The aim of this study was to evaluate the association between SARS-CoV-2 vaccination and the occurrence of thrombotic complications in patients admitted to intensive care for severe COVID-19 pneumonia.
DesignObservational, descriptive, prospective, multicentre study.
SettingIntensive care units of five university hospitals.
PatientsA total of 255 patients admitted to the intensive care unit (ICU) with SARS-CoV-2 pneumonia, confirmed by RT-PCR in throat swab or tracheal aspirate, starting the date the first vaccinated patient against SARS-CoV-2 was admitted in one of the participating ICUs, were included in the analysis.
Main variables of interestVaccination status against SARS-CoV-2 and thrombotic events.
Results18.8% of patients had received some form of vaccination. Thrombotic events occurred in 21.2% of patients. Lack of vaccination was associated with thrombotic events (OR 5.024; 95% CI: 1.104−23.123; p = 0.0037) and death (OR 5.161; 95% CI: 1.075–24.787; p = 0.04). ICU mortality was not associated with the occurrence of thrombotic complications.
ConclusionsIn this series of patients, vaccination against SARS-CoV-2 reduced the risk of thrombotic events and mortality in patients with severe COVID-19 admitted to the ICU. Thrombotic complications did not alter ICU mortality.
El objetivo de este estudio fue evaluar la asociación entre la vacunación frente al SARS-CoV-2 y la aparición de complicaciones trombóticas en pacientes ingresados en cuidados intensivos por neumonía grave por COVID-19.
DiseñoEstudio observacional, descriptivo, prospectivo y multicéntrico.
ÁmbitoUnidades de cuidados intensivos de cinco hospitales universitarios.
PacientesSe incluyeron en el análisis 255 pacientes ingresados en la Unidad de Cuidados Intensivos (UCI) con neumonía por SARS-CoV-2, confirmada mediante la técnica RT-PCR en frotis faríngeo o aspirado traqueal, desde la fecha de ingreso del primer paciente vacunado frente al SARS-CoV-2 en una de las UCI participantes.
Principales variables de interésVacunación frente al SARS-CoV-2 y eventos trombóticos.
ResultadosEl 18,8% de los pacientes había recibido algún tipo de vacunación. Se produjeron eventos trombóticos en el 21,2% de los pacientes. La falta de vacunación se asoció con eventos trombóticos (OR 5,024; IC 95%: 1,104−23,123; p = 0,0037) y con muerte (OR 5,161; IC 95%:1,075−24,787; p = 0,04). La mortalidad en UCI no se asoció con la aparición de complicaciones trombóticas.
ConclusionesEn esta serie de pacientes, la vacunación frente al SARS-CoV-2 redujo el riesgo de eventos trombóticos y la mortalidad en pacientes con COVID-19 grave ingresados en UCI. Las complicaciones trombóticas no alteraron la mortalidad en UCI.
The emergence of the SARS-CoV-2 viral pandemic in early 2020 has raised the challenge of a hitherto unknown disease whose main feature is the induction of acute respiratory failure, associated or not with acute respiratory distress syndrome (ARDS). This disease is associated with several complications, including thrombotic events.1–7
Deep vein thrombosis and pulmonary thromboembolism are common in critically ill patients and their detection depends on the introduction of diagnostic tests and the severity of the disease.8,9 A meta-analysis of 49 studies involving 18,093 critically ill patients showed that the incidence of venous thromboembolic disease was 17%, rising to 33% in studies with systematic screening and 9.8% in intensive care units (ICUs) where diagnosis was based on clinical assessment.10
Thromboembolic complications in patients with severe COVID-19 infection can be explained by factors such as sepsis, disseminated intravascular coagulation, microthrombosis and thrombotic microangiopathy, all of which are related to endothelial inflammation caused by COVID-19.11
Patients with severe COVID-19 have a thrombophilic state associated with elevated levels of numerous thrombosis biomarkers, which are used as direct markers of infection severity and prognosis.1
The available vaccines have been shown to be highly safe and effective, preventing serious illness and ICU admission, although the prothrombotic effect of some has been extensively studied.12 Although studies have reported cardiovascular and cerebrovascular disease as adverse effects of these vaccines, the incidence of serious reactions remains very low.13
Among all the variables that increase the risk of thrombotic events in patients with COVID-19, to date the effect of previous vaccination has not been evaluated. The aim of this study was to evaluate the association of the vaccination with the presence of thrombotic events in patients with COVID-19 admitted to the ICU, given the uncertainty about its prothrombotic effects in this setting.
Patients and methodDesignAn observational, descriptive, prospective, multicentre study was designed. Demographic variables, comorbidities that may be associated with thrombosis risk, anticoagulant treatment received prior to ICU admission, modality of oxygen therapy, thrombotic events occurring during ICU admission, and previous vaccination status against SARS-CoV-2 were recorded.
Thrombotic events included venous thrombosis, pulmonary embolism, catheter thrombosis, arterial thrombosis, stroke, and acute coronary syndrome (ACS).
