To analyze the efficacy of negative fluid balance in hypoxemic patients with an elevated extravascular lung water index (EVLWI).
DesignA retrospective observational study was made.
SettingIntensive Care Unit of Virgen de las Nieves Hospital (Spain).
ParticipantsForty-four patients participated in the study.
InterventionsWe analyzed our database of hypoxemic patients covering a period of 11 consecutive months. We included all hemodynamically stable and hypoxemic patients with EVLWI>9ml/kg. The protocol dictates a negative fluid balance between 500 and 1500ml/day. We analyzed the impact of this negative fluid balance strategy upon pulmonary, hemodynamic, and renal function.
Main variables of interestDemographic data, severity scores, clinical, hemodynamic, pulmonary, metabolic and renal function data.
ResultsThirty-three patients achieved negative fluid balance (NFB group) and 11 had a positive fluid balance (PFB group). In the former group, PaO2/FiO2 improved from 145 (IQR 106, 200) to 210mmHg (IQR 164, 248) (p<0.001), and EVLWI decreased from 14 (11, 18) to 10ml/kg (8, 14) (p<0.001). In the PFB group, EVLWI also decreased from 11 (10, 14) to 10ml/kg (8, 14) at the end of the protocol (p=0.004).
For these patients there were no changes in oxygenation, with a PaO2/FiO2 of 216mmHg (IQR 137, 260) at the beginning versus 205mmHg (IQR 99,257) at the end of the study (p=0.08).
ConclusionThree out of four hypoxic patients with elevated EVLWI tolerated the NFB protocol. In these subjects, the improvement of various analyzed physiological parameters was greater and faster than in those unable to complete the protocol.
Patients who did not tolerate the protocol were usually in more severe condition, though a larger sample would be needed to detect specific characteristics of this group.
Analizar la eficacia del balance hídrico negativo en pacientes hipoxémicos y con Agua Pulmonar Extravascular Indexada (EVLWI) elevada.
DiseñoEstudio retrospectivo y observacional.
ÁmbitoUnidad de Cuidados Intensivos del Hospital Virgen de las Nieves.
Participantes44 pacientes.
IntervencionesSe analizó la base de datos de pacientes hipoxémicos durante 11 meses consecutivos. Se incluyeron los pacientes hipoxémicos, hemodinámicamente estables y con EVLWI>9ml/kg. El protocolo dicta un balance hídrico negativo entre 500 y 1500ml/día. Se analizó el impacto de esta estrategia de balance negativo en la función respiratoria, hemodinámica y renal.
Variables de interés principalesDatos demográficos, escalas de gravedad y datos clínicos hemodinámicos, respiratorios, metabólicos y de función renal.
Resultados33 pacientes lograron balance hídrico negativo (Grupo BHN) y 11 tuvieron balance hídrico positivo (Grupo BHP). En el grupo BHN la PaO2/FiO2 pasó de 145 (IQR 106,200) a 210 (IQR 164, 248) mmHg (p<0.001), el EVLWI descendió de 14 (11, 18) a 10 (8, 14) ml/kg (p<0.001). En el grupo BHP, el EVLWI también descendió de 11(10, 14) a 10 (8, 14) ml/kg al final del protocolo (p=0.004); en este último grupo no hubo cambios estadísticamente significativos en la oxigenación y la PaO2/FiO2 pasó de 216 (IQR 137, 260) a 205 (IQR 99, 257) mmHg (p=0.08).
ConclusiónTres de cada cuatro pacientes hipoxémicos y con EVLWI elevados toleraron el protocolo; en ellos, la mejora de diversos parámetros analizados fue mayor y más rápida que en los pacientes que no hicieron balance negativo. Los pacientes que no toleraron el protocolo fueron los más graves aunque se necesitaría una muestra mayor para determinar las características específicas en estos.
