The prolonged administration of sedatives and opiates (SED-OPI)—especially in patients ventilated due to SARS-CoV-2-related ARDS—in whom elevated requirements for these drugs have been reported1—is often associated with phenomena of tolerance and dependency. Deprivation syndrome can occur when reducing the dose blocking or delaying the process of weaning from mechanical ventilation (MV).2,3
The concept of «difficult sedation» (DS) includes these problems and different management strategies have been proposed such as sequential sedation with SED-OPI rotation or the administration of alpha2-agonists and/or antipsychotics.4,5 These strategies rarely contemplate the administration of methadone, possibly because, unlike it happens in the pediatric population, this drug is not very much used in adult critically ill patients.6,7 Methadone is a long semi-life opiate available in solution for its enteral administration with high bioavailability that has the capacity of blocking the NMDA receptors whose activation is highly involved in the development of tolerance, and hyperalgesia.8,9
The objective of this retrospective, observational, and cohort study is to describe the experience gained with the use of methadone to control DS in ventilated patients due to COVID-19-related ARDS in whom the use of common drugs has failed and admitted between March 2020 and May 2020. Approval from the hospital clinical research ethics committee was obtained to review the patients’ health records and analyze those patients who received methadone for, at least, 48 h to control DS. DS was considered as the impossibility to reduce the dose of SED-OPI to start weaning from MV or RASS scores > 1 despite high doses of SED-OPI4 and/or the presence of uncontrolled pain with the usual opiates.
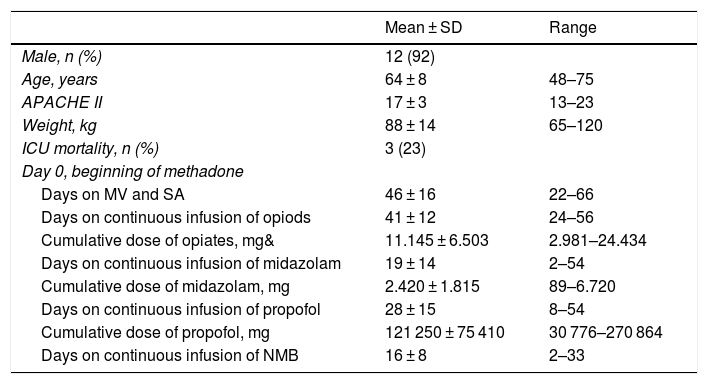
Information from demographic and clinical data, duration, and cumulative dose of SED-OPI at the beginning of treatment and 5 days before and 5 days after starting treatment was obtained (Table 1). The dose of opiates is expressed in mg of IV morphine being the equianalgesic dose ratio as follows: 100 μg of fentanyl = 100 μg of remifentanil = 10 mg of IV morphine. Registries from the nurse records of the RASS scales, the numeric visual scale or ESCID, and the side effects due to methadone were analyzed. Methadone was administered as an enteral solution at 1% and prepared by the hospital pharmacy unit. The decision to use methadone was made by the treating physician and once the routine therapeutic strategy had failed.
Demographic and clinical characteristics of patients. Duration and cumulative dose of sedatives-opiates and neuromuscular blockers at the beginning of treatment with methadone.
| Mean ± SD | Range | |
|---|---|---|
| Male, n (%) | 12 (92) | |
| Age, years | 64 ± 8 | 48–75 |
| APACHE II | 17 ± 3 | 13–23 |
| Weight, kg | 88 ± 14 | 65–120 |
| ICU mortality, n (%) | 3 (23) | |
| Day 0, beginning of methadone | ||
| Days on MV and SA | 46 ± 16 | 22–66 |
| Days on continuous infusion of opiods | 41 ± 12 | 24–56 |
| Cumulative dose of opiates, mg& | 11.145 ± 6.503 | 2.981–24.434 |
| Days on continuous infusion of midazolam | 19 ± 14 | 2–54 |
| Cumulative dose of midazolam, mg | 2.420 ± 1.815 | 89–6.720 |
| Days on continuous infusion of propofol | 28 ± 15 | 8–54 |
| Cumulative dose of propofol, mg | 121 250 ± 75 410 | 30 776–270 864 |
| Days on continuous infusion of NMB | 16 ± 8 | 2–33 |
&, dose in mg of IV morphine; ICU, intensive care unit; MV, mechanical ventilation; NMB, neuromuscular blockers; SA, sedatives, and analgesics; SD, standard deviation.