Selection of patientsAll patients aged 18 years and older who were admitted to the ICU for severe pneumonia secondary to SARS-CoV-2 infection in one of the participating five university hospitals between 8 February 2021, the date the first vaccinated patient was admitted to one of the participating ICUs, and 16 March 2022 were included.
The date of the first vaccinated admission was chosen so that the other treatments administered were more similar in all ICUs. Given the time that has elapsed since the start of the pandemic, this allowed for greater homogeneity in effective treatments for SARS-CoV-2 pneumonia.
SARS-CoV-2 infection was confirmed by RT-PCR from either throat exudate or tracheobronchial aspirate. Pneumonia was diagnosed by chest X-ray or chest computed tomography.
Reasons for exclusion were anticoagulation prior to hospitalisation, history of arterial or venous thrombotic events in any organ and lack of data on SARS-CoV-2 vaccination status.
Exclusion criteriaPatients receiving anticoagulation prior to hospitalisation, with a history of arterial or venous thrombotic events in any organ, or who lacked data on SARS-CoV-2 vaccination status were excluded from the study.
To avoid confounding by anticoagulant therapy, patients who had not received anticoagulant prophylaxis at least 48 h prior to ICU admission were also excluded. The reason for this exclusion was to consider that these patients were exposed to a higher thrombotic risk during their admission to the ICU because they had not received prophylactic doses of anticoagulants.
Anticoagulants regimensThe anticoagulant regimens administered in the ICU, all with subcutaneous enoxaparin, were prophylactic (40 mg/24 h), intermediate dose (1 mg/kg/24 h) and therapeutic dose (1 mg/kg/12 h).
Therapeutic doses were administered when a thrombotic event was diagnosed during ICU admission or in the presence of other diagnosis requiring anticoagulation (for example, due to the new onset of persistent atrial fibrillation in the ICU). The intermediate dose was given to patients with an indication for full anticoagulation who were considered to be at high risk of bleeding.
SARS-CoV-2 vaccinationThe number of doses and the type of vaccine administered were recorded. Vaccination was defined as having received at least one dose of any of the COVID-19 vaccines marketed in Spain at the time of vaccination (Pfizer’s Comirnaty®, Moderna’s Spikevax®, AstraZeneca's Vaxzevria®, and Janssen's Jcovden®), with at least 14 days elapsing between vaccine administration and the onset of COVID-19 symptoms.
ObjectivesThe primary objective of the study was to determine the association between thrombotic events in the ICU and previous vaccination. Secondary objectives were to assess the association of thrombosis with other clinical variables.
Statistical analysisTo describe the main variables of the study, the total number of cases or the percentage of qualitative variables was used. Quantitative variables were described as median and interquartile range.
Incidence was expressed as incidence of each type of thrombosis, cumulative total incidence (arterial plus venous), and incidence of the competing risk adjusted composite variable with 95% confidence interval (CI). Risk was expressed as odds ratio (OR) and 95% CI.
The sampling technique used was consecutive non-probability sampling.
The χ² test was used to compare categorical variables (with Fisher’s exact test or Yates correction where appropriate). Student’s t-test or analysis of variance was used to compare quantitative variables.
Univariate analysis was performed and included baseline characteristics, anticoagulant treatment received before and during ICU admission, type of oxygen therapy, duration of symptoms, duration of pre-ICU stay, duration of ICU stay and vaccination status.
Each of the variables collected was independently contrasted with the outcome variable (thrombosis) by logistic regression. Variables with a p-value < 0.05 in the univariate analysis were included in the multivariate analysis.
Residual analysis was used to check that the data fit well with the assumptions of the regression model. All analyses were two-tailed and statistical significance was assumed when the p-value was < 0.05.
The SPSS 26.0 package (IBM Corporation, Armonk, USA) was used for statistical analysis.
Ethical issuesThe study was approved by the research ethics committee of two participating hospitals and was valid for the rest of the hospitals according to Spanish law.
During the SARS-CoV-2 pandemic, obtaining informed consent for the handling of clinical information was not considered necessary due to the health emergency and the need to obtain all clinical data necessary to successfully treat the disease.