The negative fluid balance is associated with improvements in oxygenation1–3 and a shorter stay in the Intensive Care Unit (ICU)4; however, negative fluid balance strategies may have a theoretical risk of renal and other organ hypoperfusion, although available data do not support this assumption.5 More than two decades ago, the determination of extravascular lung water index (EVLWI) was used as a useful tool in the management of fluid therapy in critically ill patients.6,7 Then the method for determining EVLW was double indicator.
Currently, the determination of EVLW can be performed by a method that although invasive, is much simpler, transpulmonary thermodilution requiring central venous catheter and a femoral artery. In hemodynamically stable, hypoxemic patients the inclusion of EVLWI in the fluid therapy decision tree has an impact on the amount of fluid administered.8 Although the evidence is not completely clear, it seems logical to attempt a negative fluid balance to avoid volume overload. EVLWI is a parameter that provides essential physiological information,9 such as the degree of pulmonary edema. Traditionally, quantification of pulmonary edema was performed using chest radiography but their accuracy is low.10 In patients with increased pulmonary capillary permeability, achieving a negative fluid balance decreases the capillary pulmonary pressure (CPP) thereby decreasing the amount of fluid filtered into the interstitium.11 In our center, the fluid therapy protocol for hemodynamically stable, hypoxemic patients includes a negative fluid balance with the goal of lowering EVLWI and improving gas exchange. While there is little literature on the topic, De Laet et al.3 found that a negative fluid balance after renal replacement therapy yields minimal drops in EVLWI; however, this study was performed in a heterogeneous group of critically ill patients, some with normal EVLWI.
The objective of our study was to analyze the efficacy of negative fluid balance in hypoxemic patients with elevated levels of EVLWI.
Patients and methodsWe analyzed our database (from June 2011 to May 2012) of patients managed according to the fluid therapy protocol at our institution. The institutional review board of the hospital waived the need for informed consent.
We included all patients that were hypoxemic (PaO2/FiO2<300mmHg), mechanically ventilated, and hemodynamically stable in the post-resuscitation phase, and that were being monitored with a thermodilution catheter PICCO (Pulsion Medical System Munich, Germany). The thermodilution catheter is inserted in the femoral artery and this method also requires the placement of a central venous catheter (CVC) in the superior vena cava.
The CVC is used to infuse three boluses of 15–20ml of normal saline at a temperature <8°C.
The PICCO monitor includes software capable of calculating the EVLWI according the predicted body weight, along with static and dynamic hemodynamic parameters. Although the PICCO system is recalibrated throughout the day, hemodynamic parameters and lung water measurements were collected between 8:30 and 9:30 AM after the first daily calibration and simultaneously with the rest of respiratory and metabolic data.
The fluid balance protocol was then applied according to EVLWI levels:
- •
If the EVLWI was >9ml/kg then fluid restriction and/or a furosemide infusion at a rate of 2mg/h were initiated. This regimen was modified accordingly to achieve a goal of negative fluid balance of 500 to 1500ml per day.
- •
If the EVLWI was ≤9ml/kg, then monitoring was continued and fluid therapy was prescribed according to other clinical parameters: urinary output, blood pressure, Cardiac index (CI), preload, afterload, and contractility indices provided by the PICCO monitor. If EVLWI increased above the threshold level of 9ml/kg, then the negative fluid balance protocol was initiated.
The daily fluid balance was calculated as the difference between water intake (nutrition, blood products, saline infusions, medications) and output (urine, gastrointestinal, and insensible). In critically ill patients insensible losses play an important role when calculating fluid balance. These losses were calculated using the formula, IL (ml)=400×BSA (m2)×24h. BSA=body surface area was calculated according to the Dubois formula12 BSA (m2)=0.007184×weight (kg)×height (cm). If fever was present, the IL increased by 10% for every C degree above 37°C per hour. If the patient was being mechanically ventilated the total IL was divided by 2.
