To determine the changes in intubation procedures of critically ill patients without SARS-CoV-2 infection induced during the COVID-19 pandemic.
DesignSecondary Analysis of the INTUPROS Prospective Multicenter Observational Study on Intubation in Intensive Care Units (ICUs).
Setting43 Spanish ICUs between April 2019 and October 2020.
Patients1515 Non-COVID-19 patients intubated before and during the pandemic.
InterventionsNone.
Main variables of interestIntubation procedures and medication, first-pass success rate, complications, and mortality.
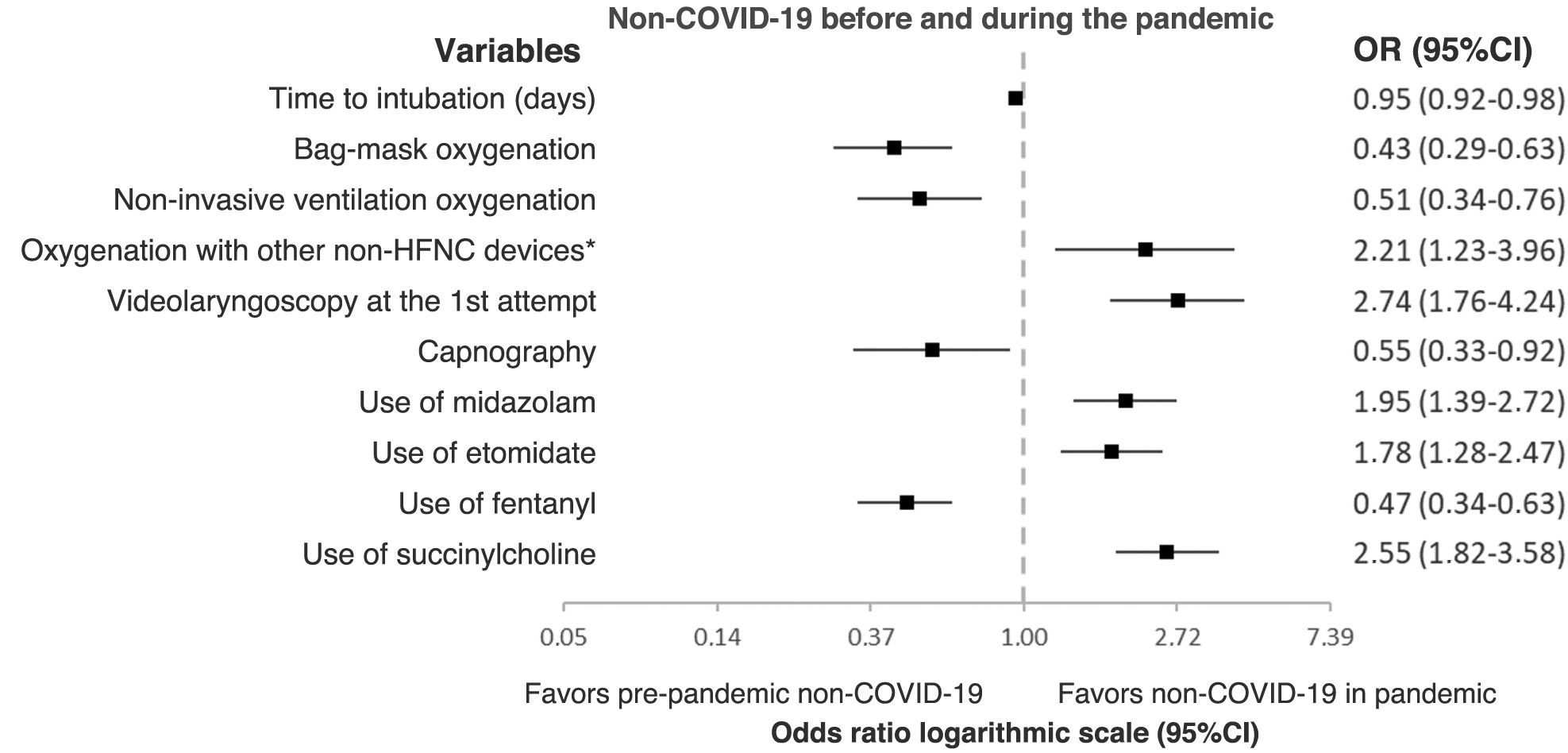
Results1199 patients intubated before the pandemic and 316 during the pandemic were analyzed. During the pandemic, there were fewer days until intubation (OR 0.95 95% CI [0.92−0.98]), reduced resuscitation bag (OR 0.43 95% CI [0.29−0.63]) and non-invasive ventilation oxygenation (OR 0.51 95% CI [0.34−0.76]), reduced use of capnography (OR 0.55 95% CI [0.33−0.92]) and fentanyl (OR 0.47 95% CI [0.34−0.63]). On the other hand, there was an increase in oxygenation with non-HFNC devices (OR 2.21 95% CI [1.23–3.96]), in use of videolaryngoscopy on the first-pass (OR 2.74 95% CI [1.76–4.24]), and greater use of midazolam (OR 1.95 95% CI [1.39–2.72]), etomidate (OR 1.78 95% CI [1.28–2.47]) and succinylcholine (OR 2.55 95% CI [1.82–3.58]). The first-pass success was higher (68.5% vs. 74.7%; P=.033). There were no pre-post differences in major complications (34.7% vs. 34.8%; P=.970) and in-hospital mortality (42.7% vs. 38.6%; P=.137).
ConclusionsThe COVID-19 pandemic modified intubation procedures in non-COVID-19 patients, changing the oxygenation strategy, the medication and the use of videolaryngoscopy, with no impact on complications or mortality.
Determinar los cambios en los procedimientos de intubación que la pandemia COVID-19 generó en la atención de los pacientes críticos sin infección por SARS-CoV-2.
DiseñoAnálisis secundario del estudio prospectivo multicéntrico observacional INTUPROS sobre intubación en unidades de cuidados intensivos (UCI).
Ámbito43 UCI españolas entre abril 2019 y octubre 2020.
Pacientes1515 pacientes No-COVID-19 intubados antes y durante la pandemia.
IntervencionesNinguna.
Variables de interés principalesProcedimientos y medicación para la intubación, tasa de intubación a la primera, complicaciones y mortalidad.
ResultadosSe analizan 1199 pacientes intubados antes de la pandemia y 316 en pandemia. En pandemia, hubo menos días hasta la intubación (OR 0,95 IC 95% [0,92–0,98]), menor oxigenación con balón (OR 0,43 IC 95% [0,29–0,63]) y ventilación no invasiva (OR 0,51 IC 95% [0,34–0,76]), menor uso de capnografía (OR 0,55 IC 95% [0,33–0,92]) y de fentanilo (OR 0,47 IC 95% [0,34–0,63]). Por contra, hubo mayor oxigenación con dispositivos no ONAF (OR 2,21 IC 95% [1,23–3,96]), mayor videolaringoscopia al primer intento (OR 2,74 IC 95% [1,76–4,24]), y mayor uso de midazolam (OR 1,95 IC 95%[1,39–2,72]), etomidato (OR 1,78 IC 95%[1,28–2,47]) y succinilcolina (OR 2,55 IC 95%[1,82–3,58]). La tasa de intubación a la primera fue superior (68,5% vs.74,7%; P=,033). No hubo diferencias pre-post en complicaciones mayores (34,7% vs. 34,8%; P=,970) y mortalidad hospitalaria (42,7% vs. 38,6%; P=,137).
