We aimed to compare the effect of 0.9% sodium chloride (SC) versus ringer’s lactate (RL) in the resolution of diabetic ketoacidosis (DKA).
DesignOpen randomized trial.
SettingA medical ICU from November 2022 to September 2023.
PatientsAll patients older than 16 years admitted to the ICU for severe DKA.
InterventionThe enrolled patient was assigned to receive RL or 0.9% SC according to the randomization scheme. Insulin therapy protocol was conducted similarly for all patients.
Main variables of interestResolution of DKA at H48 defined by a composite endpoint (glycemia < 11 mmol/l, bicarbonates > 15 mmol/l or pH > 7.30 and anion gap < 16), change in base excess, insulin needs, fluid administration volume, electrolytes (sodium, potassium, chloride), ICU length of stay, and 28-day mortality.
Results88 patients were included: SC arm (n = 42) versus RL arm (n = 46). No significant differences were observed in diabetic ketoacidosis resolution, other variables of interest or in the subgroup analysis. The monitored biomarkers showed in the RL group: a better improvement of bicarbonate at H12 (p = 0.05), best potassium level both at H24 (p = 0.008) and H48 (0.041), lower chloride at H48 (p = 0.002) and higher glycemia at H24.
ConclusionRL did not lead to faster resolution of diabetic ketoacidosis but decreased the risk of hyperchloremia and hypokalemia without increasingthe chance of hyperlactatemia.
Clinical Trial registration numberNCT05808972.
Comparar el efecto del cloruro de sodio al 0,9 % (CS) frente Ringer lactate (RL) en la resolución de la cetoacidosis diabética (CAD).
DiseñoEnsayo abierto y aleatorizado.
ÁmbitoUnidad de Cuidados Intensivos (UCI) Médicos desde noviembre de 2022 hasta septiembre de 2023.
PacientesTodos los pacientes mayores de 16 años ingresados en la UCI por CAD grave.
IntervenciónLos pacientes incluidos fueron asignados a recibir RL o CS al 0,9 % según el esquema de aleatorización. El protocolo de terapia con insulina se llevó a cabo de manera similar para todos los pacientes.
Variables de interésprincipalesResolución de la CAD a las 48 horas, definida por un criterio compuesto (glucemia <11 mmol/l, bicarbonato >15 mmol/l o pH > 7,30 y brecha aniónica <16), cambio en el exceso de base, necesidades de insulina, volumen de administración de líquidos, electrolitos (sodio, potasio, cloruro), duración de la estancia en UCI y mortalidad a los 28 días.
ResultadosSe incluyeron 88 pacientes: brazo SC (n = 42) versus brazo RL (n = 46). No se observaron diferencias significativas en la resolución de la cetoacidosis diabética ni en otros criterios de valoración, ni en el análisis por subgrupos. El monitoreo de biomarcadores mostró en el grupo RL: una mejor mejoría del bicarbonato a las 12 horas (p = 0,05), mejores niveles de potasio tanto a las 24 horas (p = 0,008) como a las 48 horas (p = 0,041), menor nivel de cloruro a las 48 horas (p = 0,002) y una mayor glucemia a las 24 horas.
ConclusionesEl RL no condujo a una resolución más rápida de la cetoacidosis diabética, pero redujo el riesgo de hipercloremia e hipopotasemia sin aumentar el riesgo de hiperlactatemia.
Número de registro del ensayo clínicoNCT05808972.
Diabetic ketoacidosis (DKA) is an acute, major, life-threatening complication of diabetes characterized by hyperglycemia, ketoacidosis, and ketonuria.1 DKA can lead to intensive care unit (ICU) admission due to the major hydroelectrolytic disorders.2 Correction of the underlying cause, rehydration with intravenous fluids, insulin therapy, and electrolyte replacement are the cornerstones of DKA management.1 Although there is widespread agreement about replacing insulin and electrolytes,3–5 it is still unclear which fluid is best for managing DKA.