Inclusion and exclusion criteriaHemodynamically stable patients were defined as those with mean arterial blood pressures >70mmHg and producing >1ml/kg/h of urine requiring stable while on decreasing doses or no vasoactive drugs. Exclusion criteria included the following: increasing requirements of vasoactive drugs over the previous 12h, at risk renal function according to the RIFLE criteria13 and electrolyte derangements (Na>150mEq/l of K<2.5mEq/L). Once all these parameters were corrected the patient was again evaluated for inclusion in the protocol.
The negative fluid balance protocol was stopped once EVLWI≤9ml/kg or the negative fluid balance resulted in adverse events, such as hemodynamic or renal compromise according to the previously mentioned parameters. Similarly, if after seven days of follow-up oxygenation did not improve, the physician responsible for the patient decided whether to continue or stop the protocol.
Demographic data collected included: age, gender, diagnosis on admission to the ICU, past medical history, APACHE III score and ISS (injury severity score) for trauma patients.
Clinical data included: FiO2, PaO2 and their ration, PEEP (positive end-expiratory pressure), EVLWI, number of chest X-ray quadrant affected, hemodynamic, metabolic and renal function data. Compliance with the protocol was also recorded as a ratio of the negative fluid balance planned days/negative fluid balance achieved days.
The protocol was considered successful when there was a significant improvement in oxygenation or radiological appearance. It was considered safe when there were no hemodynamic or renal adverse events.
The data were analyzed using SPSS version 18.0. Quantitative variables were described with median and interquartile range. Qualitative variables were described with absolute and relative frequencies. The Wilcoxon test and Mann Whitney test was used for comparison of quantitative variables and Chi-square test to compare qualitative variables. A p-value <0.05 was considered significant.
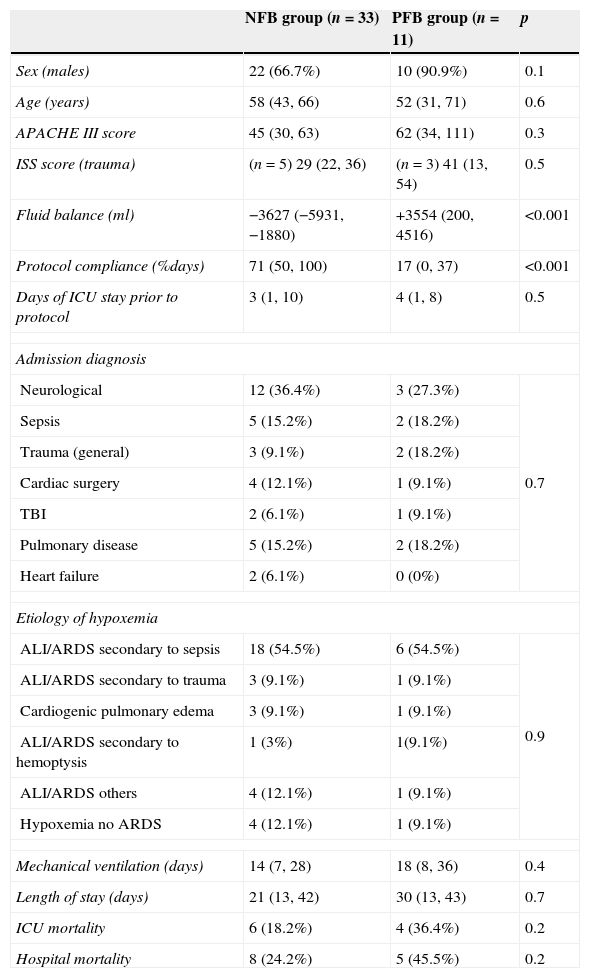
ResultsA total of 44 patients were included in the analysis. Thirty-three achieved negative fluid balance (NFB) and 11 had a positive fluid balance (PFB). Demographics, acuity scores, and other general characteristics are presented in Table 1. Compliance with the protocol was 71% in the NFB group and 17% in the PFB group (p<0.01).