ConclusionesLa pandemia COVID-19 modificó los procedimientos de intubación en pacientes No-COVID-19, cambiando la estrategia de oxigenación, la medicación utilizada y el uso de videolaringoscopia, sin generar impacto en complicaciones o mortalidad.
Intubation of critically ill patients is a high-risk procedure associated with a high rate of complications.1,2 Recently, the Spanish multicenter, prospective observational study INTUPROS on intubation in critically ill patients at the intensive care unit (ICU) setting was published, showing a severe adverse event rate of 40.4%, primarily involving hemodynamic instability and severe hypoxemia.3,4
The COVID-19 pandemic changed intubation practices, increasing the use of high-flow nasal oxygen therapy (HFNO) prior to intubation, videolaryngoscopy (VL), neuromuscular blocking agents, intubation guides, and stylets.2,3,5–18 Although was accompanied by the need for personal protective equipment (PPE) donning, a high success rate was achieved on the first-pass.3,13,14,16,17,19 Few studies have assessed the impact of the COVID-19 pandemic on intubation habits in critically ill patients without SARS-CoV-2 infection after the start of the pandemic, focusing on the different types of VL used,20 mortality rates,21 or scheduled patients,14 all of which were retrospective studies.
The INTUPROS study was conducted from April 2019 through October 2020, so it prospectively included patients before the start of the pandemic, patients with COVID-19, and patients without COVID-19 after the pandemic began. The objective of this secondary analysis is to determine the differences in intubation procedures that the COVID-19 pandemic created in the management of critically ill patients without a SARS-CoV-2 infection.
MethodologyThis is a secondary analysis of a multicenter, prospective cohort observational study conducted in 43 Spanish ICUs (INTUPROS study). A detailed description of the methodology is provided in the original publication and its supplementary electronic material.3 Patients were consecutively included in 6-month periods in each ICU from April 16th 2019 through October 31st 2020. The study protocol was updated in March 2020 to include the presence of COVID-19 as the reason for admission.
The Virgen del Rocío and Virgen Macarena Hospitals Ethics Committee (Seville, Spain) approved the study back on January 14th 2019 (1149-N-18), which was later ratified at each participant center. Given the observational design of the study and the short observation period of the intubation event (events occurring up to 30min after the procedure), informed consent was not deemed necessary. The Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) endorsed the study.
Inclusion and exclusion criteria: Patients older than 18 years old admitted to intensive care units who were intubated were included, excluding intubations due to cardiac arrest and those performed outside the ICU setting, even if they occurred during the patient's admission process. The procedure was performed by members of the intensive care medical staff or intensive care residents.
Variables: Demographic variables, type and reason for admission, severity on admission measured by APACHE II22 and SOFA23 scores, reason for intubation, organ dysfunction on the day of intubation, use of enteral nutrition and non-invasive ventilation or HFNO before intubation, pre-oxygenation methods before intubation, ramped position, facilitating devices (stylets, Frova® Guide or Eschmann® Guide), Sellick maneuver, use of laryngoscope, VL, or other devices (supraglottic, fiberoptic bronchoscopy, tracheostomy), drugs used, and physiological variables before and after intubation (heart rate, blood pressure, oxygen saturation, and respiratory rate) were analyzed. Whether the operator was a member of the medical staff or a resident was also recorded, along with the MACOCHA intubation difficulty scale24 and the Cormack glottis visualization difficulty scale. The length of the ICU stay, 28-day and in-hospital mortality rates were assessed. A database was created in RedCap software (Research Electronic Data Capture, Vanderbilt University, Nashville, TN, USA). The study was conducted following the international STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for observational studies.
Design: This secondary analysis primarily aims to compare intubation procedures between non-COVID-19 patients before and after the pandemic started, to assess possible pandemic-induced changes in intubation practices. Secondary endpoints include evaluating the possible impact on complications and mortality after intubation. Post-COVID status was considered from the declaration of the state of alarm in March 2020,25 although the researchers cannot guarantee that there was a sudden change in procedures from that date.
Statistical analysis: After applying the Kolmogorov-Smirnov test to quantitative variables, which did not guarantee normal distribution, the Mann-Whitney U test was used for univariate analysis, and data were expressed as median and quartiles. Categorical variables were analyzed using the chi-square test or Fisher's exact test as appropriate, and data were expressed as numbers and percentages. Two-tailed comparisons were drawn with a significance level of P<.05. A multivariate analysis was performed using stepwise logistic regression, including variables with a significance level<0.20, using the odds ratio criterion. Global validity was analyzed using Nagelkerke's R2, and the Hosmer-Lemeshow test was applied to assess goodness of fit. A variance inflation factor (VIF)<4 and a tolerance test>0.25 were considered indicators of low multicollinearity. The study was performed using the SPSS® version 22 statistical package (Chicago, IL, USA).
ResultsA total of 322 out of the 1837 patients included in the original analysis were diagnosed with COVID-19, as opposed to 1515 who were non-COVID-19 patients (1199 pre-pandemic and 316 during the pandemic). The flow diagram is detailed in Fig. S1 of the Supplementary Electronic Data (SED).