For several decades, the most widely used crystalloid solution was normal saline (0.9% sodium chloride solution).6,7 Furthermore, current international guidelines suggest that 0.9% sodium chloride (0.9% SC) should be the preferred as replacement fluid for DKA.8 However, recent research raises questions about the possible negative effects of sodium chloride administration, mainly the increased acidemia by hyperchloremia.9 Hence, balanced crystalloids, which replace chloride anions with lactate or acetate, are becoming increasingly popular as alternatives to 0.9% SC because of the potential negative effects of its high chloride content.10 These substitutes are more similar in chemical composition to human plasma than to sodium chloride. Reduced chloride levels and increased strong ion differential in vivo are implicated in this compositional alignment.11
The debate between SC and RL in the management of DKA is far from being resolved; as indicated in several recent reports.12–16 The recent meta-analysis that encompassed 11 trials (including one in pediatric population) not found a significant difference between balanced crystalloids and normal saline for the time to resolution of DKA, major adverse kidney events, and incidence of hypokalemia.12 However, there was a significant reduction in the post-resuscitation chloride among patients received balanced crystalloids.12 While both crystalloids are safe for treating DKA, balanced solutions were associated with improved post-resuscitation electrolyte balance and preventing hyperchloremic metabolic acidosis according to the recent meta-analysis of Gupta et al.13 Other meta-analysis indicates that the use of balanced solutions resolves DKA faster than 0.9% SC with a mean difference of 5 h,14 reduced post resuscitation hyperchloremia and hypernatremia, and increased bicarbonate level.14,15 The prospective intervention trial aiming to compare the effectiveness of Sterofundin as balanced solution versus NS in the management of DKA showed a superiority of sterofundin (shorter mean time to DKA resolution, less total intravenous fluid, less parenteral insulin, and shorter hospital stays).16
We hypothesized that RL may result in a faster DKA resolution and less electrolyte imbalances, namely chloride, potassium, sodium, and bicarbonate. Therefore, clinical guidelines could be directed toward how to treat DKA with RL instead of NS. Herein, we sought to evaluate the effect of RL comparatively to 0.9% SC on the resolution of severe DKA as well as on the electrolytes variations.
Patients and methodsDesign, setting, population and ethical statusOpen-label randomized trial in patients older than 16 years who were admitted in the medical ICU of la Rabta University Hospital (Tunis, Tunisia) for severe DKA from November 2022 to September 2023. Severe DKA was diagnosed by a blood glucose level > 14 mmol/l, an arterial pH < 7.25 (or serum bicarbonate ≤ 15 mmol/l) with an anion gap > 16 and ketonuria. Non inclusion criterion was: patients aged less than 16 years. Patients who started to receive rehydration solution in emergency department or other structure before ICU admission and those enrolled into other trials were excluded.
In accordance with ethical principles of international guidelines (declaration of Helsinki, Council for International Organizations of Medical Sciences (CIOMS), International Conference on Harmonization (ICH), and Good Clinical Practice (GCP) Guidelines, the protocol and other relevant documents were reviewed and approved by the institutional Biomedical Ethics Committee of La Rabta Hospital (reference: 07/2022). A written and informed consent was obtained from patients or their legal representative.
Protocol studyOnce DKA was diagnosed (in front of a blood glucose > 14 mmol/l, an arterial pH < 7.25 (or serum bicarbonate ≤ 15 mmol/l) with an anion gap > 16 and ketonuria), the enrolled patient was assigned to receive RL or 0.9% SC according to the randomization scheme. This last was prepared beforehand on an electronic list by the succession of four blocks of random permutations as follows ABAB, BABA, AABB, and BBAA where A corresponded to RL and B to 0.9% SC. The patient assignment was sequential and the allocation 1/1 was regularly checked. Only patients were blinded to the treatment. In addition to randomization and its regular checking, we used the restriction method to decrease the impact of confounding variables on our research (sex, age, diabetes kind, co morbidities, origin of decompensation, and baseline metabolic status). This implies that we selected the sample by including only certain patients that have similar characteristics/values of potential confounding variables.
The insulin therapy protocol was conducted similarly for all patients. Insulin was administered via intravenous electric syringe (Actrapid HM®, Novorapide®) (1 ml = 100 IU): 0.5 ml ( = 50 IU) diluted in 50 ml of normal Saline to obtain a solution of 1 ml = 1 IU with infusion rate at 0.1 IU/kg/h.