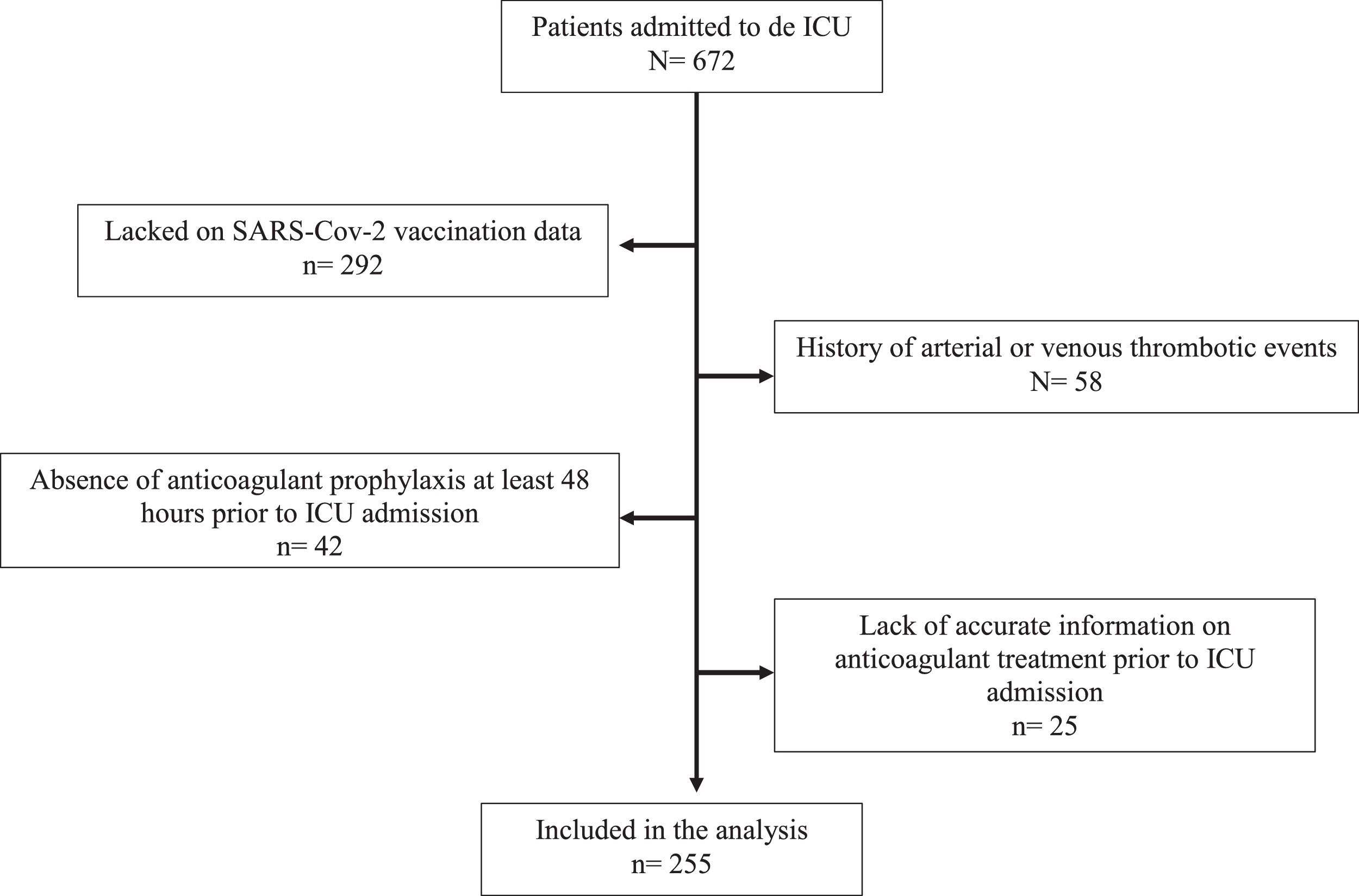
ResultsDuring the study period, 672 patients were admitted to the ICU due to SARS-CoV-2 pneumonia in the five participant hospitals. After applying the exclusion criteria, 280 patients remained for evaluation (patients selection shown in Fig. 1). For 25 of these, there was no accurate information on anticoagulant treatment prior to ICU admission. Finally, 255 patients were included in the analysis. Forty-eight patients (18.8%) met the vaccination criteria on admission to the ICU.
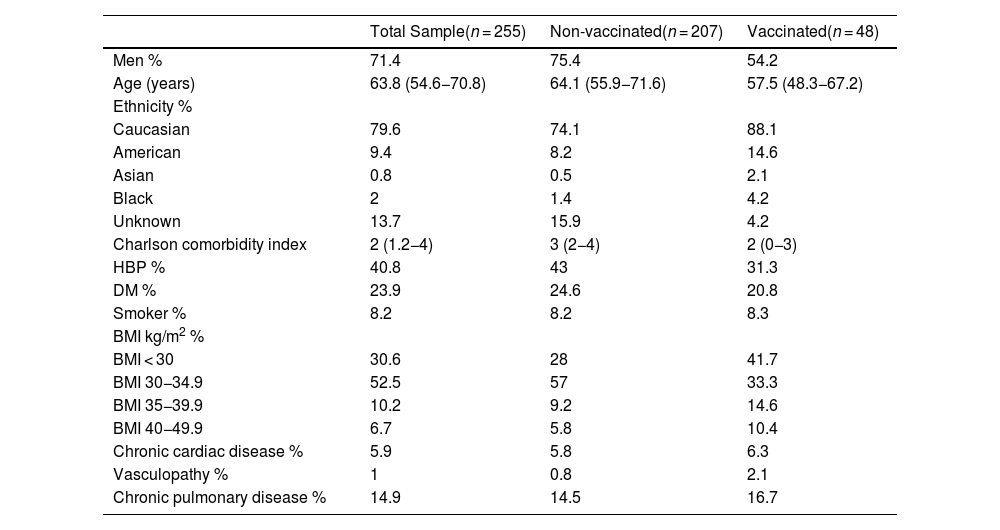
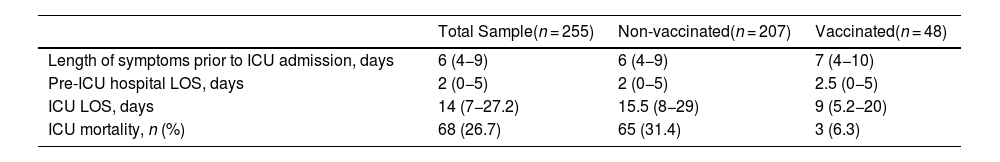
Table 1 shows the demographic and baseline characteristics of the patients. 71.4% of patients were male. The median age was 63.8 years. The median duration of COVID-19 symptoms prior to ICU admission was 6 days. The median length of stay (LOS) in the ICU was 14 days (Table 2).