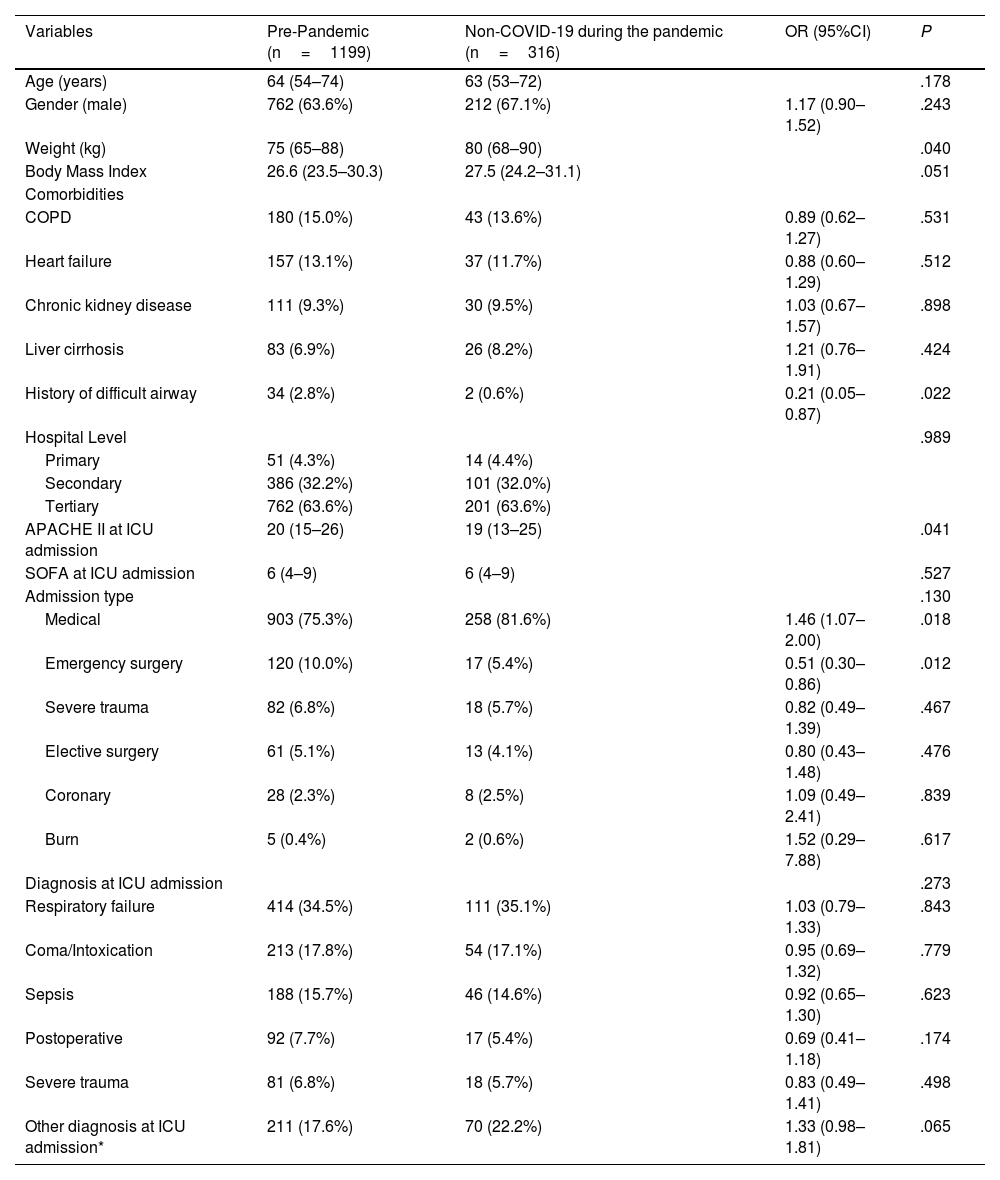
Table 1 shows the comparison of demographic characteristics, comorbidities, type of hospital, type of admission, severity scores, and diagnosis on admission. Non-COVID-19 patients during the pandemic had a higher weight, fewer difficult airway events, lower APACHE II scores on admission, a higher proportion of admissions, and fewer emergency surgical admissions.
Admission characteristics of non-COVID-19 patients pre- and during the pandemic.
| Variables | Pre-Pandemic (n=1199) | Non-COVID-19 during the pandemic (n=316) | OR (95%CI) | P |
|---|---|---|---|---|
| Age (years) | 64 (54–74) | 63 (53–72) | .178 | |
| Gender (male) | 762 (63.6%) | 212 (67.1%) | 1.17 (0.90–1.52) | .243 |
| Weight (kg) | 75 (65–88) | 80 (68–90) | .040 | |
| Body Mass Index | 26.6 (23.5–30.3) | 27.5 (24.2–31.1) | .051 | |
| Comorbidities | ||||
| COPD | 180 (15.0%) | 43 (13.6%) | 0.89 (0.62–1.27) | .531 |
| Heart failure | 157 (13.1%) | 37 (11.7%) | 0.88 (0.60–1.29) | .512 |
| Chronic kidney disease | 111 (9.3%) | 30 (9.5%) | 1.03 (0.67–1.57) | .898 |
| Liver cirrhosis | 83 (6.9%) | 26 (8.2%) | 1.21 (0.76–1.91) | .424 |
| History of difficult airway | 34 (2.8%) | 2 (0.6%) | 0.21 (0.05–0.87) | .022 |
| Hospital Level | .989 | |||
| Primary | 51 (4.3%) | 14 (4.4%) | ||
| Secondary | 386 (32.2%) | 101 (32.0%) | ||
| Tertiary | 762 (63.6%) | 201 (63.6%) | ||
| APACHE II at ICU admission | 20 (15–26) | 19 (13–25) | .041 | |
| SOFA at ICU admission | 6 (4–9) | 6 (4–9) | .527 | |
| Admission type | .130 | |||
| Medical | 903 (75.3%) | 258 (81.6%) | 1.46 (1.07–2.00) | .018 |
| Emergency surgery | 120 (10.0%) | 17 (5.4%) | 0.51 (0.30–0.86) | .012 |
| Severe trauma | 82 (6.8%) | 18 (5.7%) | 0.82 (0.49–1.39) | .467 |
| Elective surgery | 61 (5.1%) | 13 (4.1%) | 0.80 (0.43–1.48) | .476 |
| Coronary | 28 (2.3%) | 8 (2.5%) | 1.09 (0.49–2.41) | .839 |
| Burn | 5 (0.4%) | 2 (0.6%) | 1.52 (0.29–7.88) | .617 |
| Diagnosis at ICU admission | .273 | |||
| Respiratory failure | 414 (34.5%) | 111 (35.1%) | 1.03 (0.79–1.33) | .843 |
| Coma/Intoxication | 213 (17.8%) | 54 (17.1%) | 0.95 (0.69–1.32) | .779 |
| Sepsis | 188 (15.7%) | 46 (14.6%) | 0.92 (0.65–1.30) | .623 |
| Postoperative | 92 (7.7%) | 17 (5.4%) | 0.69 (0.41–1.18) | .174 |
| Severe trauma | 81 (6.8%) | 18 (5.7%) | 0.83 (0.49–1.41) | .498 |
| Other diagnosis at ICU admission* | 211 (17.6%) | 70 (22.2%) | 1.33 (0.98–1.81) | .065 |
Quantitative variables are expressed as median (25th–75th percentile); categorical variables expressed as absolute number and percentage.
APACHE: Acute Physiology, Age, Chronic Health Evaluation score; COPD: chronic obstructive pulmonary disease; SOFA: Sequential Organ Failure Assessment score; ICU: intensive care unit; OR: odds ratio; CI: confidence interval.