The total fluid volume (whether RL or SC) was estimated as a deficit of between 70–100 ml/kg (justified clinically by a fluid loss of approximately 6–9 l)17 and administrated over 48 h. The half of resuscitation fluid was administered within first 8–12 h of presentationat 15–20 ml/kg/H then re-evaluate.17 Schematically simpler, fluid solution (SC or RL) was distributed as follows: 1–3 l during the first hour, 1 l during the second hour, 1 l during the following 2 h, then 1 l every 4 h, depending on the degree of dehydration. This staged strategy in the prescription of liquids had a goal to avoid the rapidly reversing of plasma osmotic gradient, including the risk of cerebral edema, since hyperglycemia is corrected faster than ketoacidosis (6 and 12 h, respectively).17 When capillary glycemia dropped to <2.5 g/l, fluid was switched to 5% glucose serum to allow continued parenteral insulin perfusion and avoid hypoglycemia.
For all patients, the blood samples were taken for glycemia, arterial Blood gas, electrolytes (sodium, potassium, chloride), and lactate. These study samples were taken at baseline, H6, H12, H24 and 48 h later.
EndpointsThe primary endpoint was resolution of DKA at H48 defined as a composite criterion (glycemia < 11 mmol/l, bicarbonates > 15 mmol/l or pH > 7.30 and anion gap < 16). Secondary were: resolution of DKA at 24, change in base excess to ≥ −3 meq/l (H24 and H 48), insulin needs within 48 h, fluid administration volume, electrolytes variations over 48 h of monitoring, ICU length of stay (LOS), and 28-days mortality.
Sample size estimation and analysisRamanan et al.18 compared plasmalyte (PL) versus SC in the management of DKA and showed that at 24 h, DKA resolution occurred in 69% among PL arm and 36% among SC arm (p = 0.002). by referring to this online sample size calculator: https://www.easymedstat.com/ with a type 1 risk of 5% and type 2 risk of 20%, a bilateral test and a dropout rate estimated at 10%, we expected that at least 39 patients per group would be necessary to detect a statistical difference.