General descriptors.
| NFB group (n=33) | PFB group (n=11) | p | |
|---|---|---|---|
| Sex (males) | 22 (66.7%) | 10 (90.9%) | 0.1 |
| Age (years) | 58 (43, 66) | 52 (31, 71) | 0.6 |
| APACHE III score | 45 (30, 63) | 62 (34, 111) | 0.3 |
| ISS score (trauma) | (n=5) 29 (22, 36) | (n=3) 41 (13, 54) | 0.5 |
| Fluid balance (ml) | −3627 (−5931, −1880) | +3554 (200, 4516) | <0.001 |
| Protocol compliance (%days) | 71 (50, 100) | 17 (0, 37) | <0.001 |
| Days of ICU stay prior to protocol | 3 (1, 10) | 4 (1, 8) | 0.5 |
| Admission diagnosis | |||
| Neurological | 12 (36.4%) | 3 (27.3%) | 0.7 |
| Sepsis | 5 (15.2%) | 2 (18.2%) | |
| Trauma (general) | 3 (9.1%) | 2 (18.2%) | |
| Cardiac surgery | 4 (12.1%) | 1 (9.1%) | |
| TBI | 2 (6.1%) | 1 (9.1%) | |
| Pulmonary disease | 5 (15.2%) | 2 (18.2%) | |
| Heart failure | 2 (6.1%) | 0 (0%) | |
| Etiology of hypoxemia | |||
| ALI/ARDS secondary to sepsis | 18 (54.5%) | 6 (54.5%) | 0.9 |
| ALI/ARDS secondary to trauma | 3 (9.1%) | 1 (9.1%) | |
| Cardiogenic pulmonary edema | 3 (9.1%) | 1 (9.1%) | |
| ALI/ARDS secondary to hemoptysis | 1 (3%) | 1(9.1%) | |
| ALI/ARDS others | 4 (12.1%) | 1 (9.1%) | |
| Hypoxemia no ARDS | 4 (12.1%) | 1 (9.1%) | |
| Mechanical ventilation (days) | 14 (7, 28) | 18 (8, 36) | 0.4 |
| Length of stay (days) | 21 (13, 42) | 30 (13, 43) | 0.7 |
| ICU mortality | 6 (18.2%) | 4 (36.4%) | 0.2 |
| Hospital mortality | 8 (24.2%) | 5 (45.5%) | 0.2 |
NFB, negative fluid balance; PFB, positive fluid balance; p, level of significance; ISS, injury severity score; TBI, traumatic brain injury; ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
Quantitative variables are expressed as median and interquartile range, qualitative variables as frequencies and percentages.
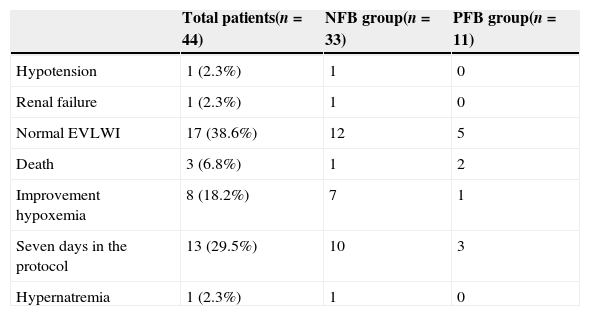
The reasons for termination of the protocol can be seen in Table 2.
Protocol termination cause.
| Total patients(n=44) | NFB group(n=33) | PFB group(n=11) | |
|---|---|---|---|
| Hypotension | 1 (2.3%) | 1 | 0 |
| Renal failure | 1 (2.3%) | 1 | 0 |
| Normal EVLWI | 17 (38.6%) | 12 | 5 |
| Death | 3 (6.8%) | 1 | 2 |
| Improvement hypoxemia | 8 (18.2%) | 7 | 1 |
| Seven days in the protocol | 13 (29.5%) | 10 | 3 |
| Hypernatremia | 1 (2.3%) | 1 | 0 |
NFB, negative fluid balance; PFB, positive fluid balance.