Sample baseline characteristics.
| Total Sample(n = 255) | Non-vaccinated(n = 207) | Vaccinated(n = 48) | |
|---|---|---|---|
| Men % | 71.4 | 75.4 | 54.2 |
| Age (years) | 63.8 (54.6−70.8) | 64.1 (55.9−71.6) | 57.5 (48.3−67.2) |
| Ethnicity % | |||
| Caucasian | 79.6 | 74.1 | 88.1 |
| American | 9.4 | 8.2 | 14.6 |
| Asian | 0.8 | 0.5 | 2.1 |
| Black | 2 | 1.4 | 4.2 |
| Unknown | 13.7 | 15.9 | 4.2 |
| Charlson comorbidity index | 2 (1.2−4) | 3 (2−4) | 2 (0−3) |
| HBP % | 40.8 | 43 | 31.3 |
| DM % | 23.9 | 24.6 | 20.8 |
| Smoker % | 8.2 | 8.2 | 8.3 |
| BMI kg/m2 % | |||
| BMI < 30 | 30.6 | 28 | 41.7 |
| BMI 30−34.9 | 52.5 | 57 | 33.3 |
| BMI 35−39.9 | 10.2 | 9.2 | 14.6 |
| BMI 40−49.9 | 6.7 | 5.8 | 10.4 |
| Chronic cardiac disease % | 5.9 | 5.8 | 6.3 |
| Vasculopathy % | 1 | 0.8 | 2.1 |
| Chronic pulmonary disease % | 14.9 | 14.5 | 16.7 |
Variables are expressed as median (interquartile range), except for categorical variables, which are expressed as percentages. HBP: hypertension; DM: diabetes mellitus. BMI: body mass index.
Items related to symptoms, LOS and ICU mortality.
| Total Sample(n = 255) | Non-vaccinated(n = 207) | Vaccinated(n = 48) | |
|---|---|---|---|
| Length of symptoms prior to ICU admission, days | 6 (4−9) | 6 (4−9) | 7 (4−10) |
| Pre-ICU hospital LOS, days | 2 (0−5) | 2 (0−5) | 2.5 (0−5) |
| ICU LOS, days | 14 (7−27.2) | 15.5 (8−29) | 9 (5.2−20) |
| ICU mortality, n (%) | 68 (26.7) | 65 (31.4) | 3 (6.3) |
Variables expressed as median (interquartile range). ICU: intensive care unit. LOS: length of stay. ICU mortality expressed as rate and percentage.
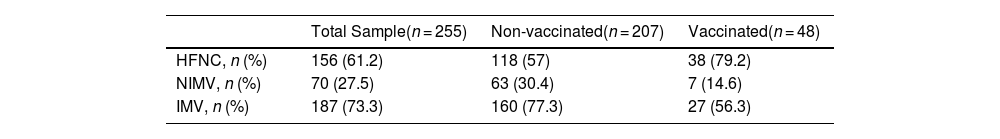
One hundred and fifty-six (61.2%) patients received high-flow nasal cannula oxygen (HFNC) delivering gas flow at 50−70 L/min and 50–100% FIO2 by a nasal canula, seventy (27.5%) received non-invasive mechanical ventilation (NIMV) through an oronasal mask and one hundred and eighty-seven (73%) were intubated and received invasive mechanical ventilation (IMV) (Table 3).
Modalities of oxygen therapy.
| Total Sample(n = 255) | Non-vaccinated(n = 207) | Vaccinated(n = 48) | |
|---|---|---|---|
| HFNC, n (%) | 156 (61.2) | 118 (57) | 38 (79.2) |
| NIMV, n (%) | 70 (27.5) | 63 (30.4) | 7 (14.6) |
| IMV, n (%) | 187 (73.3) | 160 (77.3) | 27 (56.3) |
Variables expressed as rate and percentage. HFNC: high-flow nasal cannula; NIV: non-invasive mechanical ventilation; IMV: invasive mechanical ventilation.