Continuous variables were reported as median and interquartile range (IQR) and compared using the non parametric test of Mann–Whitney. Categorical variables were reported as frequencies and proportions and compared with the Chi2 test.This trial followed the Consolidated Standards of Reporting Trials (CONSORT).
A subgroup analysis for key outcomes was performed. The outcomes considered were Resolution of DKA at H48, Change in base excess ≥ −3 meq/l at H48, Received fluid over 48H, and Insulin needs within 48 h. Stratification was based on age (inferior versus superior than 25 years), diabetes kind (type 1 vs 2 vs inaugural), Severity of DKA (assessed by baseline pH: <7.20 vs >7.20), and severity illness (assessed by SAPS II severity score <18 vs >18). The cutoffs set (25 years for age, 7.20 for pH, and 18 for SPAS II) were those found as medians in our results. All analyses were performed by SPSS 20 and followed a per-protocol approach. A p-value <5% was considered for statistical significance.
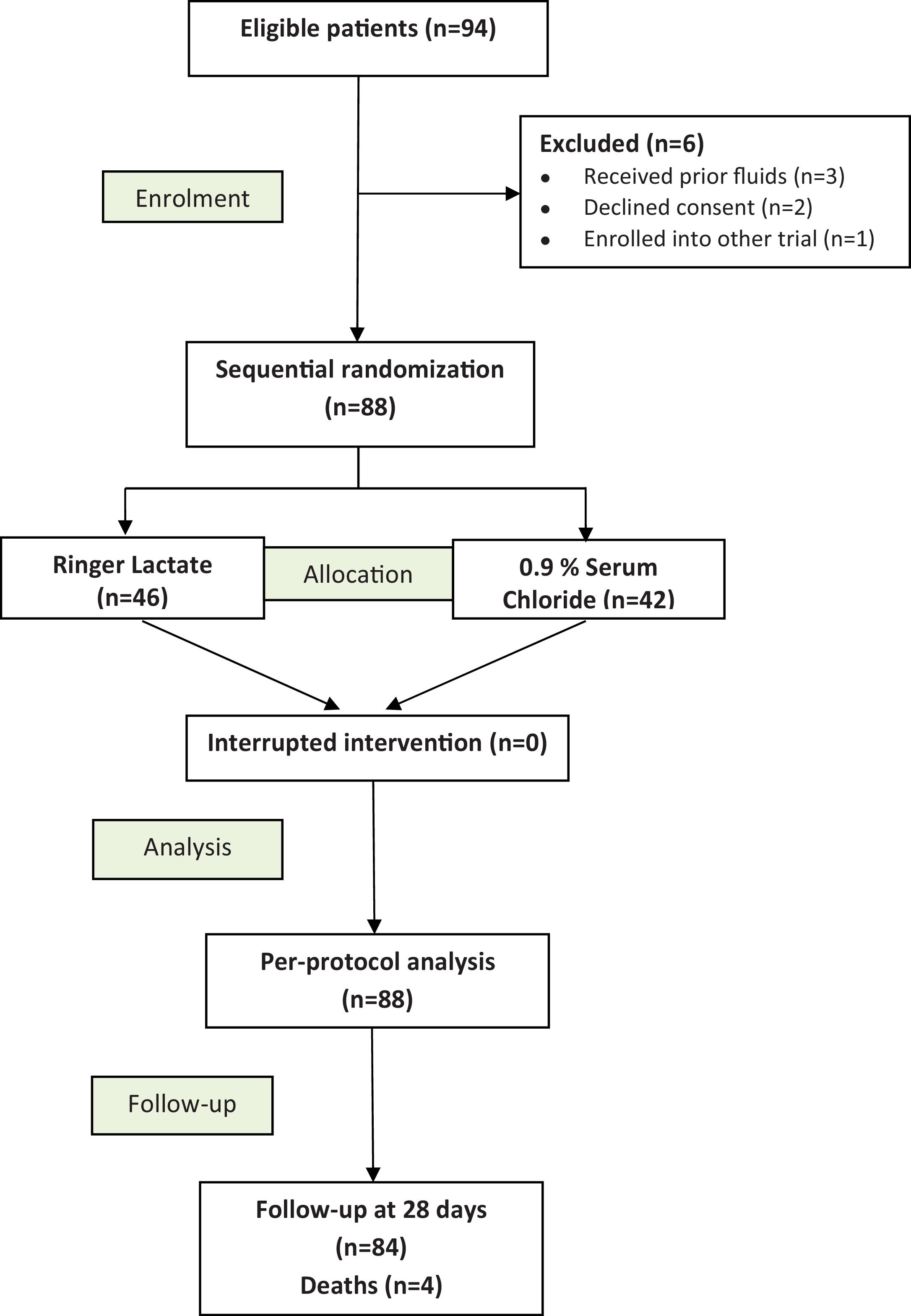
ResultsPatient’s flowA total of 94 diagnosed DKA in 94 eligible patients were assigned in our study protocol. Among them, six were excluded: 3 have previously received fluids, 2 cases of unapproved consent by legal representatives, and one patient was enrolled into other trial. Finally, 88 patients were included: RL (n = 46) and 0.9% SC (n = 42). The CONSORT flow diagram is displayed in Fig. 1.
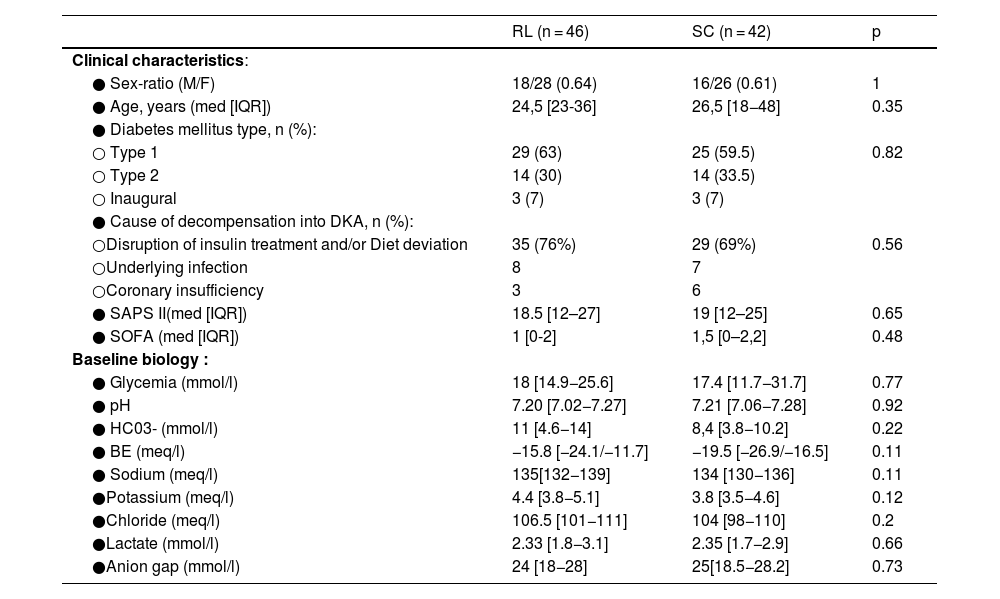