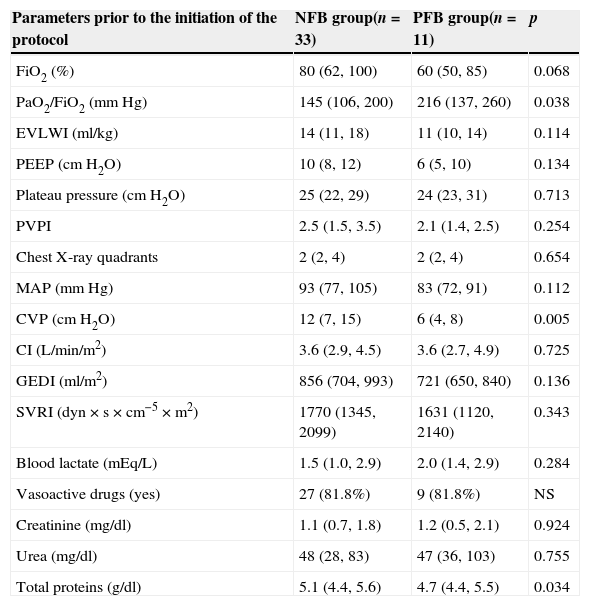
When comparing the hemodynamic and respiratory parameters between groups at the beginning of the protocol they were statistically different. The NFB group had a significantly lower PaO2/FiO2, 145 vs. 216mmHg (p=0.04), a higher CVP, +12 vs.+6cmH2O (p<0.01) and total protein higher than the PFB group, 5.1 vs. 4.7g/dl (p=0.04) (Table 3).
Comparison of the hemodynamic and pulmonary parameters before enrollment.
| Parameters prior to the initiation of the protocol | NFB group(n=33) | PFB group(n=11) | p |
|---|---|---|---|
| FiO2 (%) | 80 (62, 100) | 60 (50, 85) | 0.068 |
| PaO2/FiO2 (mmHg) | 145 (106, 200) | 216 (137, 260) | 0.038 |
| EVLWI (ml/kg) | 14 (11, 18) | 11 (10, 14) | 0.114 |
| PEEP (cmH2O) | 10 (8, 12) | 6 (5, 10) | 0.134 |
| Plateau pressure (cmH2O) | 25 (22, 29) | 24 (23, 31) | 0.713 |
| PVPI | 2.5 (1.5, 3.5) | 2.1 (1.4, 2.5) | 0.254 |
| Chest X-ray quadrants | 2 (2, 4) | 2 (2, 4) | 0.654 |
| MAP (mmHg) | 93 (77, 105) | 83 (72, 91) | 0.112 |
| CVP (cmH2O) | 12 (7, 15) | 6 (4, 8) | 0.005 |
| CI (L/min/m2) | 3.6 (2.9, 4.5) | 3.6 (2.7, 4.9) | 0.725 |
| GEDI (ml/m2) | 856 (704, 993) | 721 (650, 840) | 0.136 |
| SVRI (dyn×s×cm−5×m2) | 1770 (1345, 2099) | 1631 (1120, 2140) | 0.343 |
| Blood lactate (mEq/L) | 1.5 (1.0, 2.9) | 2.0 (1.4, 2.9) | 0.284 |
| Vasoactive drugs (yes) | 27 (81.8%) | 9 (81.8%) | NS |
| Creatinine (mg/dl) | 1.1 (0.7, 1.8) | 1.2 (0.5, 2.1) | 0.924 |
| Urea (mg/dl) | 48 (28, 83) | 47 (36, 103) | 0.755 |
| Total proteins (g/dl) | 5.1 (4.4, 5.6) | 4.7 (4.4, 5.5) | 0.034 |
NFB, negative fluid balance; PFB, positive fluid balance; p, level of significance; EVLWI, extravascular lung water index; PEEP, positive end-expiratory pressure; PVPI, pulmonary vascular permeability index; MAP, mean arterial pressure; CVP, central venous pressure; CI, cardiac index; GEDI, global end diastolic volume index; SVRI, systemic vascular resistance index; NS, not significant.