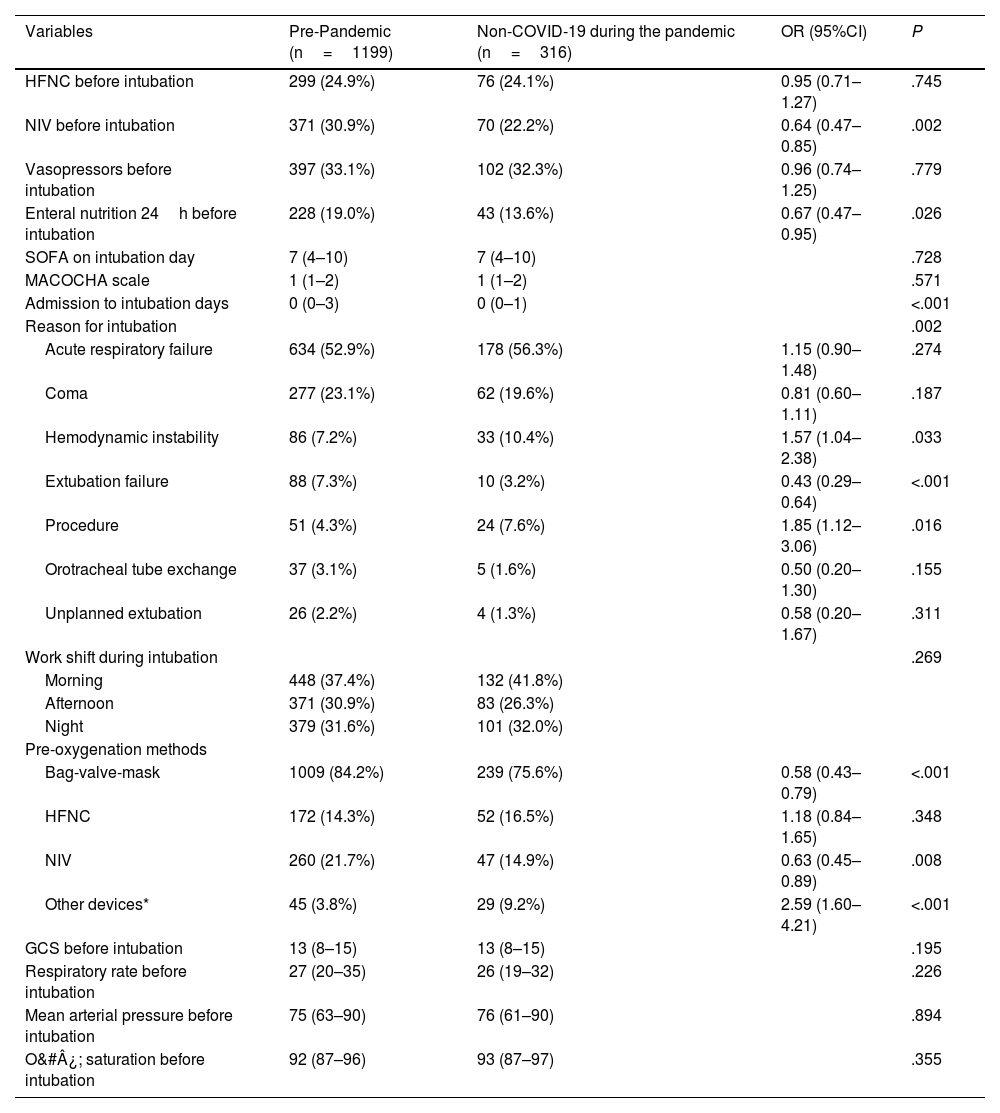
Table 2 compares the procedures prior to intubation, reasons for intubation, and pre-intubation conditions. During the pandemic, non-COVID-19 patients had less prior use of non-invasive ventilation (NIV) and enteral nutrition in the 24h prior, shorter admissions before intubation, more frequent instability and procedural reasons for intubation, and fewer failed extubation cases. Reintubation as a cause of intubation (failed extubation, or change of endotracheal tube) was significantly lower in the non-COVID-19 group during the pandemic (OR, 0.44 [95%CI, 0.27–0.72]; P=.001). There was less use of NIV and resuscitation bags for pre-oxygenation and more use of other non-HFNO devices.
Prior procedures, reason, and clinical status before intubation: comparison of non-COVID-19 patients before and during the pandemic.
| Variables | Pre-Pandemic (n=1199) | Non-COVID-19 during the pandemic (n=316) | OR (95%CI) | P |
|---|---|---|---|---|
| HFNC before intubation | 299 (24.9%) | 76 (24.1%) | 0.95 (0.71–1.27) | .745 |
| NIV before intubation | 371 (30.9%) | 70 (22.2%) | 0.64 (0.47–0.85) | .002 |
| Vasopressors before intubation | 397 (33.1%) | 102 (32.3%) | 0.96 (0.74–1.25) | .779 |
| Enteral nutrition 24h before intubation | 228 (19.0%) | 43 (13.6%) | 0.67 (0.47–0.95) | .026 |
| SOFA on intubation day | 7 (4–10) | 7 (4–10) | .728 | |
| MACOCHA scale | 1 (1–2) | 1 (1–2) | .571 | |
| Admission to intubation days | 0 (0–3) | 0 (0–1) | <.001 | |
| Reason for intubation | .002 | |||
| Acute respiratory failure | 634 (52.9%) | 178 (56.3%) | 1.15 (0.90–1.48) | .274 |
| Coma | 277 (23.1%) | 62 (19.6%) | 0.81 (0.60–1.11) | .187 |
| Hemodynamic instability | 86 (7.2%) | 33 (10.4%) | 1.57 (1.04–2.38) | .033 |
| Extubation failure | 88 (7.3%) | 10 (3.2%) | 0.43 (0.29–0.64) | <.001 |
| Procedure | 51 (4.3%) | 24 (7.6%) | 1.85 (1.12–3.06) | .016 |
| Orotracheal tube exchange | 37 (3.1%) | 5 (1.6%) | 0.50 (0.20–1.30) | .155 |
| Unplanned extubation | 26 (2.2%) | 4 (1.3%) | 0.58 (0.20–1.67) | .311 |
| Work shift during intubation | .269 | |||
| Morning | 448 (37.4%) | 132 (41.8%) | ||
| Afternoon | 371 (30.9%) | 83 (26.3%) | ||
| Night | 379 (31.6%) | 101 (32.0%) | ||
| Pre-oxygenation methods | ||||
| Bag-valve-mask | 1009 (84.2%) | 239 (75.6%) | 0.58 (0.43–0.79) | <.001 |
| HFNC | 172 (14.3%) | 52 (16.5%) | 1.18 (0.84–1.65) | .348 |
| NIV | 260 (21.7%) | 47 (14.9%) | 0.63 (0.45–0.89) | .008 |
| Other devices* | 45 (3.8%) | 29 (9.2%) | 2.59 (1.60–4.21) | <.001 |
| GCS before intubation | 13 (8–15) | 13 (8–15) | .195 | |
| Respiratory rate before intubation | 27 (20–35) | 26 (19–32) | .226 | |
| Mean arterial pressure before intubation | 75 (63–90) | 76 (61–90) | .894 | |
| O&#¿; saturation before intubation | 92 (87–96) | 93 (87–97) | .355 | |
Quantitative variables are expressed as median (25th–75th percentile); categorical variables as absolute number and percentage. GCS: Glasgow Coma Scale; MACOCHA: Risk scale for difficult intubation; HFNC: High-flow nasal cannula; SOFA: Sequential Organ Failure Assessment score; NIV: non-invasive ventilation; OR: odds ratio; CI: confidence interval.