Qualitative variables were analyzed using the X2 test or Fisher’s exact test. Quantitative variables were analyzed using the Mann–Whitney U test.
During the study period, a total of 92 patients required MV due to SARS-CoV-2-related ARDS. Fourteen patients received, at least, 1 dose of methadone, 13 of whom met the study criteria. Methadone was started after 46 ± 16 days on MV, and the administration of SED-OPI with a range from 21 to 66 days. The early daily dose was 45 ± 23 mg distributed in 2–3 doses; then it was adjusted between 0.1 mg/kg and 0.4 mg/kg every 8 or 12 h depending on each patient’s response.
Methadone was started in 9 patients due to the impossibility of reducing the dose of SED-OPI to start the process of weaning from MV or due to the presence of RASS scores > 1 despite high doses of SED-OPI. In addition to propofol, 6 patients were on alpha2-agonists, 4 were infused with midazolam, and 5 with cisatracurium. In 4 patients, treatment was indicated due to uncontrolled pain following tolerance to high doses of opiates and despite multimodal analgesia.
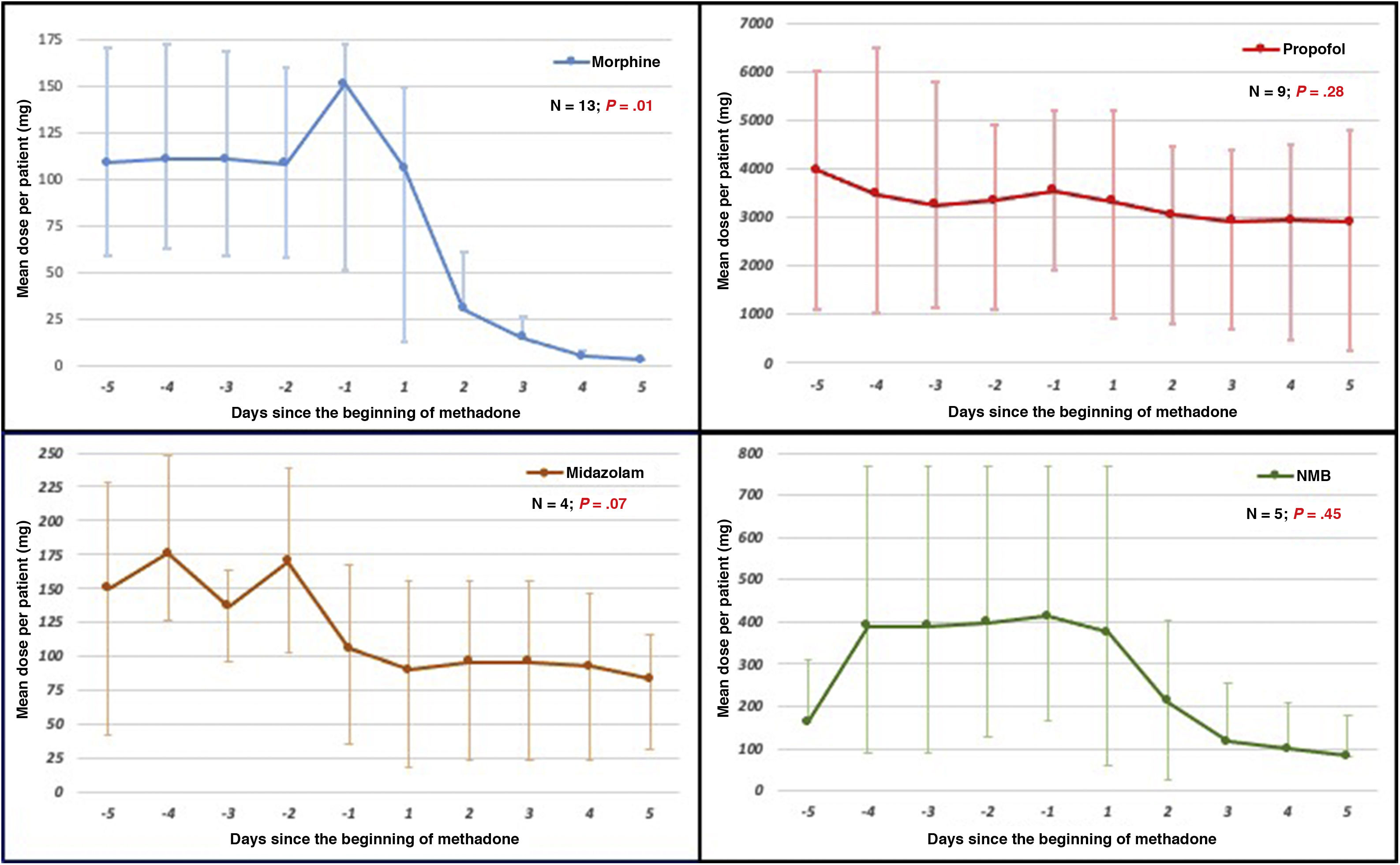
In 11 out of 13 patients (85%) the quality of sedoanalgesia improved and values of −2 and 0 were obtained in the RASS score, and pain went under control with a numerical visual scale < 4. Also, the dose of drugs used was reduced to adapt to MV (Fig. 1) with progression towards weaning from the ventilator. After 5 days of treatment, conventional opiates were withdrawn in 11 patients and the dose was reduced by 64% in 2 patients. In 5 out of 9 patients the dose of propofol was reduced in 68% ± 26% while in 3 out of 4 patients the dose of midazolam was reduced in 51% ± 31%. Also, cisatracurium was withdrawn in 3 patients while in the remaining 2—although still needed—it stopped progression to weaning from MV: in 1 patient the dose of cisatracurium was reduced by 50% and in the other patient the dose of midazolam was reduced by 56%.
Dose of sedatives, opiates, and neuromuscular blockers from day -5 of the beginning of methadone until day +5 of treatment. Doses expressed as median and interquartile range (IQR25-75). Statistical analysis between the cumulative doses on the 5 previous days and the cumulative doses 5 days after treatment.