Quantitative variables are expressed as median and interquartile range, qualitative variables as frequencies and percentages.
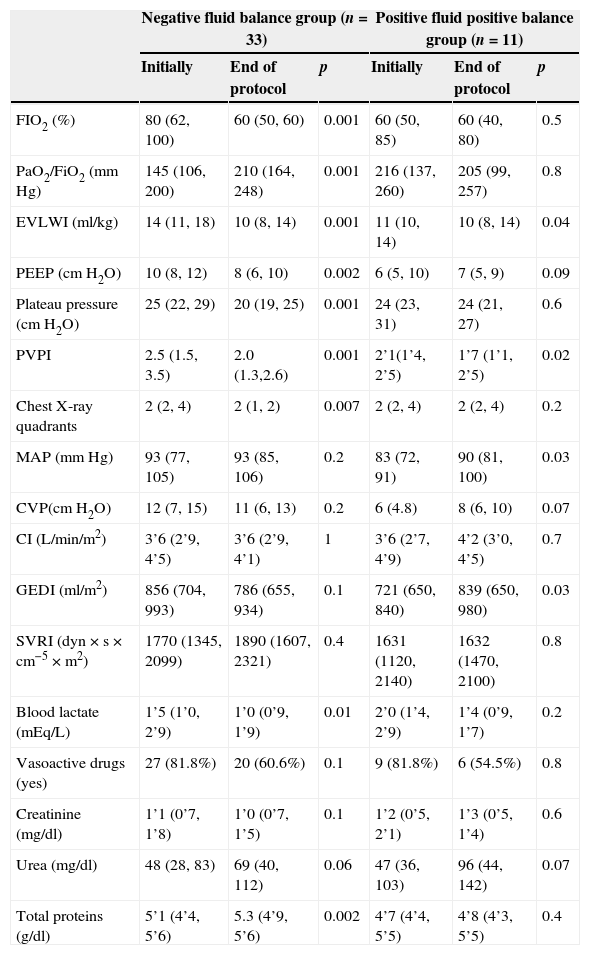
The 33 patients who achieved NFB experienced a clear improvement in oxygenation; the PaO2/FiO2 increased from 145 (106, 200) mmHg to 210 (164, 268) mmHg at the end of the protocol (p<0.01). The EVLWI decreased from 14(11, 18) ml/kg to 10 (8, 14) ml/kg. PEEP level, plateau pressure, and PVPI also experienced statistically significant improvements (Table 4).
Pulmonary, hemodynamic and metabolic parameters in the two groups at the beginning and conclusion of the protocol.
| Negative fluid balance group (n=33) | Positive fluid positive balance group (n=11) | |||||
|---|---|---|---|---|---|---|
| Initially | End of protocol | p | Initially | End of protocol | p | |
| FIO2 (%) | 80 (62, 100) | 60 (50, 60) | 0.001 | 60 (50, 85) | 60 (40, 80) | 0.5 |
| PaO2/FiO2 (mm Hg) | 145 (106, 200) | 210 (164, 248) | 0.001 | 216 (137, 260) | 205 (99, 257) | 0.8 |
| EVLWI (ml/kg) | 14 (11, 18) | 10 (8, 14) | 0.001 | 11 (10, 14) | 10 (8, 14) | 0.04 |
| PEEP (cmH2O) | 10 (8, 12) | 8 (6, 10) | 0.002 | 6 (5, 10) | 7 (5, 9) | 0.09 |
| Plateau pressure (cmH2O) | 25 (22, 29) | 20 (19, 25) | 0.001 | 24 (23, 31) | 24 (21, 27) | 0.6 |
| PVPI | 2.5 (1.5, 3.5) | 2.0 (1.3,2.6) | 0.001 | 2’1(1’4, 2’5) | 1’7 (1’1, 2’5) | 0.02 |
| Chest X-ray quadrants | 2 (2, 4) | 2 (1, 2) | 0.007 | 2 (2, 4) | 2 (2, 4) | 0.2 |
| MAP (mmHg) | 93 (77, 105) | 93 (85, 106) | 0.2 | 83 (72, 91) | 90 (81, 100) | 0.03 |
| CVP(cmH2O) | 12 (7, 15) | 11 (6, 13) | 0.2 | 6 (4.8) | 8 (6, 10) | 0.07 |
| CI (L/min/m2) | 3’6 (2’9, 4’5) | 3’6 (2’9, 4’1) | 1 | 3’6 (2’7, 4’9) | 4’2 (3’0, 4’5) | 0.7 |
| GEDI (ml/m2) | 856 (704, 993) | 786 (655, 934) | 0.1 | 721 (650, 840) | 839 (650, 980) | 0.03 |
| SVRI (dyn×s×cm−5×m2) | 1770 (1345, 2099) | 1890 (1607, 2321) | 0.4 | 1631 (1120, 2140) | 1632 (1470, 2100) | 0.8 |
| Blood lactate (mEq/L) | 1’5 (1’0, 2’9) | 1’0 (0’9, 1’9) | 0.01 | 2’0 (1’4, 2’9) | 1’4 (0’9, 1’7) | 0.2 |
