Primary: To evaluate the level of sedation, use, daily doses, and duration of analgosedative drugs in COVID-19 patients on mechanical ventilation (MV) using a standardized protocol, comparing survivors and non-survivors. Secondary: To identify independent predictors of hospital mortality.
DesignRetrospective cohort study.
SettingMedical-surgical ICU.
PatientsAdults with SARS-CoV-2 infection requiring invasive MV and continuous infusion of analgosedation and/or neuromuscular blocking agents (NMBAs) for at least 48 h.
InterventionsNone.
Main variables of interestLevel of sedation, use, daily doses, and duration of analgosedative drugs; hospital mortality and associated factors.
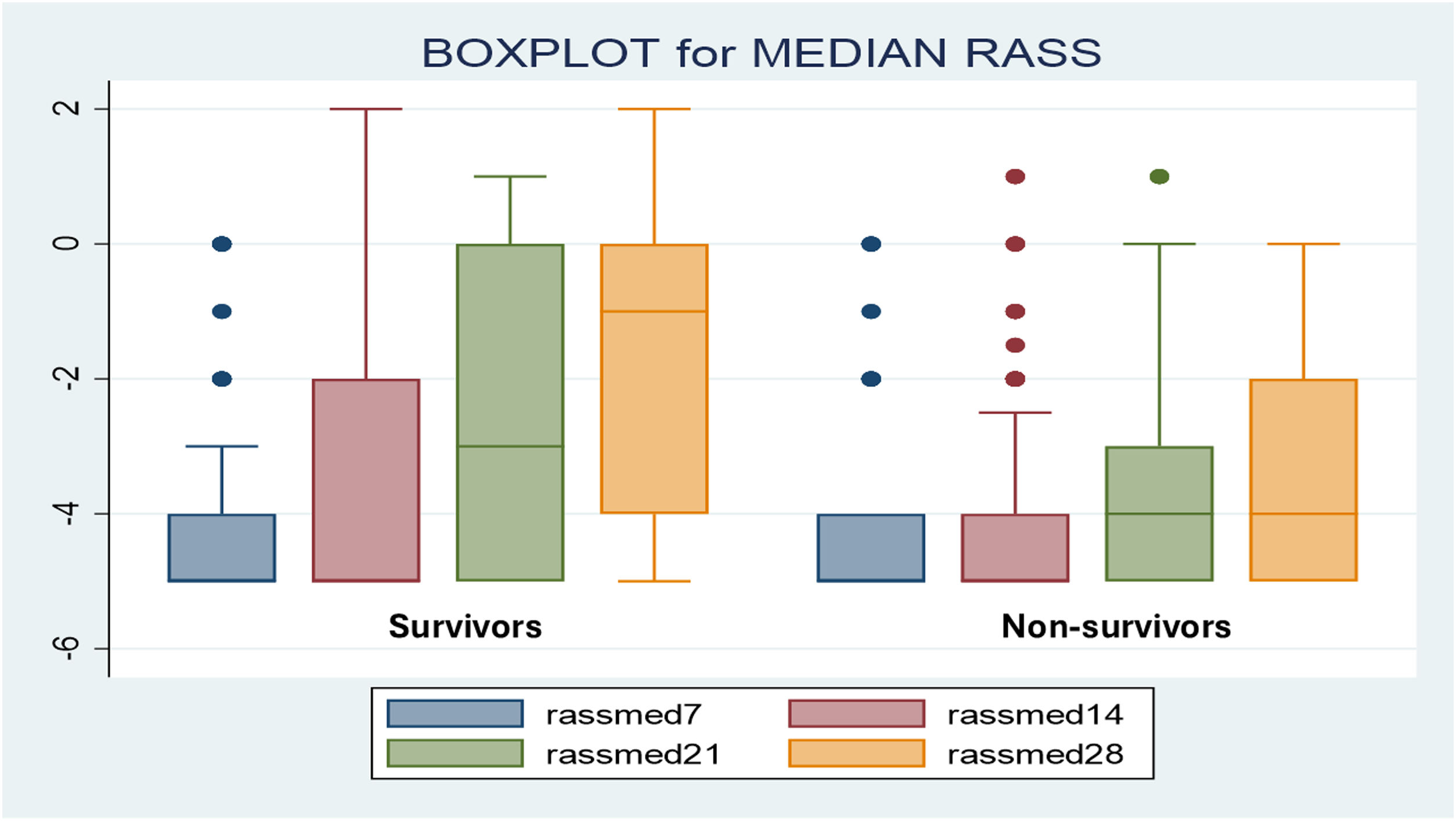
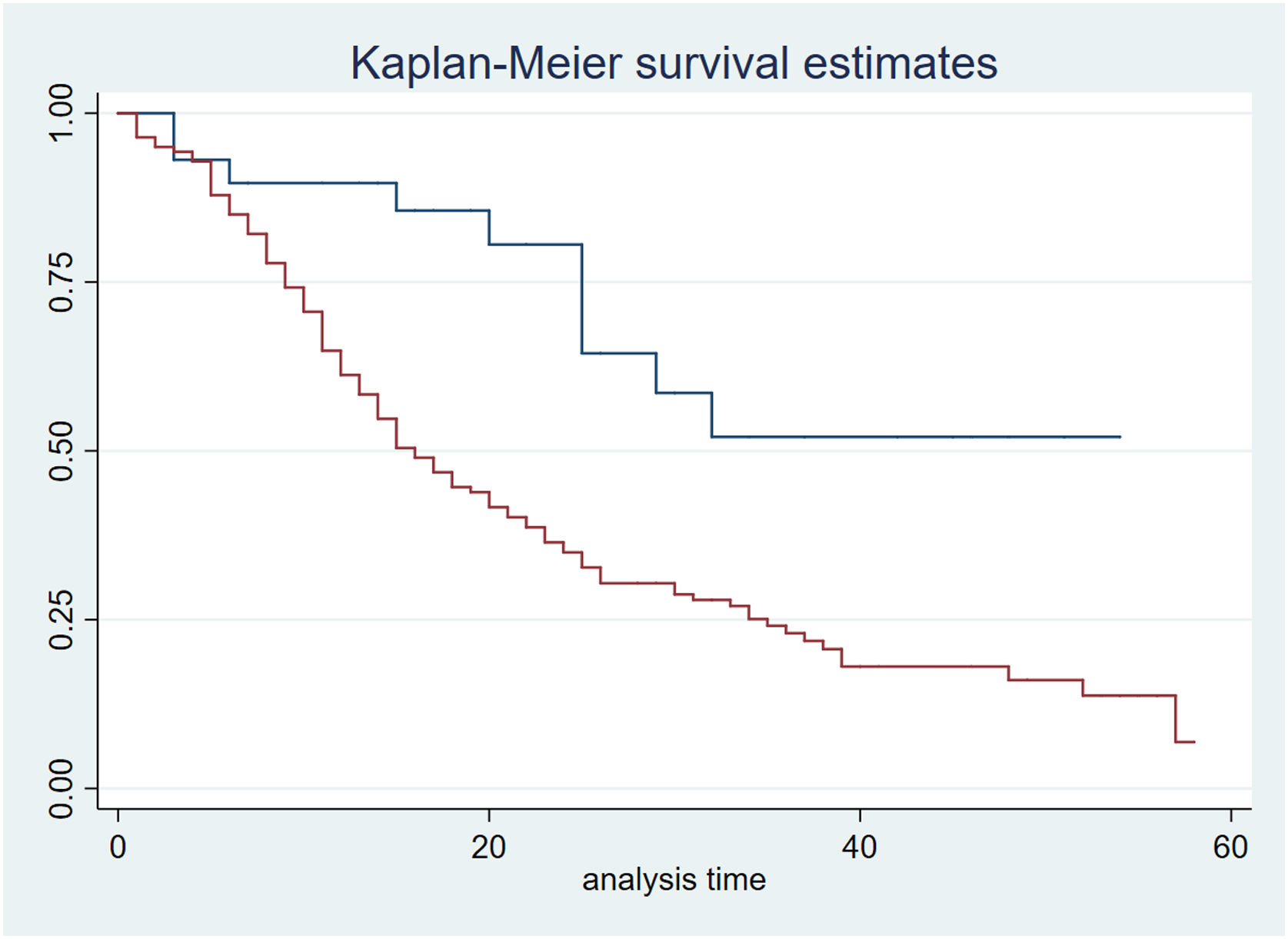
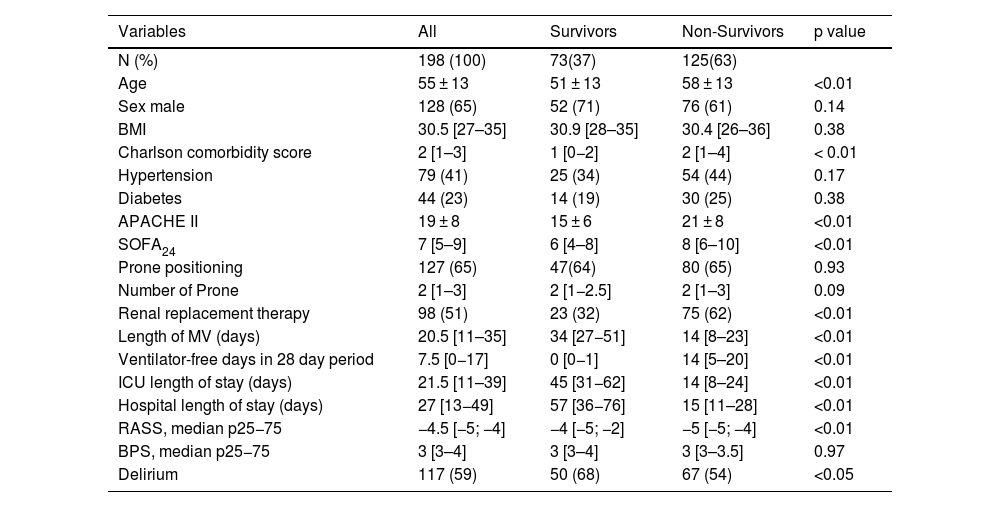
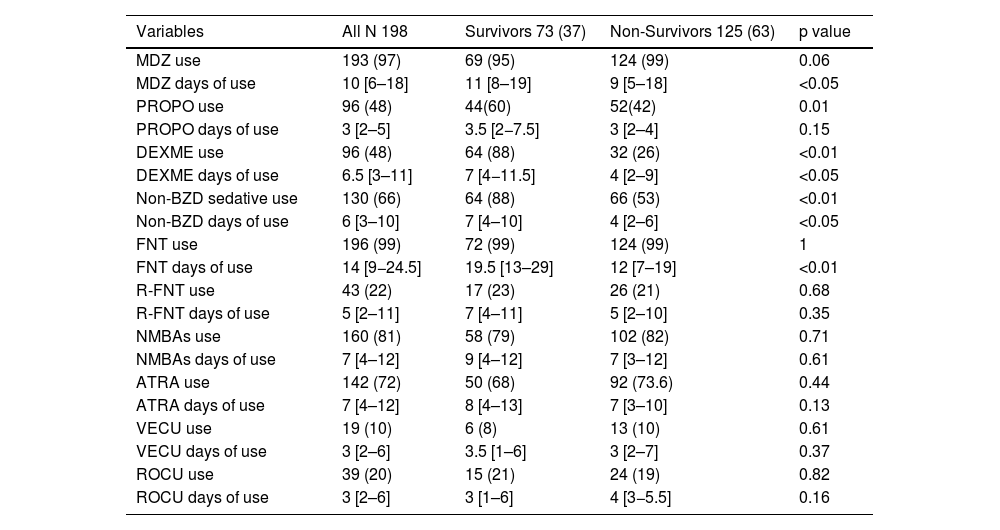
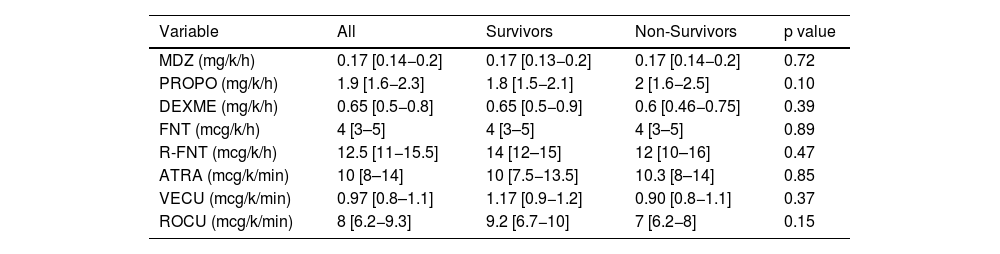