Baseline dataOverall, our series was young (median age = 25 years) with a female predominance (n = 54 (61.5%)) and the type of diabetes mellitus was mostly of type 1 in 54 cases. The most common causes were disruption of insulin treatment and/or diet deviation (64/88), and underlying infectionwhich was mainly of urinary origin (11 out of 15). DKA was inaugural for diabetes mellitus in 6 patients. The laboratory findings showed a median pH at 7.20, median HCO3- at 9.7 mmol/l, and anion gap at 24 mmol/l. All baseline clinical characteristics and laboratory parameters (glycemia, pH, bicarbonates, BE, electrolytes, lactates, anion gap) were comparable between RL and SC groups (Table 1).
Comparison of baseline characteristics.
| RL (n = 46) | SC (n = 42) | p | |
|---|---|---|---|
| Clinical characteristics: | |||
| ● Sex-ratio (M/F) | 18/28 (0.64) | 16/26 (0.61) | 1 |
| ● Age, years (med [IQR]) | 24,5 [23-36] | 26,5 [18−48] | 0.35 |
| ● Diabetes mellitus type, n (%): | |||
| ○ Type 1 | 29 (63) | 25 (59.5) | 0.82 |
| ○ Type 2 | 14 (30) | 14 (33.5) | |
| ○ Inaugural | 3 (7) | 3 (7) | |
| ● Cause of decompensation into DKA, n (%): | |||
| ○Disruption of insulin treatment and/or Diet deviation | 35 (76%) | 29 (69%) | 0.56 |
| ○Underlying infection | 8 | 7 | |
| ○Coronary insufficiency | 3 | 6 | |
| ● SAPS II(med [IQR]) | 18.5 [12–27] | 19 [12–25] | 0.65 |
| ● SOFA (med [IQR]) | 1 [0-2] | 1,5 [0–2,2] | 0.48 |
| Baseline biology : | |||
| ● Glycemia (mmol/l) | 18 [14.9−25.6] | 17.4 [11.7−31.7] | 0.77 |
| ● pH | 7.20 [7.02−7.27] | 7.21 [7.06−7.28] | 0.92 |
| ● HC03- (mmol/l) | 11 [4.6−14] | 8,4 [3.8−10.2] | 0.22 |
| ● BE (meq/l) | −15.8 [−24.1/−11.7] | −19.5 [−26.9/−16.5] | 0.11 |
| ● Sodium (meq/l) | 135[132−139] | 134 [130−136] | 0.11 |
| ●Potassium (meq/l) | 4.4 [3.8−5.1] | 3.8 [3.5−4.6] | 0.12 |
| ●Chloride (meq/l) | 106.5 [101−111] | 104 [98−110] | 0.2 |
| ●Lactate (mmol/l) | 2.33 [1.8−3.1] | 2.35 [1.7−2.9] | 0.66 |
| ●Anion gap (mmol/l) | 24 [18−28] | 25[18.5−28.2] | 0.73 |
RL: ringer lactate, SC: serum chloride, DKA: diabetic ketoacidosis, med: median, IQR: interquartile range, SAPS: Simplified Acute Physiology Score, SOFA: sequential Organ Failure Assessment.