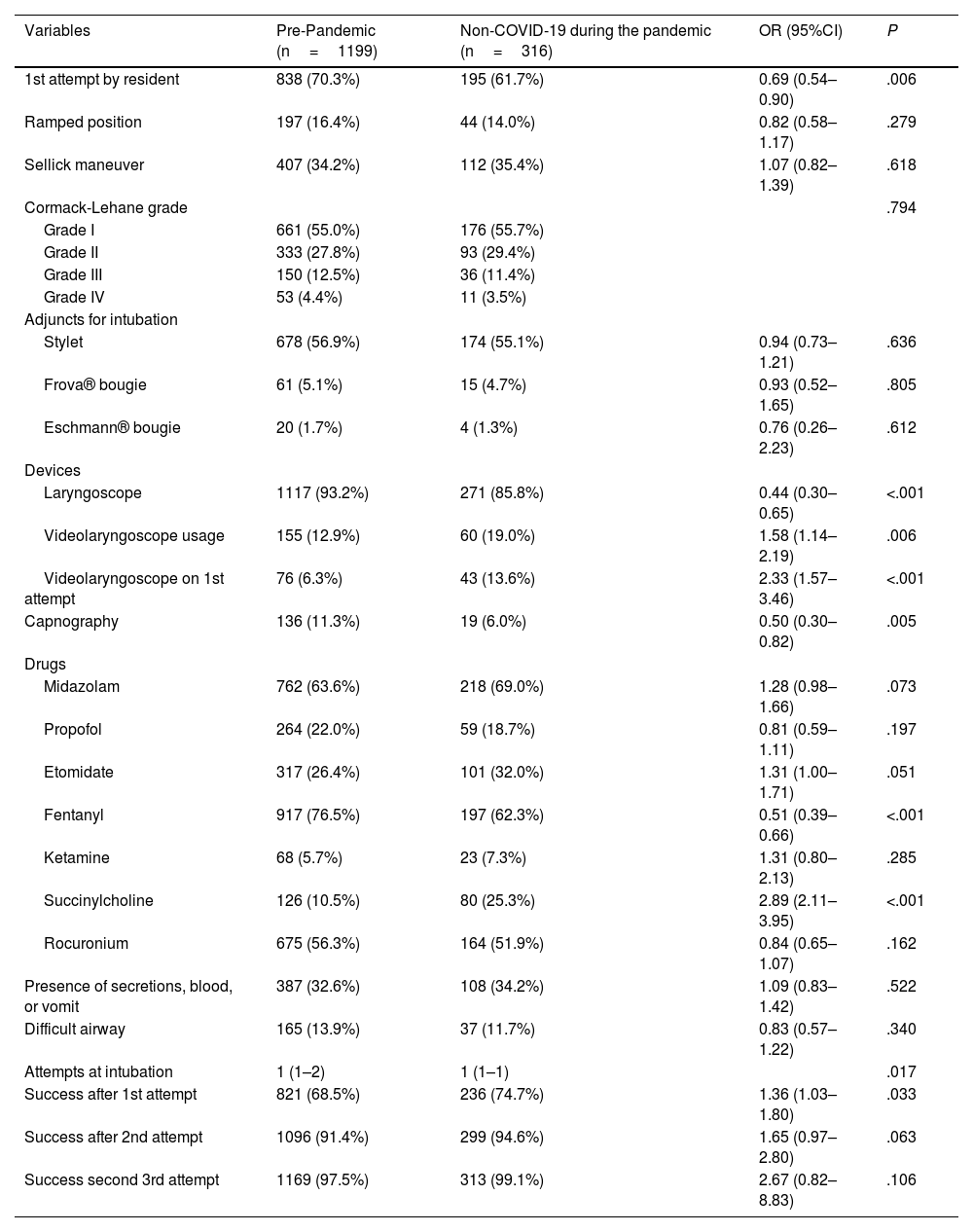
Table 3 compares the devices, maneuvers, and drugs used in the intubation procedure, as well as the findings, number of attempts, and operator performing the intubation. Notably, non-COVID-19 patients during the pandemic had a lower frequency of residents as the first operator, lower use of the laryngoscope, and greater use of VL both overall and at first attempt, with less use of capnography, achieving a higher first-pass intubation success rate. The use of capnography in non-COVID-19 patients before (OR 2.31 [95%CI, 1.29–4.15]) and during the pandemic (OR 3.95 [95%CI, 1.56–9.98]) was higher when VL was used. There was also greater use of neuromuscular blocking agents, especially succinylcholine, and less use of fentanyl. Fig. S2 shows the changes in drug usage between pre-pandemic patients, COVID-19 patients, and non-COVID-19 patients during the pandemic.
Devices, maneuvers, drugs used during intubation, and findings: non-COVID-19 patients before and during the pandemic.
| Variables | Pre-Pandemic (n=1199) | Non-COVID-19 during the pandemic (n=316) | OR (95%CI) | P |
|---|---|---|---|---|
| 1st attempt by resident | 838 (70.3%) | 195 (61.7%) | 0.69 (0.54–0.90) | .006 |
| Ramped position | 197 (16.4%) | 44 (14.0%) | 0.82 (0.58–1.17) | .279 |
| Sellick maneuver | 407 (34.2%) | 112 (35.4%) | 1.07 (0.82–1.39) | .618 |
| Cormack-Lehane grade | .794 | |||
| Grade I | 661 (55.0%) | 176 (55.7%) | ||
| Grade II | 333 (27.8%) | 93 (29.4%) | ||
| Grade III | 150 (12.5%) | 36 (11.4%) | ||
| Grade IV | 53 (4.4%) | 11 (3.5%) | ||
| Adjuncts for intubation | ||||
| Stylet | 678 (56.9%) | 174 (55.1%) | 0.94 (0.73–1.21) | .636 |
| Frova® bougie | 61 (5.1%) | 15 (4.7%) | 0.93 (0.52–1.65) | .805 |
| Eschmann® bougie | 20 (1.7%) | 4 (1.3%) | 0.76 (0.26–2.23) | .612 |
| Devices | ||||
| Laryngoscope | 1117 (93.2%) | 271 (85.8%) | 0.44 (0.30–0.65) | <.001 |
| Videolaryngoscope usage | 155 (12.9%) | 60 (19.0%) | 1.58 (1.14–2.19) | .006 |
| Videolaryngoscope on 1st attempt | 76 (6.3%) | 43 (13.6%) | 2.33 (1.57–3.46) | <.001 |
| Capnography | 136 (11.3%) | 19 (6.0%) | 0.50 (0.30–0.82) | .005 |
| Drugs | ||||
| Midazolam | 762 (63.6%) | 218 (69.0%) | 1.28 (0.98–1.66) | .073 |
| Propofol | 264 (22.0%) | 59 (18.7%) | 0.81 (0.59–1.11) | .197 |
| Etomidate | 317 (26.4%) | 101 (32.0%) | 1.31 (1.00–1.71) | .051 |
| Fentanyl | 917 (76.5%) | 197 (62.3%) | 0.51 (0.39–0.66) | <.001 |
| Ketamine | 68 (5.7%) | 23 (7.3%) | 1.31 (0.80–2.13) | .285 |
| Succinylcholine | 126 (10.5%) | 80 (25.3%) | 2.89 (2.11–3.95) | <.001 |
| Rocuronium | 675 (56.3%) | 164 (51.9%) | 0.84 (0.65–1.07) | .162 |
| Presence of secretions, blood, or vomit | 387 (32.6%) | 108 (34.2%) | 1.09 (0.83–1.42) | .522 |
| Difficult airway | 165 (13.9%) | 37 (11.7%) | 0.83 (0.57–1.22) | .340 |
| Attempts at intubation | 1 (1–2) | 1 (1–1) | .017 | |
| Success after 1st attempt | 821 (68.5%) | 236 (74.7%) | 1.36 (1.03–1.80) | .033 |
| Success after 2nd attempt | 1096 (91.4%) | 299 (94.6%) | 1.65 (0.97–2.80) | .063 |
| Success second 3rd attempt | 1169 (97.5%) | 313 (99.1%) | 2.67 (0.82–8.83) | .106 |
Quantitative variables are expressed as median (25th–75th percentile); categorical variables as absolute number and percentage. OR: odds ratio; CI: confidence interval.
