To analyze our experience with extracorporeal membrane oxygenation (ECMO) therapy for acute respiratory distress syndrome (ARDS) treatment during the COVID-19 pandemic.
DesignRetrospective, observational, single center study.
SettingThird-level hospital in Spain.
PatientsAdult patients with COVID-19 ARDS treated with an ECMO system in our center between March 2020 and March 2023.
InterventionsRetrospective collection of variables during hospital admission and follow-up.
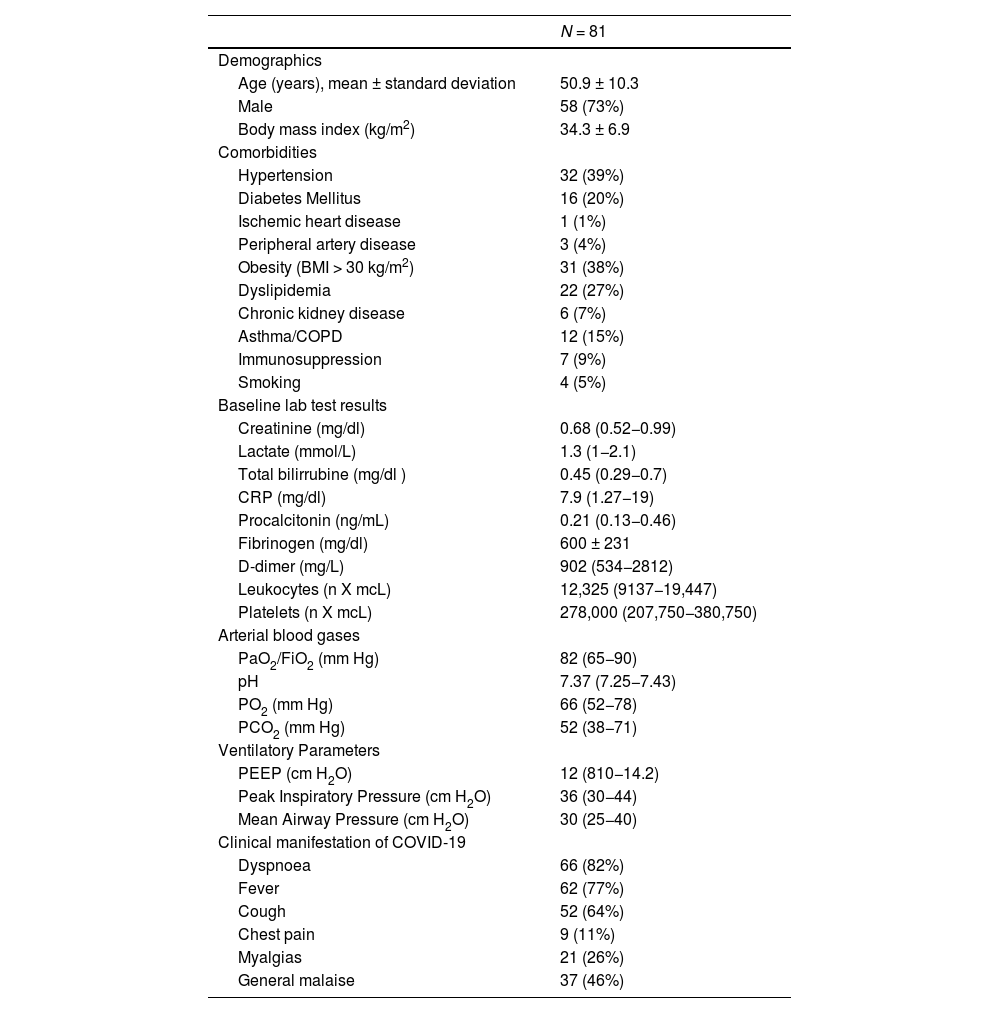
Main Variables of InterestDemographic variables, clinical history, variables related to ECMO therapy, COVID-19 wave number, in-hospital mortality, adverse events, ICU and hospital length of stay, and functional status at follow-up were collected.