| Vasoactive drugs (yes) | 27 (81.8%) | 20 (60.6%) | 0.1 | 9 (81.8%) | 6 (54.5%) | 0.8 |
| Creatinine (mg/dl) | 1’1 (0’7, 1’8) | 1’0 (0’7, 1’5) | 0.1 | 1’2 (0’5, 2’1) | 1’3 (0’5, 1’4) | 0.6 |
| Urea (mg/dl) | 48 (28, 83) | 69 (40, 112) | 0.06 | 47 (36, 103) | 96 (44, 142) | 0.07 |
| Total proteins (g/dl) | 5’1 (4’4, 5’6) | 5.3 (4’9, 5’6) | 0.002 | 4’7 (4’4, 5’5) | 4’8 (4’3, 5’5) | 0.4 |
EVLWI, extravascular lung water index; PEEP, positive end-expiratory pressure; PVPI, pulmonary vascular permeability index; MAP, mean arterial; CVP, central venous pressure; CI, cardiac index; GEDI, global end diastolic volume index; SVRI, systemic vascular resistance index.
Quantitative variables are expressed as median and interquartile range, qualitative variables as frequencies and percentages.
Patients with negative fluid balance did not experience changes in hemodynamic or metabolic parameters with the exception of a significant drop in lactic acid levels from 1.5 (1.0, 2.9) mEq/L to 1.0 (0.9, 1.9) mEq/L (p=0.01) and total proteins which increased from 5.1 (4.4, 5.6) to 5.3 (4.9, 5.6) g/dl, (p<0.01). Other hemodynamic and metabolic parameters are presented in Table 4.
The eleven patients with positive fluid balance experienced a moderate but statistically significant drop in EVLWI from 11 (10, 14) to 10 (8, 14) ml/kg at the end of the protocol (p<0.01). The PVPI also dropped from 2.1 (1.4, 2.5) to 1.7 (1.1, 2.5) at the end of the protocol (p<0.01) (Table 4). The blood pressure increased from 83 (72, 91) to 90 (81, 100) mmHg (p=0.03) and GEDI (global end diastolic index) also increased from 721 (650, 840) ml/m2 to 839 (6 with p=0.03) (Table 4).
In five patients the creatinine increased significantly and they were diagnosed with non-oliguric acute renal failure. None required renal replacement treatment. Two of the patients belonged to the NFB group and three to the PFB group. Two of these patients died, one in each group.
The standarized mortality ratio of the total sample has been 0.86 (CI 95%, 0.72–0.99).