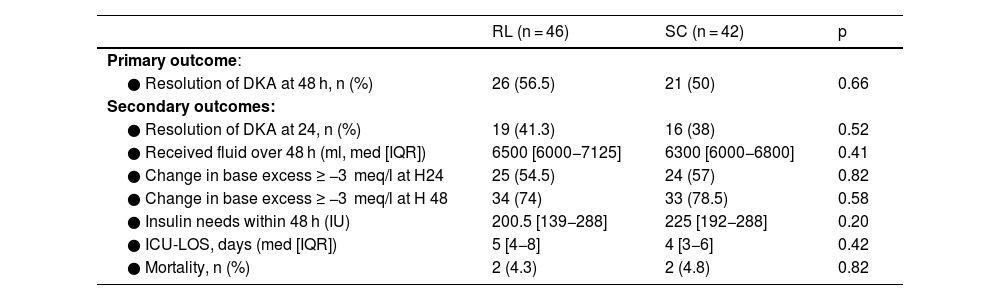
At H48, resolution of DKA occurred in 56.5% among RL patients versus 50% among 0.9% SC patients without statistical difference (p = 0.66). Regarding the secondary endpoints, no difference was highlighted for fluid administration (p = 0.36), change in base excess to ≥−3 meq/l, insulin needs (p = 0.2) or for follow-up outcomes (Table 2). The results of outcomes analyses by subgroups are displayed in Fig. 2 as forest plots for the binary outcomes (DKA Resolution at H48 and Change in base excess ≥ −3 meq/l at H48) and in Table 3 for the quantitative outcomes (Received fluid and Insulin needs over 48 H) with more complement data in Additional material 1. There was no significant difference. Nevertheless, there was a trend towards significance of SC superiority in change in base excess ≥ −3 meq/l at H48 for type 2 diabetes strata (OR = 1.39 [0.88–6.45]) and SAPS II > 18 (OR = 1.40 [0.9–6.2]).
Comparison of outcome parameters between the study groups.
| RL (n = 46) | SC (n = 42) | p | |
|---|---|---|---|
| Primary outcome: | |||
| ● Resolution of DKA at 48 h, n (%) | 26 (56.5) | 21 (50) | 0.66 |
| Secondary outcomes: | |||
| ● Resolution of DKA at 24, n (%) | 19 (41.3) | 16 (38) | 0.52 |
| ● Received fluid over 48 h (ml, med [IQR]) | 6500 [6000−7125] | 6300 [6000−6800] | 0.41 |
| ● Change in base excess ≥ −3 meq/l at H24 | 25 (54.5) | 24 (57) | 0.82 |
| ● Change in base excess ≥ −3 meq/l at H 48 | 34 (74) | 33 (78.5) | 0.58 |
| ● Insulin needs within 48 h (IU) | 200.5 [139−288] | 225 [192−288] | 0.20 |
| ● ICU-LOS, days (med [IQR]) | 5 [4−8] | 4 [3−6] | 0.42 |
| ● Mortality, n (%) | 2 (4.3) | 2 (4.8) | 0.82 |
RL: ringer lactate, SC: serum chloride, DKA: diabetic ketoacidosis, IU: international unit, ICU: intensive care unit, LOS: length of stay, mmol: millimoles, meq: milliequivalent, L: liter.
Analysis of Outcomes (DKA resolution of at H48 and Change in base excess ≥ −3 meq/l at H48) by subgroups.
RL: ringer lactate, SC: sodium chloride, DKA: diabetic ketoacidosis SAPS: Simplified Acute Physiology Score, BE: bas excess.
Legends: Comparison of subgroups according to age (< versus ≥25 years), diabetes kind (type 1 vs 2 vs inaugural), Severity of DKA (assessed by baseline pH: <7.20 vs >7.20), and severity illness (assessed by SAPS II severity score <18 vs >18) showed a similarity in the effect of RL and SC for the studied endpoints. However, there was a trend towards significance of SC superiority on the change in base excess ≥ −3 meq/l at H48 for type 2 diabetes strata (OR = 1.39 [0.88–6.45]) and SAPS II > 18 (OR = 1.40 [0.9–6.2]).