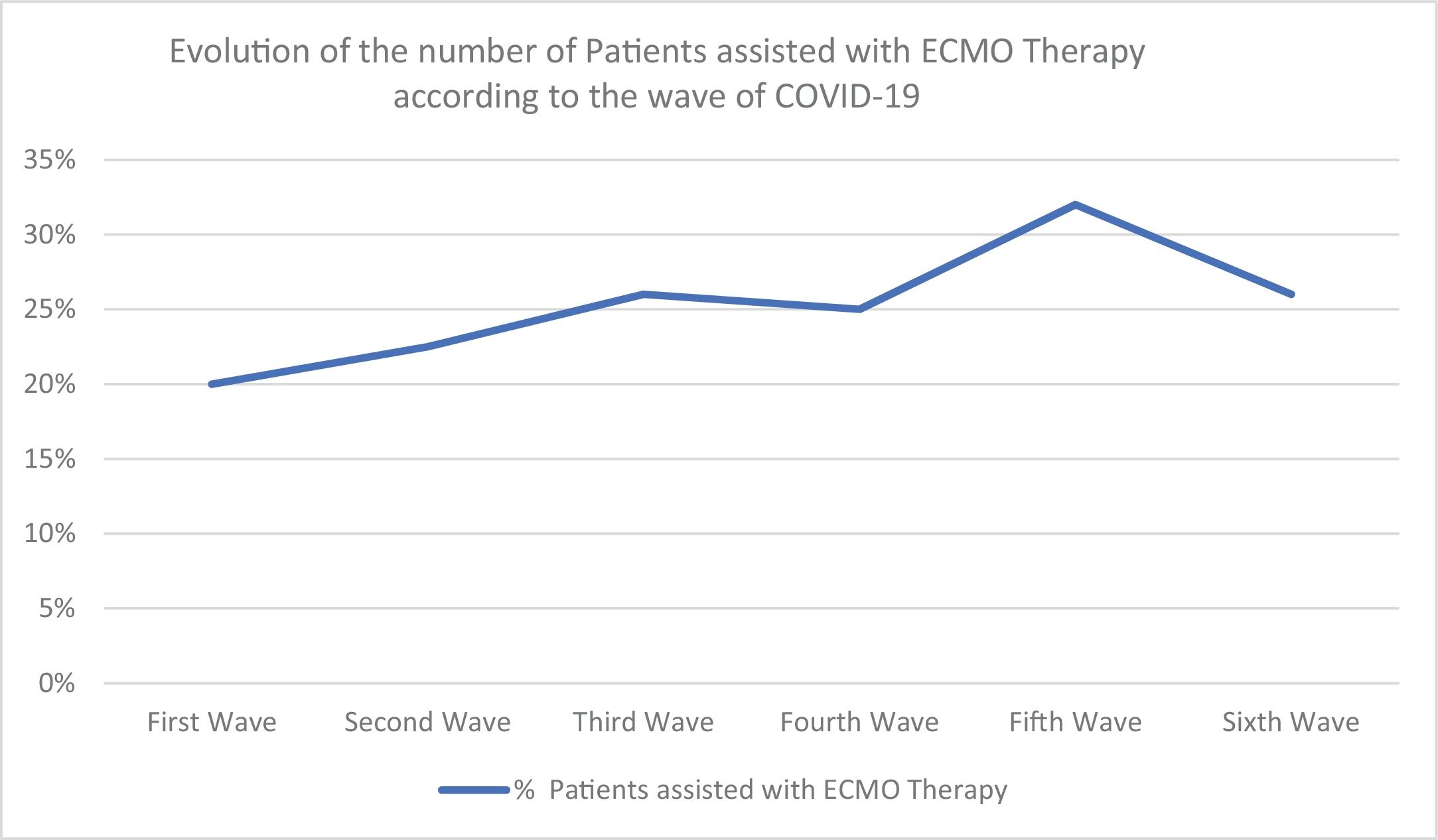
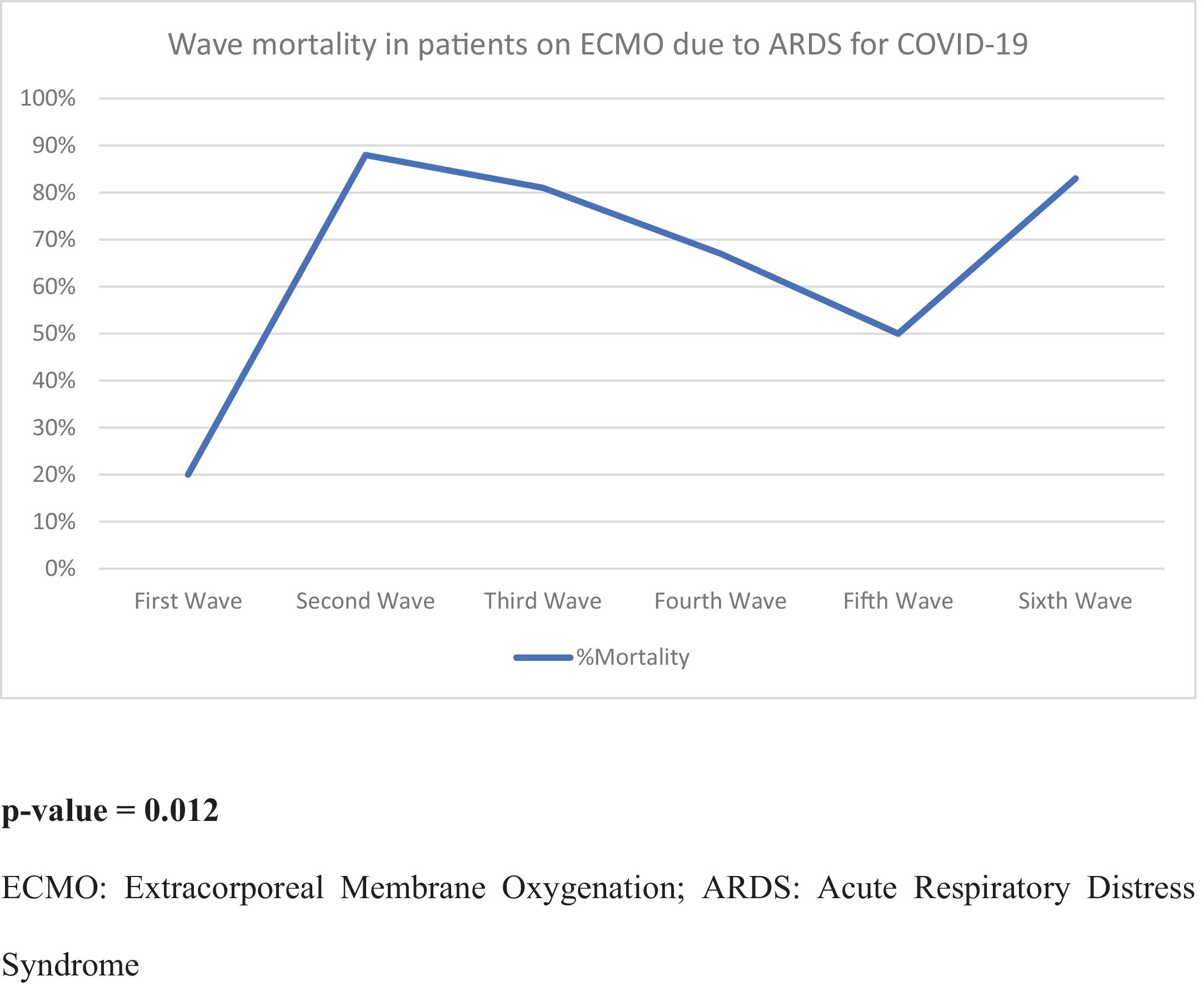
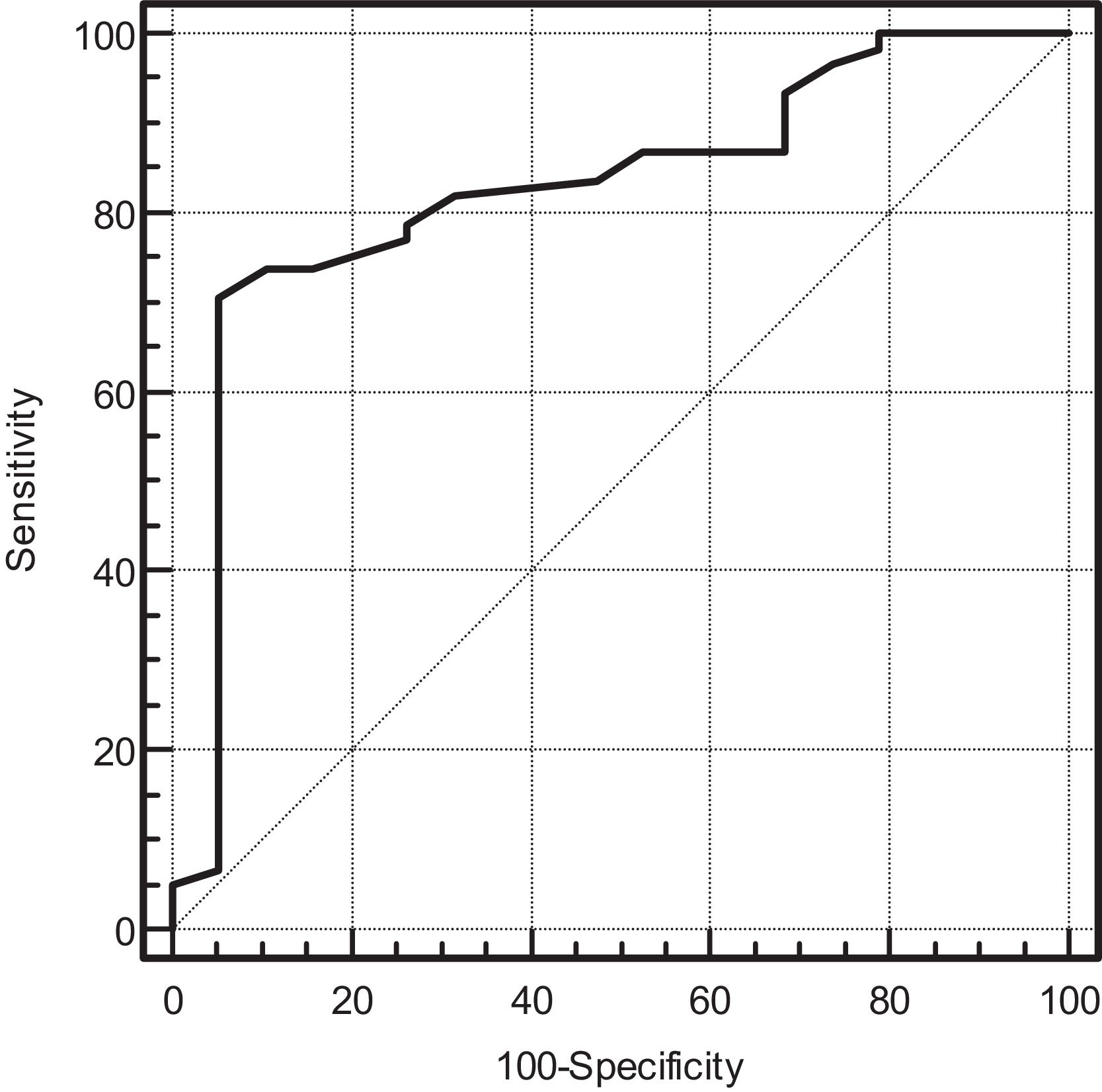
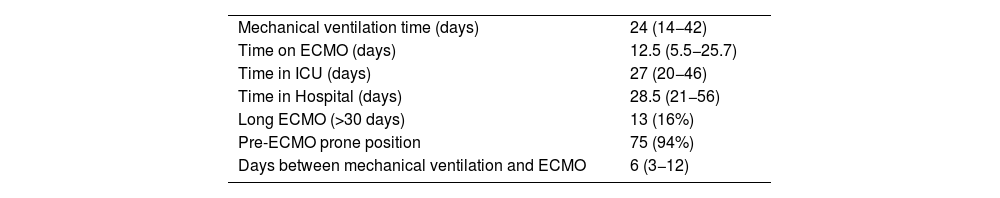
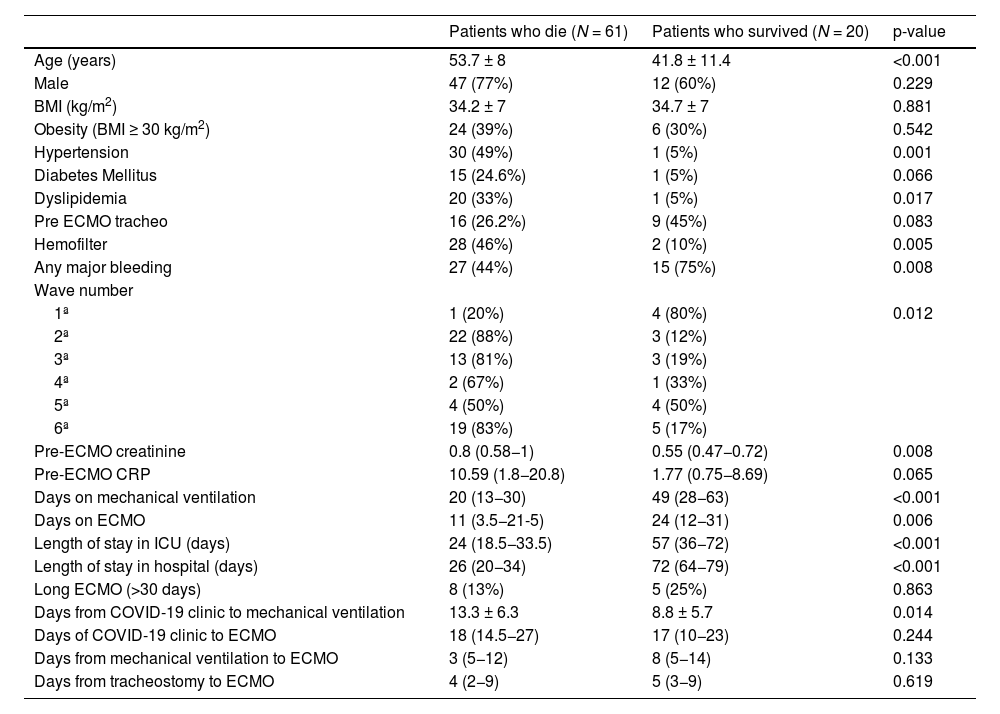
ResultsEighty-one patients were included. Of these, 61 patients (75%) died during hospitalization. Patients who died were older and had more comorbidities. During the second, third, and sixth waves, mortality was higher. In the multivariate analysis, the only independent predictor of mortality was age (OR 1.24 95% CI (1.027–1.5, P = 0.025). After discharge, 40% of patients had difficulties returning to normal life due to respiratory failure requiring oxygen and arthropathies.