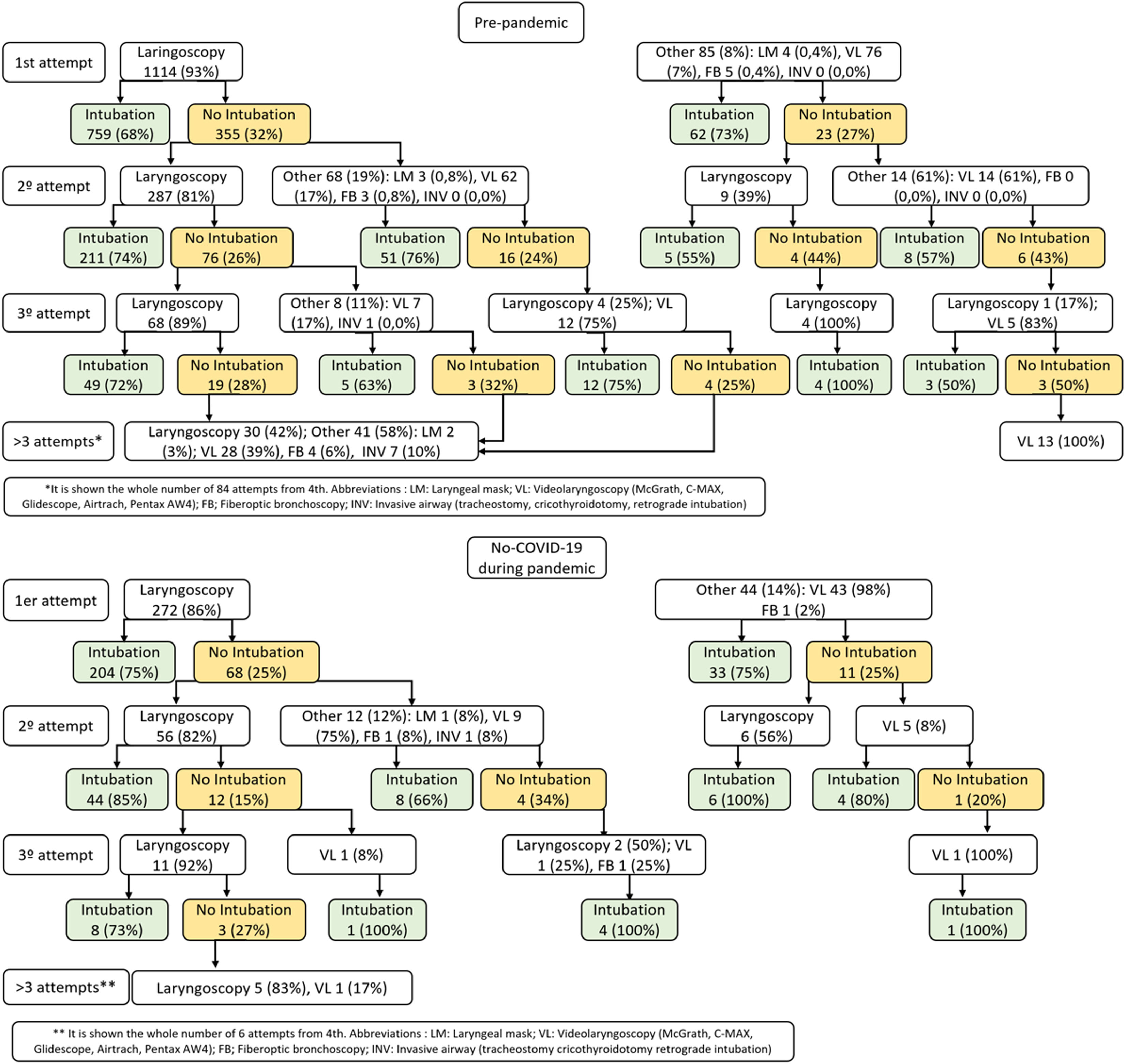
Fig. 1 shows the differences in intubation strategies by attempts between the pre-pandemic and pandemic phases for non-COVID-19 patients, highlighting the greater use of VL at first attempt during the pandemic. The intubation success rate with laryngoscope improved during the pandemic, both at the first attempt (75% vs 68%; P=.029) and in the cumulative first 3 attempts (76% vs 69%; P<.001) [Fig. S3].
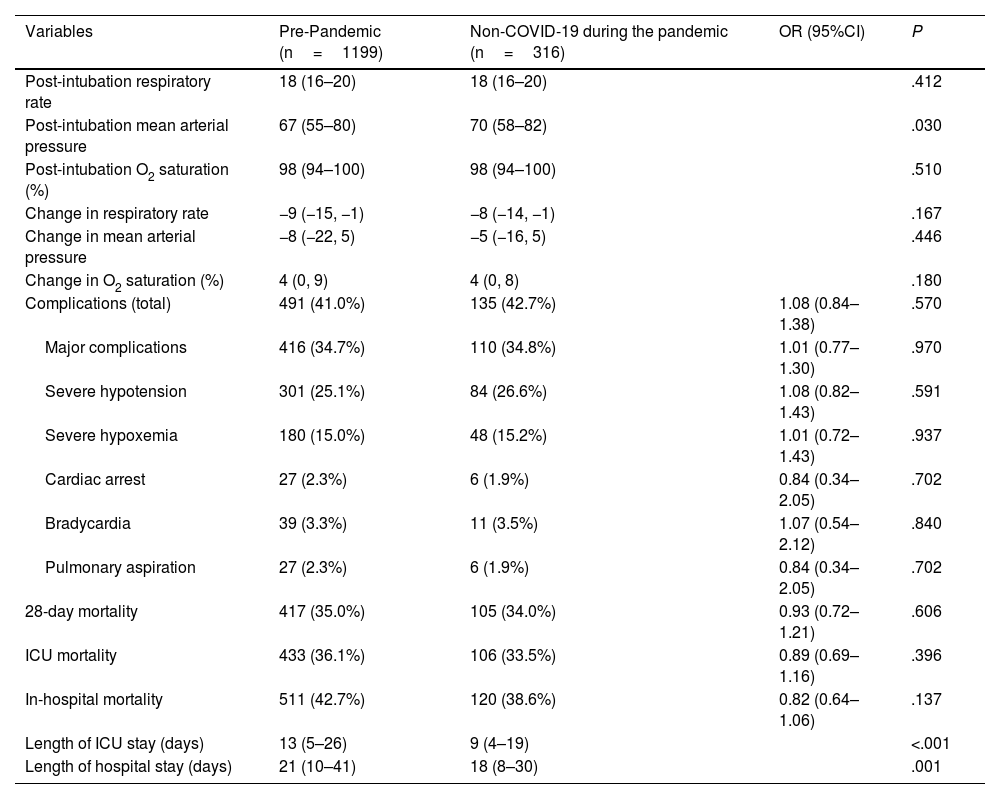
Table 4 compares the post-intubation vital signs, immediate complications, and 28-day mortality rate at the ICU and hospital settings. A significantly higher mean blood pressure was detected after intubation in non-COVID-19 patients during the pandemic, with no other differences being reported in the remaining variables. Moreover, the length of ICU and hospital stays was significantly shorter during the pandemic.