ResultsAmong 198 patients (nurse-to-patient ratio 1:2.4; 65% staff turnover), median global RASS was –4.5. Kaplan–Meier analysis showed lower survival with deeper sedation. Fentanyl (99%) and midazolam (97%) were the most used, followed by NMBAs (81%), propofol and dexmedetomidine (48%). Non-benzodiazepine sedatives were precribed more in survivors (88%) than non-survivors (53%) (p < 0.01). Survivors had more days of fentanyl, midazolam, and dexmedetomidine; no differences in NMBA use or drug doses were observed. Mortality was 63%. Independent predictors of mortality included APACHE II, SOFA24, Charlson score, median RASS, and non-benzodiazepine sedative use.
ConclusionsStandardized protocols emphasizing the ACD components of the ABCDEF bundle, along with appropriate use of analgosedation and NMBAs despite limited staffing, effectively supported the management of sedation without significant dose differences between survivors and non-survivors. Sedation level and the use of non-benzodiazepine sedatives were independently associated with better outcomes, highlighting the importance of the light sedation and the ABCDEF bundle.
Primario: Evaluar nivel de sedación, uso, dosis diarias y duración de fármacos analgosedantes en pacientes con COVID-19 en ventilación mecánica (VM) utilizando protocolos estandarizados, comparando sobrevivientes y no sobrevivientes. Secundario: Identificar predictores independientes de mortalidad hospitalaria.
DiseñoCohorte retrospectiva
ÁmbitoUCI médico-quirúrgica.
PacientesAdultos con SARS-CoV-2, VM invasiva e infusión continua de analgosedación y/o bloqueantes neuromusculares (BNMs) ≥48 horas.
IntervencionesNinguna.
Variables de interés principalesNivel de sedación, uso, dosis diarias y duración de analgosedantes; mortalidad hospitalaria y factores asociados.
ResultadosSe incluyeron 198 pacientes. Relación enfermero-paciente 1:2,4; recambio de enfermería 65%. El RASS global fue −4.5. La sedación profunda se asoció con menor supervivencia (Kaplan–Meier). Fentanilo (99%) y midazolam (97%) fueron los más utilizados; seguidos por BNMs (81%), propofol y dexmedetomidina (48%). Los sedantes no benzodiacepínicos se usaron en 88% de los sobrevivientes versus 53% de los no sobrevivientes (p < 0,01). Los sobrevivientes tuvieron significativamente más días con fentanilo, midazolam y dexmedetomidina, sin diferencias con BNMs ni en las dosis de todos los fármacos. Mortalidad 63%. Los predictores independientes de mortalidad incluyeron APACHE II, SOFA24, Charlson, RASS mediana y uso de sedantes no benzodiacepínicos.
ConclusionesProtocolos estandarizados que enfatizan los componentes ACD del paquete ABCDEF y el uso adecuado de la analgosedación y BNMs, incluso con personal limitado, permitieron una gestión efectiva de la sedación sin diferencias significativas en dosis entre sobrevivientes y no sobrevivientes. El nivel de sedación y los sedantes no benzodiacepínicos se asociaron independientemente con mejores resultados, destacando la importancia de sedación ligera paquetes ABCDEF.
Article
Go to the members area of the website of the SEMICYUC (www.semicyuc.org )and click the link to the magazine.