Our study confirms the utility of methadone to control DS refractory to treatment with other drugs during prolonged sedation of patients with ARDS. Although some authors do not recommend the use of methadone in critically ill patients for its potential side effects,10 we found no relevant complications in our study. A total of 3 patients showed one-time hypotension with the first dose of methadone probably due to its fast absorption. With the dose used in our patients no QT interval prolongations or serotoninergic clinical signs were reported. The dose used can be a good guide on its use. However, we cannot recommend a dose of administration because of the variable responses reported that depend on ventilation, time, and dose administered of other opiates—both the cumulative dose and the dose administered at the beginning of treatment—and on the integrity of intestinal absorption, hepatic metabolism, and the possibility of interactions at P450 level. To minimize the appearance of complications a moderate approach is to avoid exceeding daily doses of 100 mg and also its use in patients treated with drugs that can extend the QT interval or predispose to serotoninergic clinical signs.
In conclusion, after the study findings, we believe that methadone is a drug that should be taken into consideration to treat the clinical signs of DS following the administration of high and prolonged doses of SED-OPI drugs during MV in patients with ARDS.
Conflicts of interestDr. Chamorro-Jambrina, Dr. Alcántara-Carmona, and Dr. Romera-Ortega received speaking fees from Orion-Pharma on behalf of their scientific lectures. The remaining authors declared no conflicts of interest whatsoever.
Please cite this article as: Fernández-Tobar R, Chamorro-Jambrina C, Pérez-Torres M, Castiñeiras-Amor B, Alcántara-Carmona S, Romera-Ortega MA. Metadona como fármaco de rescate para el control de la sedoanalgesia difícil en pacientes con SDRA asociado a infección por SARS-CoV-2. Med Intensiva. 2022;46:279–281.