ConclusionIn-hospital mortality increased during the pandemic. Older age was the only independent predictor of mortality. After discharge, no deaths were recorded during the first 18 months of follow-up, although 40% of surviving patients had respiratory and motor sequelae making it difficult for them to return to a normal life.
Analizar nuestra experiencia con la terapia de soporte con membrana de oxigenación extracorpórea (ECMO) para tratar el síndrome de distrés respiratorio agudo (SDRA) durante la pandemia de COVID-19.
DiseñoEstudio retrospectivo y observacional de un único centro.
ÁmbitoHospital de tercer nivel en España.
PacientesPacientes adultos con COVID-19 tratados con sistema ECMO por SDRA en nuestro centro entre Marzo 2020 y Marzo 2023.
IntervencionesRecogida retrospectiva de variables durante el ingreso y el seguimiento.
Variables de interés principalesSe recogieron variables demográficas, antecedentes clínicos, variables relacionadas con la terapia ECMO, numero de ola de COVID-19, mortalidad hospitalaria, eventos adversos, duración de estancia en UCI y en hospital y estado funcional en el seguimiento.
ResultadosSe incluyeron 81 pacientes. De ellos 61 pacientes (75%) fallecieron durante la hospitalización. Los pacientes que fallecieron tenían más edad y más comorbilidad. Durante la segunda, tercera y sexta olas la mortalidad fue mayor. En el análisis multivariante, el único predictor independiente de mortalidad fue la edad (OR 1.24 IC 95% (1.027–1.5, P = 0.025). Después del alta, el 40% de los pacientes presentaban dificultades para retornar a una vida normal por insuficiencia respiratoria que precisaba de oxígeno y artropatías.
ConclusiónLa mortalidad hospitalaria aumentó durante la pandemia. Tener mayor edad fue el único predictor independiente de mortalidad. Tras el alta, ningún paciente falleció durante los primeros 18 meses de seguimiento, aunque el 40% de los pacientes supervivientes presentan secuelas respiratorias y motoras que dificultan su regreso a una vida normal.
Article
Go to the members area of the website of the SEMICYUC (www.semicyuc.org )and click the link to the magazine.