiology in severe traumaA damage control technique such as manual compression or surgical packing can be considered.13 After accessing the bleeding vessel, emboligenic material is released or stents are placed in order to reinforce the damaged arterial wall, as in aortic traumatisms.51 Among other advantages, this approach reduces the need for organ resection and non-therapeutic laparotomies, avoids the stress of surgery, and lessens the transfusion needs and costs.32,51
The information provided by MDCT is crucial for deciding between nonsurgical management and hemostatic intervention and, in the latter, among endovascular, surgical or mixed accesses. In this regard, nonsurgical management requires the absence of active bleeding, while the presence of such bleeding or of a pseudoaneurysm requires hemostatic intervention (Figs 2 and 4). In performing embolization, angioCT provides orientation on the bleeding point and offers very useful guidance that helps reduce room time, the radiation dose and the contrast medium requirements. With this approach, nonsurgical management can be made of up to 95% of all bleeding traumatisms.52 It will be difficult to establish first level evidence for endovascular techniques in trauma, though there is level 2 and level 3 evidence warranting their safety, efficacy, rapidity and cost-effectiveness.44,52
Diagnostic and therapeutic management of traumatic hemorrhageAlthough neurological damage is the leading cause of post-trauma mortality, bleeding accounts for 30–40% of the deaths and for 80% of early in-hospital mortality.53 In addition to exsanguination, hypotension secondary to acute blood loss is directly responsible for secondary brain damage and contributes to the development of multiorgan failure.1,20,53,54 It is regarded as the first cause of potentially avoidable post-trauma mortality.1,20
Time is a crucial factor in the control of traumatic hemorrhage.13 In patients with abdominal trauma, the mortality rate increases 1% for every three minutes of treatment delay.55 In the presence of hemodynamic instability, each hour of delay in applying endovascular hemostatic care increases the risk of death by 47%.56
Bleeding control is carried out simultaneous to the management of hypovolemic shock, with the purpose of minimizing the risk of mortality and secondary compartmental syndrome.54 In practice, the selection of patients amenable to immediate surgery is usually established according to the characteristics of trauma and the degree of response to treatment, with the inevitably limited information afforded by inconclusive diagnostic techniques such as radiography and FAST.5,54 Incomplete information and the need for a rapid response complicate the decision, as evidenced by the fact that mistakes in the control of traumatic hemorrhage constitute the main cause of error resulting in death.57 Hypotension does not necessarily imply active bleeding, and may be due to other causes of shock (obstructive, neurogenic), or constitute a collateral effect of analgesia/anesthesia.54 Bleeding may have ceased, with the persistence of hypotension, and absence of the latter or even a response to treatment likewise does not rule out early-stage hemorrhagic shock.27 Lastly, and although pelvic fracture is more often the cause of severe bleeding, the bleeding site may have some other location (intra- or retroperitoneal), or may be multiple–in which case the management approach will differ.20,50
Surgery, seen from the “damage control” perspective, has been the main treatment option in hemorrhagic shock, allowing access to the bleeding body cavity and control of bleeding by suturing, direct compression or surgical packing. However, surgery is often unable to control the bleeding, and endoluminal techniques therefore must also be used. Angiography is the technique of choice in the case of arterial bleeding, particularly when the latter is of pelvic or extraperitoneal origin–though its usefulness is limited when there are a number of bleeding foci, or when bleeding is of venous origin. In these cases open surgery is the treatment of choice.50 When the angiography room and operating room are located at a considerable distance from the emergency care area, both the decision to move an unstable patient to either room and the order in which transfer is carried out have an impact in terms of morbidity–mortality.50
The ATLS protocol acknowledges the usefulness of angiographic embolization,5 but not the use of CT in unstable patients. However, it is precisely in these patients where the technique is especially useful, and in an adequately equipped trauma center, hemodynamic instability should be the main indication of WBCT, not an obstacle for such exploration. The risk of postponing surgery for a few minutes is probably less than the risk of performing surgery on a “blind” basis.1,11–13,33,41 The only way to stabilize a patient with active bleeding is to identify and stop the source of bleeding. It makes no sense to postpone CT, and eventually embolization or surgery, in wait of “spontaneous” stabilization of the patient. If the patient condition disadvises transfer to the CT room, immediate transfer to the operating room or angiography room should be carried out–though things there might not be exactly as imagined.
Trauma centersThe difference in mortality rate among STPs can reach 20% between countries with similar economic resources, depending on whether an organized trauma care system is available or not. In this respect, there is evidence that such patients might be receiving suboptimal care in low complexity hospitals.1,2,4,32 The great majority of STPs are taken to hospital outside routine working hours, when the experienced professionals are no longer on duty, and this likewise increases morbidity–mortality (the so-called “weekend effect”).58 Severe traumatisms are infrequent, representing less than 0.2% of the activity of the Emergency Care Department, and many hospital centers do not see one case a week.1,58 The best results (mean stay, ICU, mortality) are obtained when patient care is provided by experienced professionals, in centers attending over 650 STPs a year.59 It does not seem feasible or efficient for all hospitals to have the infrastructure, professionals and training needed for severe trauma care with the adoption of a 24/7 approach.1 In the same way as in other countries, the organization of severe trauma care within a network model, involving regional trauma centers coordinated with other second-level centers, appears essential.1,3,32,58 The indicator that should govern the functioning of both is the time to definitive treatment.
The main functions of radiology in the management of STPs have been standardized in the European setting.13,60 Basically, the aim is to afford 24/7 access to two key services: WBCT exploration, with immediate expert reporting; and interventional radiology for the control of traumatic hemorrhage.
The radiologist in charge of CT must have sufficient experience to receive STPs, apply the correct exploration protocol–including opportune modifications according to the pattern of injuries and clinical condition of the patient–and establish a first balance of injuries, as a primary CT assessment providing an orientation for initial patient management. The radiologist must make sure that the study is complete before the patient leaves the room. Lastly, following exhaustive evaluation of all the images and the opportune post-process assessment, a definitive report must be drafted. The possibility of transmitting digital images over distances in turn allows specialized teleconsultation. It is considered that MRI services should be available within 12h.13
The availability of interventional radiology is currently considered obligate in centers that attend STPs. It offers an alternative to surgery or a complement to the conservative management of some injuries.1,51 Maintaining an Interventional Radiology Unit with a 24/7 approach poses important organizational and personnel training difficulties; consequently, such units should be centralized in reference trauma hospitals. The RCR offers some recommendations and standards regarding the upgrading and equipment of angiography rooms, their location close to the emergency care area, protocols for the transfer of seriously ill and unstable patients, and a response or reaction time defined as 30min.13
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Artigas Martín JM, Martí de Gracia M, Claraco Vega LM, Parrilla Herranz P. Radiología e imagen en el traumatismo grave. Med Intensiva. 2015;39:49–59.