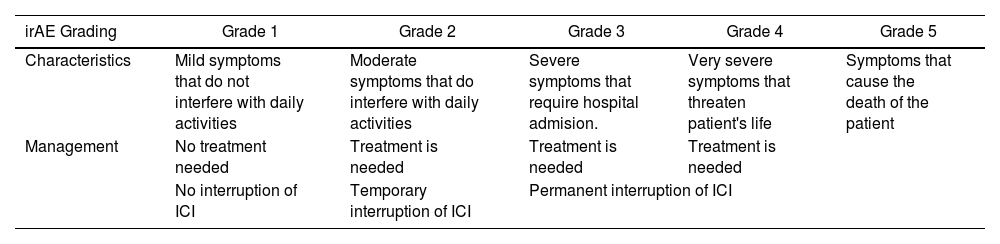
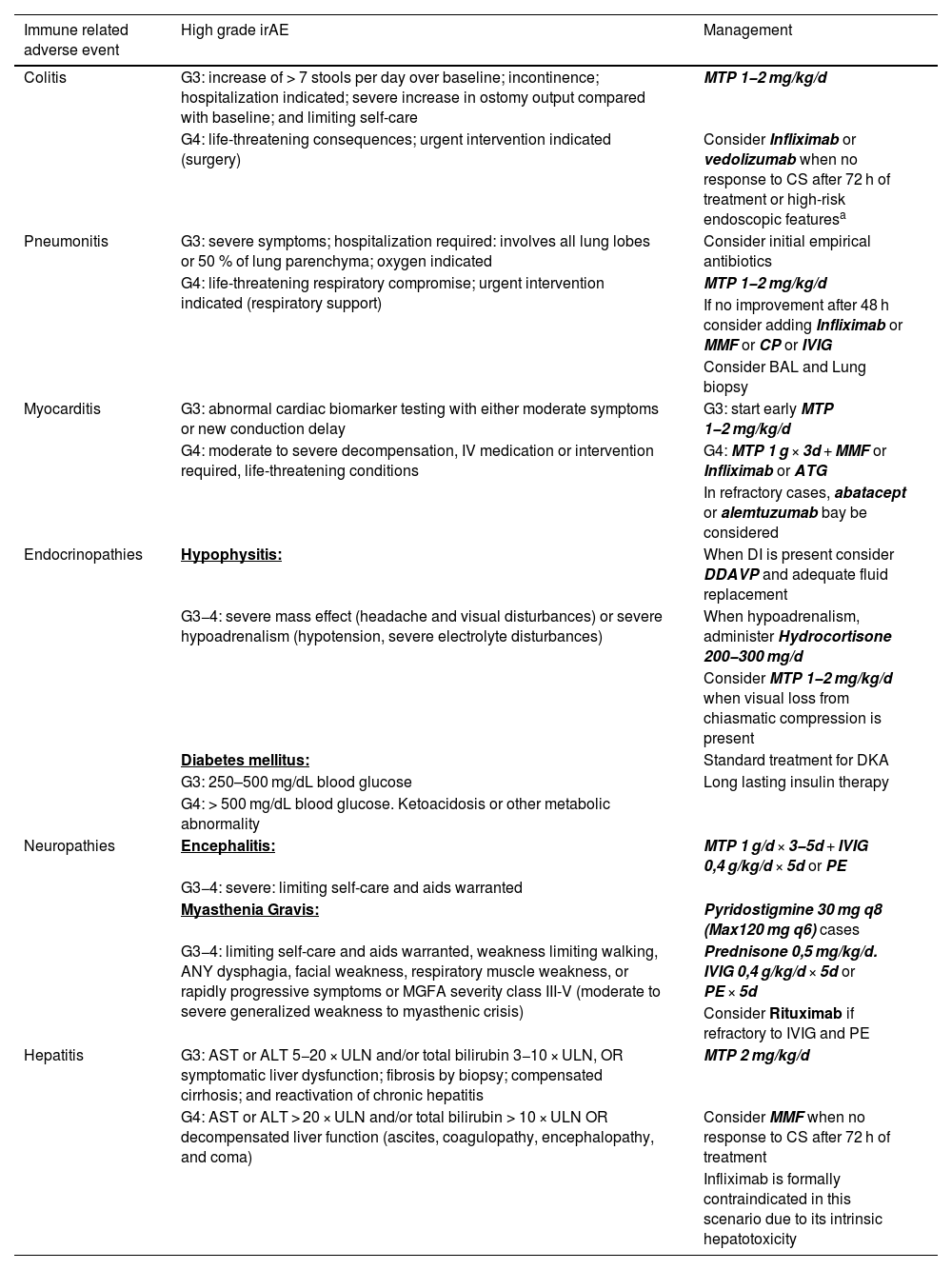
The pharmacological group of immune checkpoint-inhibitors (ICI) has revolutionized the field of oncology in the last ten years. The improvements in the survival of certain cancers thanks to these treatments comes at the cost of an increased morbidity and mortality due to certain immune related adverse events (irAE). This review will concentrate on the irAE that more frequently require intensive care unit (ICU) admission. The infectious burden of patients treated with ICI is also explored, shining light not only on the infections caused by the immunosuppression needed to manage the different irAE, but also on the specific infections arising from a unique immune dysregulation only seen in ICI treated patients.
El grupo farmacológico de los checkpoint-inhibitors (ICI) ha revolucionado la oncología en los últimos diez años. Las mejorías en el pronóstico oncológico se han acompañado de una serie de eventos adversos inmunomediados (irAE) que otorgan importante morbi-mortalidad. Esta revisión está centrada en los irAE que con mayor frecuencia pueden requerir un ingreso en una unidad de cuidados intensivos (UCI). Además, aporta un énfasis en la importancia de la patología infecciosa que pueden sufrir estos enfermos, ya sea por la inmunosupresión requerida para tratar los irAE, como por la disregulación inmune propia de los ICI.
Article
Go to the members area of the website of the SEMICYUC (www.semicyuc.org )and click the link to the magazine.