ICU LOS, HFNC therapy and duration of IMV were not associated with vaccination.
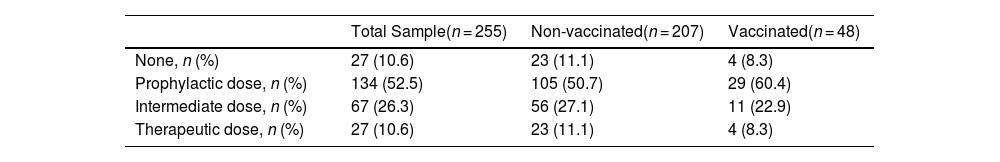
During ICU stay, 10.6% of patients received no anticoagulant, 52.5% received prophylactic doses, 26.3% received intermediate doses, and 10.6% received therapeutic anticoagulant doses. For patients with thrombotic diagnoses made after admission to the ICU or those with persistent atrial fibrillation, anticoagulation therapy was initiated at an intermediate dose or at the full therapeutic dose, respectively. These anticoagulant regimens are shown for vaccinated and non-vaccinated patients in Table 4.
Anticoagulant regimens with enoxaparin administered in the ICU.
| Total Sample(n = 255) | Non-vaccinated(n = 207) | Vaccinated(n = 48) | |
|---|---|---|---|
| None, n (%) | 27 (10.6) | 23 (11.1) | 4 (8.3) |
| Prophylactic dose, n (%) | 134 (52.5) | 105 (50.7) | 29 (60.4) |
| Intermediate dose, n (%) | 67 (26.3) | 56 (27.1) | 11 (22.9) |
| Therapeutic dose, n (%) | 27 (10.6) | 23 (11.1) | 4 (8.3) |
Variables expressed as rate and percentage.
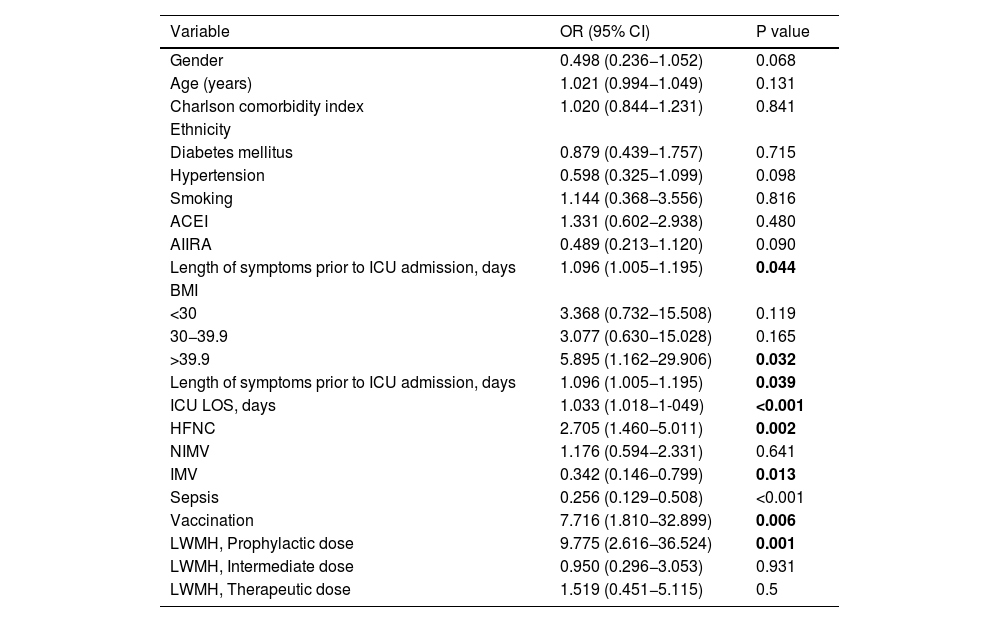
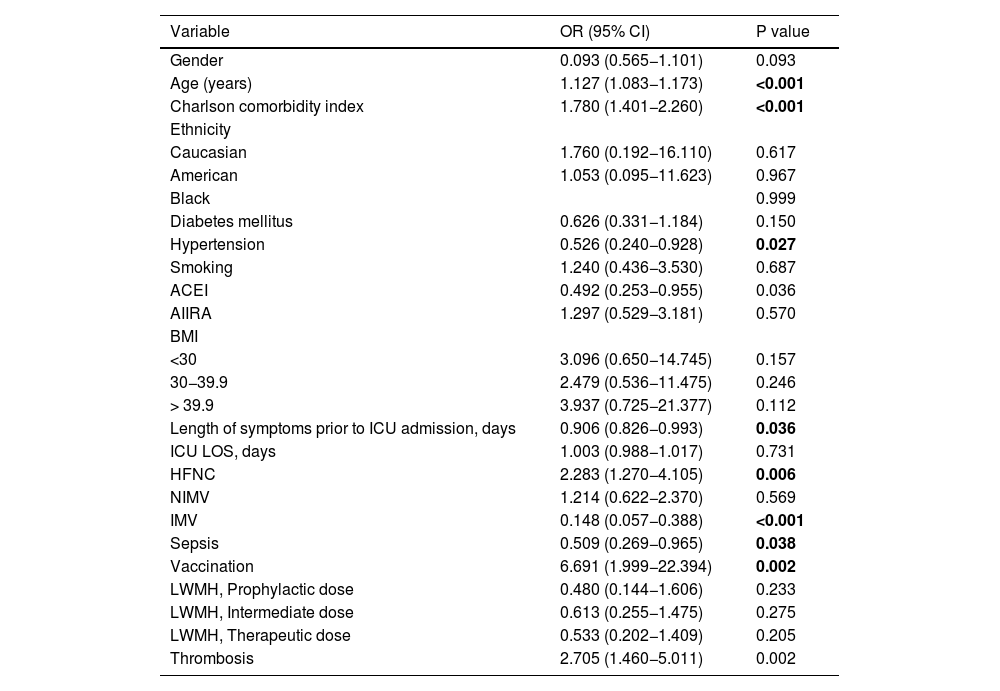
The results of the univariate analysis, with thrombosis and mortality as dependent variables, are presented in Appendix Tables A1 and A2.
Variables contrasted with the outcome variable (thrombosis) by logistic regression.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Gender | 0.498 (0.236−1.052) | 0.068 |
| Age (years) | 1.021 (0.994−1.049) | 0.131 |
| Charlson comorbidity index | 1.020 (0.844−1.231) | 0.841 |
| Ethnicity | ||
| Diabetes mellitus | 0.879 (0.439−1.757) | 0.715 |
| Hypertension | 0.598 (0.325−1.099) | 0.098 |
| Smoking | 1.144 (0.368−3.556) | 0.816 |
| ACEI | 1.331 (0.602−2.938) | 0.480 |
| AIIRA | 0.489 (0.213−1.120) | 0.090 |
| Length of symptoms prior to ICU admission, days | 1.096 (1.005−1.195) | 0.044 |
| BMI | ||
| <30 | 3.368 (0.732−15.508) | 0.119 |
| 30−39.9 | 3.077 (0.630−15.028) | 0.165 |