DiscussionOur results show that hypoxemic patients with elevated EVLWI can follow a protocol of negative fluid balance with the goal of improving their respiratory function safely and effectively. Those patients who achieved a negative fluid balance experienced a clear improvement in their respiratory function as well as a significant reduction in EVLWI without detrimental renal or cardio circulatory effects.
In hemodynamically stable patients with acute respiratory distress syndrome, negative fluid balance decreases pulmonary capillary pressure which is associated with an improved prognosis.11 In hypoproteinemic patients with acute lung injury, negative fluid balance improves oxygenation1,2; nonetheless there is scant information about its effects on EVLWI. De Laet et al.3 found that achieving negative fluid balance using renal replacement therapy resulted in small but significant reductions in EVLWI. Furthermore this negative balance has not been associated with adverse hemodynamic effects. In our study, negative fluid balance was achieved for a longer period of time (7 days), and we found not only significant reductions in EVLWI but also improvements in oxygenation.
Probably the reduction in EVLWI is both a result of negative fluid balance and the resolution of the primary pulmonary process. This aspect is supported by the data provided by the PVPI (pulmonary vascular permeability index) which can be considered as a marker of capillary permeability.14–16 It improved in both study groups.
Like other physiological parameters, the normal value for EVLWI is subject to discussion. Some authors define a normal value as ≤7ml/kg.17,18 In a recent study in humans,19 the normal value reached was 7.4±3.3ml/kg. Other authors20–22 consider the superior limit to be 10ml/kg. We chose this as our threshold since experimental and clinical experience8 supports it.
The level of compliance with the protocol had limitations (71% in the NFB group). Reasons for non-compliance were multiple but included receiving blood products and undergoing surgeries, which reflects the complexity of critically ill patients. It is not clear why some patients achieved NFB while others did not. Initial preload, central venous pressure and total proteins were statistically significantly higher in the group that finally achieved NFB. Others parameters such as GEDI and TA were also higher although the difference did not reach statistical significance.
Likewise these patients were more hypoxemic and had higher EVLWI at initiation of the protocol suggesting higher volume overload. That may be the reason why they were able to tolerate the negative fluid balance strategy better. Furthermore, although there were no statistically significant differences, there was a tendency toward higher APACHE and ISS scores in the patients that did not tolerate the protocol. It is possible that the higher acuity of this group was the reason for protocol non-compliance.
Renal function was unaffected despite the negative fluid balance, and the rise in urea was not statistically significant. These results are consistent with those achieved by other authors5 who found even a lower rate of renal failure among those patients achieving negative fluid balance. Likewise, we did not find differences in cardiovascular function expressed as use of vasoactive drugs and blood lactate levels.
We did not find statistically significant differences in length of ICU stay or in duration of mechanical ventilation between the two groups. There were no significant differences in ICU or hospital mortality although it is worth noting that the mortality in the NFB group was 24% vs. 45% in the PFB group (Table 1). We must also note that this non-statistically significant difference could be explained by the higher acuity of patients in the PFB group.
Based in all this, we think that our protocol is secure, considering also that the observed mortality in the entire sample was significantly lower than expected with a standarized mortality ratio of 0.86 (CI 95%, 0.72–0.99).
Studies with a larger number of patients are needed to clarify these questions.
Study limitationsThis is a single center, retrospective, observational study with a relatively small number of patients. There may also be some limitations related to the measurement of EVLWI. This technique is perfusion dependent, and it is not reliable in patients with large areas of non-perfused lung, as in the case of acute pulmonary embolism. However, none of the patients included in our series suffered from pulmonary embolism.
ConclusionsIn our series, three out of four patients achieved negative fluid balance and this was associated with a rapid and greater improvement in oxigenation and in other analyzed parameters than patiens who does not achieved negative flauid balance.
Conflict of interestEnrique Fernandez-Mondéjar is member of the Medical Advisory Board of Pulsion Medical Systems. The rest of the authors do not have conflict of interest.