Post-intubation vital signs, complications, and mortality: non-COVID-19 Patients before and during the pandemic.
| Variables | Pre-Pandemic (n=1199) | Non-COVID-19 during the pandemic (n=316) | OR (95%CI) | P |
|---|---|---|---|---|
| Post-intubation respiratory rate | 18 (16–20) | 18 (16–20) | .412 | |
| Post-intubation mean arterial pressure | 67 (55–80) | 70 (58–82) | .030 | |
| Post-intubation O2 saturation (%) | 98 (94–100) | 98 (94–100) | .510 | |
| Change in respiratory rate | −9 (−15, −1) | −8 (−14, −1) | .167 | |
| Change in mean arterial pressure | −8 (−22, 5) | −5 (−16, 5) | .446 | |
| Change in O2 saturation (%) | 4 (0, 9) | 4 (0, 8) | .180 | |
| Complications (total) | 491 (41.0%) | 135 (42.7%) | 1.08 (0.84–1.38) | .570 |
| Major complications | 416 (34.7%) | 110 (34.8%) | 1.01 (0.77–1.30) | .970 |
| Severe hypotension | 301 (25.1%) | 84 (26.6%) | 1.08 (0.82–1.43) | .591 |
| Severe hypoxemia | 180 (15.0%) | 48 (15.2%) | 1.01 (0.72–1.43) | .937 |
| Cardiac arrest | 27 (2.3%) | 6 (1.9%) | 0.84 (0.34–2.05) | .702 |
| Bradycardia | 39 (3.3%) | 11 (3.5%) | 1.07 (0.54–2.12) | .840 |
| Pulmonary aspiration | 27 (2.3%) | 6 (1.9%) | 0.84 (0.34–2.05) | .702 |
| 28-day mortality | 417 (35.0%) | 105 (34.0%) | 0.93 (0.72–1.21) | .606 |
| ICU mortality | 433 (36.1%) | 106 (33.5%) | 0.89 (0.69–1.16) | .396 |
| In-hospital mortality | 511 (42.7%) | 120 (38.6%) | 0.82 (0.64–1.06) | .137 |
| Length of ICU stay (days) | 13 (5–26) | 9 (4–19) | <.001 | |
| Length of hospital stay (days) | 21 (10–41) | 18 (8–30) | .001 |
Quantitative variables are expressed as median (25th–75th percentile); categorical variables as absolute values and percentage. OR: odds ratio; CI: confidence interval.
In the multivariate analysis, several variables were independently associated with intubation in non-COVID-19 patients after the pandemic (Fig. 2). Notably, the lengths of stay were shorte before intubation; a different method of pre-oxygenation with less use of resuscitation bags and NIV, and more use of other types of devices; drug changes with more use of midazolam, etomidate, and succinylcholine, and less fentanyl; and more use of VL initially, but less capnography.
DiscussionThis study shows that the COVID-19 pandemic modified intubation procedures for both COVID-19 and non-COVID-19 critically ill patients, changing pre-oxygenation strategies, drug usage, and the use of VL, at least, during the period when COVID-19 patients coexisted.
The recommendations made for intubation of critically ill COVID-19 patients at the start of the pandemic led to a change in intubation procedures.26,27 These recommendations aimed to address the severity of critical hypoxemia in these patients while trying to minimize the professional risk associated with it. In the early phases of the pandemic, diagnosis was not as fast as it later became, so many patients were intubated without having been able to rule out SARS-CoV-2 infection.28
Non-COVID-19 patients during the pandemic had higher weight than before, and although changes in lifestyle habits in Spain during the pandemic were documented,29 this is most likely a random finding. The slightly lower APACHE II score and the shift in the patient profile with more medical admissions and fewer emergency surgical procedures could have to do with organizational changes in Spanish ICUs,28 which found themselves having to handle a surge of COVID-19 patients and the involvement of other facilities for the management of non-COVID-19 critically ill patients. However, diagnoses upon admission were similar, and distribution across hospital levels was the also the same.
The reasons for intubation varied slightly, though in both periods, 75% were for respiratory failure and coma. The higher proportion of procedures and hemodynamic instability may be related to reduced accessibility during the pandemic, and the lower rate of reintubations may be due to nursing staff ratios and physician workload, which could have delayed or prevented timelier extubations.
In our study, there was greater use of VL in non-COVID-19 patients during the pandemic, which, temporarily, was associated with a greater use in COVID-19 patients,2,3,9,14–16,19,30–36 with a high first-pass intubation success rate. Our interpretation is that the increased availability of the resource, training in its use, pressure on health care workers, and the positive outcomes influenced this higher utilization rate. Additionally, in many cases, patients were intubated before confirming SARS-CoV-2 infection.
In contrast, we found a significant reduction in the use of capnography, which was already low in the overall INTUPROS study population,3 and even further reduced in non-COVID-19 patients intubated after the onset of the pandemic, contrasting with the results of other studies.1,36 This may be related to low prior adherence rates, but not with the increased use of VL, as capnography was more widely used in those patients despite direct visualization of intubation. Furthermore, the study excluded patients who were intubated for cardiorespiratory arrest, in which to the use capnography is recommended beyond confirming the proper placement of the endotracheal tube.37
The pre-oxygenation strategy was a point of controversy during the COVID-19 pandemic due to recommendations issued to protect healthcare workers from aerosol exposure, especially during intubation.38 This led to recommendations to avoid HFNO, NIV, and manual ventilation. This resulted in the use of systems generating fewer aerosols, such as non-rebreather masks or oropharyngeal cannulas. The coexistence of COVID-19 and non-COVID-19 patients in the units, delayed SARS-COV-2 diagnosis, and fear among healthcare workers in the early months may have led to changes in pre-oxygenation for intubations in non-SARS-COV-2-infected patients. This also justifies the finding of shorter time to intubation.
The multivariate analysis showed greater use of midazolam and etomidate in non-COVID-19 patients during the pandemic, with less use of fentanyl. The medication guidelines recommended for COVID-19 patients may have increased the use of midazolam and neuromuscular blocking agents,3 but the lower use of fentanyl and the higher use of etomidate in non-COVID-19 patients do not seem to have an explanation based on the analyzed data. Unlike other studies,7 no increased hypotension or higher in-hospital mortality rates were detected in patients on etomidate, whether COVID-19 or non-COVID-19, during any period.
The higher rate of first-pass intubation during the pandemic can be attributed to a greater use of VL and neuromuscular blocking agents, although it was also better with the laryngoscope, possibly due to a lower rate of first attempts by residents, who have fewer acquired skills.39 Of note, the complication and mortality rates were similar across both periods, which may be surprising given the higher first-pass intubation rate. Nonetheless, several studies have shown equally high rates of major complications in critically ill patients,1,40 even with higher first-pass intubation success rates than in our series. Reducing complications in intubation of critically ill patients remains challenging and likely cannot be solely addressed by improving first-pass intubation rates. It should be combined with appropriate timing of intubation, oxygenation devices, pharmacological measures, better use of capnography, etc.
It is also important to note that during the pandemic, there was limited access to certain drugs and medical supplies, as well as differences in resource management, which was heterogeneously distributed across the centers, possibly affecting the observed differences.