| >39.9 | 5.895 (1.162−29.906) | 0.032 |
| Length of symptoms prior to ICU admission, days | 1.096 (1.005−1.195) | 0.039 |
| ICU LOS, days | 1.033 (1.018−1-049) | <0.001 |
| HFNC | 2.705 (1.460−5.011) | 0.002 |
| NIMV | 1.176 (0.594−2.331) | 0.641 |
| IMV | 0.342 (0.146−0.799) | 0.013 |
| Sepsis | 0.256 (0.129−0.508) | <0.001 |
| Vaccination | 7.716 (1.810−32.899) | 0.006 |
| LWMH, Prophylactic dose | 9.775 (2.616−36.524) | 0.001 |
| LWMH, Intermediate dose | 0.950 (0.296−3.053) | 0.931 |
| LWMH, Therapeutic dose | 1.519 (0.451−5.115) | 0.5 |
Bold highlights results with statistical significance.
Variables contrasted with the outcome variable (mortality) by logistic regression.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Gender | 0.093 (0.565−1.101) | 0.093 |
| Age (years) | 1.127 (1.083−1.173) | <0.001 |
| Charlson comorbidity index | 1.780 (1.401−2.260) | <0.001 |
| Ethnicity | ||
| Caucasian | 1.760 (0.192−16.110) | 0.617 |
| American | 1.053 (0.095−11.623) | 0.967 |
| Black | 0.999 | |
| Diabetes mellitus | 0.626 (0.331−1.184) | 0.150 |
| Hypertension | 0.526 (0.240−0.928) | 0.027 |
| Smoking | 1.240 (0.436−3.530) | 0.687 |
| ACEI | 0.492 (0.253−0.955) | 0.036 |
| AIIRA | 1.297 (0.529−3.181) | 0.570 |
| BMI | ||
| <30 | 3.096 (0.650−14.745) | 0.157 |
| 30−39.9 | 2.479 (0.536−11.475) | 0.246 |
| > 39.9 | 3.937 (0.725−21.377) | 0.112 |
| Length of symptoms prior to ICU admission, days | 0.906 (0.826−0.993) | 0.036 |
| ICU LOS, days | 1.003 (0.988−1.017) | 0.731 |
| HFNC | 2.283 (1.270−4.105) | 0.006 |
| NIMV | 1.214 (0.622−2.370) | 0.569 |
| IMV | 0.148 (0.057−0.388) | <0.001 |
| Sepsis | 0.509 (0.269−0.965) | 0.038 |
| Vaccination | 6.691 (1.999−22.394) | 0.002 |
| LWMH, Prophylactic dose | 0.480 (0.144−1.606) | 0.233 |
| LWMH, Intermediate dose | 0.613 (0.255−1.475) | 0.275 |
| LWMH, Therapeutic dose | 0.533 (0.202−1.409) | 0.205 |
| Thrombosis | 2.705 (1.460−5.011) | 0.002 |
Bold highlights results with statistical significance.