The study has several limitations. It is a secondary study that does not answer an original research question. The period division is not exact, and as the study was conducted in 6-month cycles, one or both periods could have been included in each hospital. Furthermore, the sample size of non-COVID-19 patients during the pandemic is limited, and the heterogeneity in the case mix of patients and units may hide uncontrollable biases. Additionally, although all units were required to have intubation protocols, these were not standardized. On the other hand, the prospective and multicenter design of the study, which preceded and coincided with the onset of the pandemic, strengthens the data obtained.
ConclusionsCOVID-19 pandemic-driven procedures influenced the way non-COVID-19 critically ill patients were intubated, altering the pre-oxygenation strategy, drug used, and the utilization of videolaryngoscopy, at least, during the period when COVID-19 patients were coexisting. However, this did not result in changes in complications or mortality.
CRediT authorship contribution statementJLGG: original idea, design, analysis, writing; JTA: original idea, design, database, study monitoring, writing; FGV: original idea, design, writing; EGE: design, data collection, study monitoring, manuscript review; EMB: data collection, manuscript review; FOC: data collection, manuscript review; VSM: data collection, manuscript review; ERR: data collection, manuscript review; RABA: data collection, manuscript review; JMQ: data collection, manuscript review; MIRG: data collection, manuscript review; JGM: original idea, design, study monitoring, writing.
Informed consentThe original study was conducted after the approval of Virgen Macarena and Virgen del Rocío Hospitals Ethics Committee in Seville (Spain) which was later ratified in the various hospitals. The obtaining of informed consent was deemed unnecessary.
Declaration of Generative AI and AI-assisted technologies in the writing processNo artificial intelligence tools have been used in the generation of figures or in the creation or refinement of the text.
FundingWe received funding to work on this document.
Ana Abella-Álvarez (Hospital del Henares, Coslada, Madrid); José Manuel-Allegue (Complejo Hospitalario Universitario de Cartagena, Murcia); Rosario Amaya-Villar (Hospital Universitario Virgen del Rocío [UCI neuro-traumatológica], Sevilla); Borja Antolino-Jiménez (Hospital Parc Taulí, Sabadell); Herbert Baquerizo-Vargas (Consorci Sanitari de l'Anoia - Hospital de Igualada)Alberto Belenguer-Muncharaz (Hospital Doctor Peset, Valencia); María Dolores Bosque-Cebolla (Hospital Universitario General de Cataluña, Barcelona); Amparo Cabanillas-Carrillo (Hospital Universitario del Sureste, Arganda del Rey, Madrid); Álvaro Castellanos-Ortega (Hospital Universitario y Politécnico La Fe, Valencia); Laura Claverías-Cabrera (Hospital Joan XXIII de Tarragona); Yolanda Díaz-Buendía (Hospital Parc Salut del Mar, Barcelona); Domingo Díaz-Díaz (Hospital Infanta Leonor, Madrid); Olga Díaz-Martín (Complejo Asistencial Universitario de Salamanca); Pedro Enríquez-Giraudo (Hospital Rio Hortega, Valladolid); Ángel Estella-García (Hospital de Jerez de la Frontera); Lorena Fernández-Rodríguez (Hospital Rio Hortega, Valladolid); Lourdes Fisac-Cuadrado (Hospital Universitario de Burgos); Carmen de la Fuente-Martos (Hospital Reina Sofía de Córdoba); Emilio García-Prieto (Hospital Universitario Central de Asturias, Oviedo); Alejandro González-Castro (Hospital Marqués de Valdecilla, Santander); Natalia Gordo-Herrera (Escuela Politécnica, Universidad Francisco de Vitoria, Pozuelo de Alarcón-Madrid); María Herreros-Gonzalo (Hospital General de la Mancha Centro, Alcázar de San Juan); José Carlos Igeño-Cano (Hospital San Juan de Dios, Córdoba); Juan Ramón Jiménez-del Valle (Hospital Virgen Macarena, Sevilla); Joao Antonio Lameirao-Gaspar (Hospital Universitario Virgen del Rocío [UCI neuro-traumatológica], Sevilla); Cristina López-Martin (Hospital Reina Sofía de Córdoba); José María López-Sánchez (Hospital Universitario Virgen del Rocío [UCI general], Sevilla); Leire López de la Oliva-Calvo (Hospital del Henares, Coslada, Madrid); Mónica Magret-Iglesias (Hospital Joan XXIII de Tarragona); Sara Manrique-Moreno (Hospital Joan XXIII de Tarragona); Diego Manzano-Moratinos (Hospital Universitario de Getafe); Antoni Margarit-Ribas (Hospital Nostra Senyora de Meritxell, Andorra); Joan Ramón Masclans-Enviz (Hospital Parc Salut del Mar, Barcelona); Iván Matáix-Ponce (Hospital Universitario Virgen del Rocío [UCI general], Sevilla); Alejandro Moneo-González (Hospital 12 de Octubre, Madrid); Diana Monge-Donaire (Complejo Asistencial de Zamora); Olga Moreno-Romero (Hospital Virgen de las Nieves de Granada); Alicia Muñoz-Cantero (Hospital Universitario de Bajadoz); Camilo Nariño-Molano (Hospital Universitario de Burgos); Juan Carlos Montejo-González (Hospital 12 de Octubre, Madrid); Águeda Ojados-Muñoz (Complejo Hospitalario Universitario de Cartagena, Murcia); César Palazón-Sánchez (Hospital Reina Sofía Murcia); Carla Palencia-Amador (Hospital Mutua Tarrasa, Barcelona); Eduardo Palencia-Herrejón (Hospital Infanta Leonor, Madrid); Oscar Peñuelas-Rodríguez (Hospital Universitario de Getafe); Demetrio Pérez-Civantos (Hospital Universitario de Bajadoz); José María Pérez-Villares (Hospital Virgen de las Nieves de Granada); Andrea Ortiz-Suñer (Hospital Comarcal de Vinaroz); Jaume Revuelto Rey (Hospital Puerta del Mar, Cádiz); Gemma Rialp Cervera (Hospital Universitari Son Llatzer, Baleares); Ricardo Rivera-Fernández (Complejo Hospitalario de Jaén); María Isabel Rodríguez-Higueras (Hospital Universitario Torrecárdenas, Almería); Miriam Rodríguez-Romo (Hospital Gómez Ulla, Madrid); Diego Rodríguez-Serrano (Hospital Príncipe de Asturias, Alcalá de Henares, Madrid); Olga Rufo-Tejeiro (Hospital San Juan de Dios del Aljarafe, Sevilla); María Isabel Ruiz-García (Complejo Hospitalario de Jaén);Jesús Sánchez-Ballesteros (Hospital Rio Hortega, Valladolid); Francisco Miguel Sánchez-Silos (Hospital San Juan de Dios, Córdoba); Lorenzo Socias-Crespí (Hospital Universitari Son Llatzer, Baleares); Roser Tomás-Puig (Hospital Universitario General de Cataluña, Barcelona); María Luz Urendes-Cáceres (Hospital Mutua Tarrasa, Barcelona); Nuria Rodríguez-Farré (Hospital de la Santa Creu i Sant Pau); Carlos Vicent-Perales (Hospital Universitario y Politécnico La Fe, Valencia); and Rafael Zaragoza-Crespo (Hospital Doctor Peset, Valencia).
A list of the researchers participating in the INTUPROS Group is provided in the Appendix A.