Fifty-four patients (21.2%) developed at least one thrombotic event while in the ICU: superficial or deep vein thrombosis 19.6%, deep vein thrombosis 6.7%, pulmonary thromboembolism 9.4%, catheter thrombosis 5.1%, stroke 1.6% and ACS 0.4%.
Each day of symptoms before ICU admission increased the risk of thrombosis (P = 0.024), as did each day of ICU admission (P = 0.003). Not receiving HFNC therapy increased the risk of thrombosis (P = 0.024).
Risk of deathA longer duration of symptoms before hospital admission was associated with a lower risk of mortality (P = 0.015). The risk of death was found to be higher in older patients (P < 0.001).
Sixty-eight patients (26.7%) died in the ICU. No statistical association was found between thrombotic events and ICU mortality in the multivariate analysis.
Effects of vaccinationThe lack of vaccination significantly increased the risk of thrombosis (P = 0.0037) and the risk of death (P = 0.04).
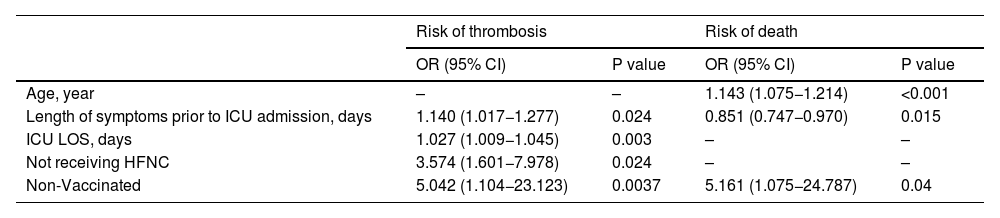
Table 5 shows a complete overview of the data obtained from the multivariate analysis.
Multivariate analysis. Variables associated with thrombosis and death.
| Risk of thrombosis | Risk of death | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, year | – | – | 1.143 (1.075−1.214) | <0.001 |
| Length of symptoms prior to ICU admission, days | 1.140 (1.017−1.277) | 0.024 | 0.851 (0.747−0.970) | 0.015 |
| ICU LOS, days | 1.027 (1.009−1.045) | 0.003 | – | – |
| Not receiving HFNC | 3.574 (1.601−7.978) | 0.024 | – | – |
| Non-Vaccinated | 5.042 (1.104−23.123) | 0.0037 | 5.161 (1.075−24.787) | 0.04 |
ICU: intensive care unit. LOS: length of stay; HFNC: high-flow nasal cannula.
No data imputation method was used in this study. On the one hand, no variable had more than 10% missing data. This is considered an acceptable threshold without loss of representativeness. No systematic pattern of missing data was observed (missing data was not associated with specific patient characteristics), so there seems to be no obvious bias. However, to ensure the integrity and quality of the analysis, cases with missing data for key variables (thrombosis, heparin use, vaccination and mortality) were excluded. This was done rather than using imputation methods to impute missing values. The removal of these cases did not significantly reduce the sample size. Thus, the statistical power of the analysis was maintained.
DiscussionTo our knowledge, this is the first study to include vaccination before ICU admission as a factor to be considered in its influence on the occurrence of thrombosis events. In addition, we excluded as a confounding factor the absence of prophylactic anticoagulation before the onset of possible thrombotic complications. A published series of patients enrolled during the fifth wave of COVID-19 in Spain (July to September 2021), distinguishing between patients with complete and incomplete vaccination, showed no differences in the incidence of pulmonary thromboembolism compared with unvaccinated patients. However, this report does not include a description of other thrombotic complications or the anticoagulation regimens administered.14 This study also showed no association of thrombosis events related to mechanical ventilation or ICU outcome variables, despite the fact that unvaccinated patients had more severe ARDS. Furthermore, our study has shown no association between thrombotic complications and ICU mortality as an additional finding.
Thrombotic complications in patients with COVID-19, especially in the most severe cases, have been the subject of particular attention, and various factors that might increase the risk of this event have been studied.2,15 The incidence of thrombosis in our series was 21.2%, slightly lower than that described by other authors in large systematic reviews at the beginning of the pandemic, before the start of vaccination.16,17 This fact leads us to conclude that the way in which this complication was diagnosed did not change significantly in actual clinical practice, from the beginning of the pandemic to the final date of our case collection.
We have found some additional risk factors related to the risk of thrombosis. The increased rate of thrombosis in patients with a larger duration of symptoms before ICU admission could be explained by the fact that most of these patients stayed at rest for a longer time. Patients presented to the ICU with a more severe illness were intubated immediately, not receiving HFNC at admission. These patients were at higher risk of thrombosis events as shown is previous studies.16,17
Vaccination against SARS-CoV-2 has been shown to reduce the incidence and severity of disease, but has not been specifically studied in the most severe patients.18 In the series we report, the risk of thrombosis in critically ill unvaccinated patients increased more than 5-fold. Thrombotic events were also associated with ICU LOS. However, this complication was not associated with mortality, which is consistent with the results of the COVID-19 Critical Care Consortium of almost 12,000 patients, in which bleeding complications, but not thrombotic complications, were associated with mortality.19 It should be noted that no patient in our study died from pulmonary thromboembolism. On the other hand, Hungaro et al. reported an increase in mortality in the presence of deep vein thrombosis and pulmonary thromboembolism in patients with varying degrees of lung damage.20 Mortality in this study may be mainly related to the extent of lung damage, as thromboembolic complications are a marker of disease severity.
Our study excluded patients who did not receive any type of anticoagulant during hospitalisation prior to ICU admission, to eliminate what could be a confounding factor in the assessment of thrombotic. The design of this study required that some form of anticoagulant therapy had been administered for at least 48 h prior to ICU admission to ensure at least adequate antithrombotic prophylaxis. Although the aim of the study was not to evaluate the different doses of anticoagulation regimen prior to ICU admission, a clinical trial of different combinations of heparins and direct anticoagulants in hospitalised patients with COVID-19 failed to improve clinical outcomes and increased bleeding with the administration of therapeutic anticoagulation compared to the use of prophylactic anticoagulation, in agreement with our findings.21
Limitations of the studyThis study has some important limitations.
First, thrombotic events diagnosis were not made by systematic screening, but by imaging tests following suspicion. However, as mentioned above, the incidence of these complications in our series is not significantly different from that described in patients with severe COVID-19.16,17 In any case, the authors acknowledge that these results are not applicable to the entire ICU population admitted for SARS-CoV-2 pneumonia, given the differences in treatments administered in such a short period of time, even among the ICUs participating in the study.
Second, identification of vaccination status in many cases was done by reviewing the electronic medical record, which may contain incomplete data collected during the pandemic.
Third, as in most countries, vaccination in Spain started with the population at highest risk of poor outcome from COVID-19 pneumonia, so the sample may not have captured the effect of the vaccine on the whole population. In addition, due to the time period studied, vaccination could not have been completed in most cases and it was not possible to distinguish patients with different degrees of immunisation.
Fourth, our study does not include the effect of vaccination on coagulation factors or markers of inflammation, which could explain some of the effects of vaccination on prothrombotic status. Changes in coagulation factors that occur acutely and are maintained at follow-up have been widely reported in patients with severe COVID-19.22,23
Fifth, the small sample of vaccinated patients and the heterogeneity of vaccines do not allow to assess their effect on prothrombotic risk, as other authors have pointed out.13
Finally, the association between longer ICU LOS and the presence of thrombotic complications is reasonably related to greater patient acuity, longer immobilisation and greater activity of inflammatory factors, although our study did not include these variables in its assessment.24
ConclusionsThese results showed that vaccination against SARS-CoV-2 was associated with a reduced risk of thrombotic events in patients with severe COVID-19. Other variables related to severity of illness, such as ICU LOS, duration of symptoms before ICU admission, and not receiving HFNC in the ICU, were also associated with the risk of thrombotic events.
In this study, the occurrence of thrombotic complications did not affect ICU mortality.
It should be noted that the diagnosis of a thrombotic event was made using imaging tests following clinical suspicion, rather than as a routine search of all patients. This is the main limitation of the study.
Further studies should investigate the relationship between SARS-CoV-2 vaccination, inflammation and coagulation factors to gain a deeper understanding of the underlying mechanisms of these findings.
CRediT authorship contribution statementAlonso-Fernández, M. Ángeles: Design of the study and patients selection
Carola Bledig: Design of the study and patients selection
Manso-Álvarez, Madian; Design of the study and patients selection
Gómez-Guardiola, Raquel: Design of the study and patients selection
Blancas García, Marina: Design of the study and patients selection
Quintana-Díaz, Manuel: Design of the study and patients selection
Martín-Parra, Carmen: Acquisition of data
López-Matamala, Blanca: Acquisition of data
Algaba-Calderón, Ángela: Acquisition of data
Marcos Neira, Pilar: Acquisition of data
Serrano-Lázaro, Ainhoa: Acquisition of data
Silva-Obregón, José Alberto: Acquisition of data
Campillo-Morales, Salvador: Database design and data control
Bartolomé, Irene: Methodology and statistical analysis
Blancas, Rafael: Redaction and final review of the manuscript
Martínez-González, Óscar: Metodology, design of the study and final review of the manuscript.