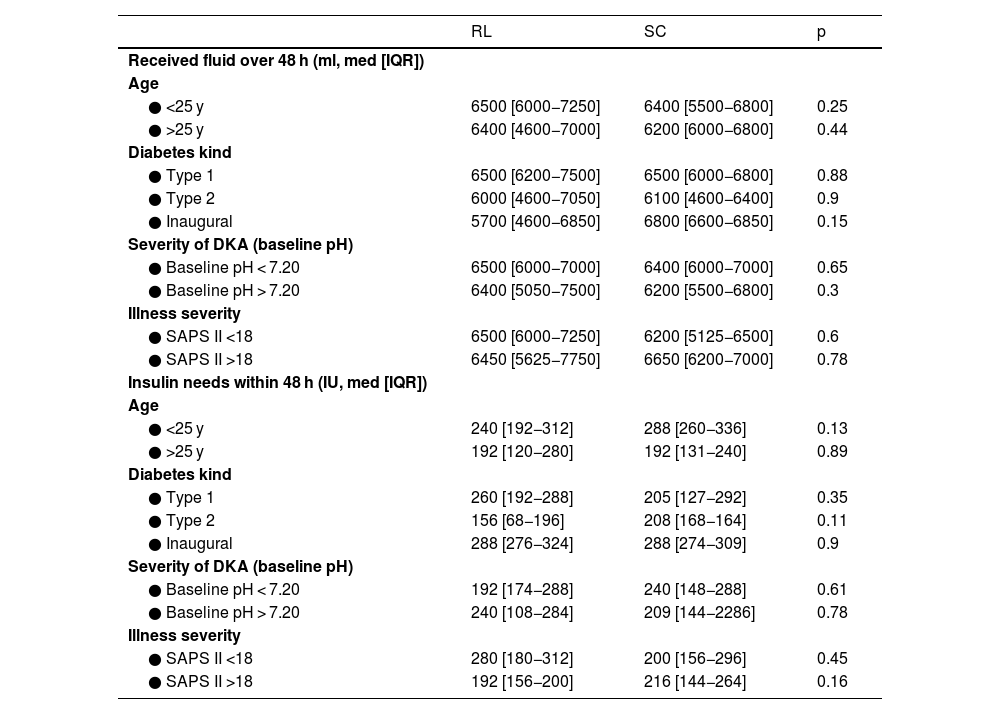
Analysis of outcomes (received fluid and insulin needs over 48 h) by subgroups.
| RL | SC | p | |
|---|---|---|---|
| Received fluid over 48 h (ml, med [IQR]) | |||
| Age | |||
| ● <25 y | 6500 [6000−7250] | 6400 [5500−6800] | 0.25 |
| ● >25 y | 6400 [4600−7000] | 6200 [6000−6800] | 0.44 |
| Diabetes kind | |||
| ● Type 1 | 6500 [6200−7500] | 6500 [6000−6800] | 0.88 |
| ● Type 2 | 6000 [4600−7050] | 6100 [4600−6400] | 0.9 |
| ● Inaugural | 5700 [4600−6850] | 6800 [6600−6850] | 0.15 |
| Severity of DKA (baseline pH) | |||
| ● Baseline pH < 7.20 | 6500 [6000−7000] | 6400 [6000−7000] | 0.65 |
| ● Baseline pH > 7.20 | 6400 [5050−7500] | 6200 [5500−6800] | 0.3 |
| Illness severity | |||
| ● SAPS II <18 | 6500 [6000−7250] | 6200 [5125−6500] | 0.6 |
| ● SAPS II >18 | 6450 [5625−7750] | 6650 [6200−7000] | 0.78 |
| Insulin needs within 48 h (IU, med [IQR]) | |||
| Age | |||
| ● <25 y | 240 [192−312] | 288 [260−336] | 0.13 |
| ● >25 y | 192 [120−280] | 192 [131−240] | 0.89 |
| Diabetes kind | |||
| ● Type 1 | 260 [192−288] | 205 [127−292] | 0.35 |
| ● Type 2 | 156 [68−196] | 208 [168−164] | 0.11 |
| ● Inaugural | 288 [276−324] | 288 [274−309] | 0.9 |
| Severity of DKA (baseline pH) | |||
| ● Baseline pH < 7.20 | 192 [174−288] | 240 [148−288] | 0.61 |
| ● Baseline pH > 7.20 | 240 [108−284] | 209 [144−2286] | 0.78 |
| Illness severity | |||
| ● SAPS II <18 | 280 [180−312] | 200 [156−296] | 0.45 |
| ● SAPS II >18 | 192 [156−200] | 216 [144−264] | 0.16 |
RL: ringer lactate, SC: serum chloride, DKA: diabetic ketoacidosis, SAPS: Simplified Acute Physiology Score.
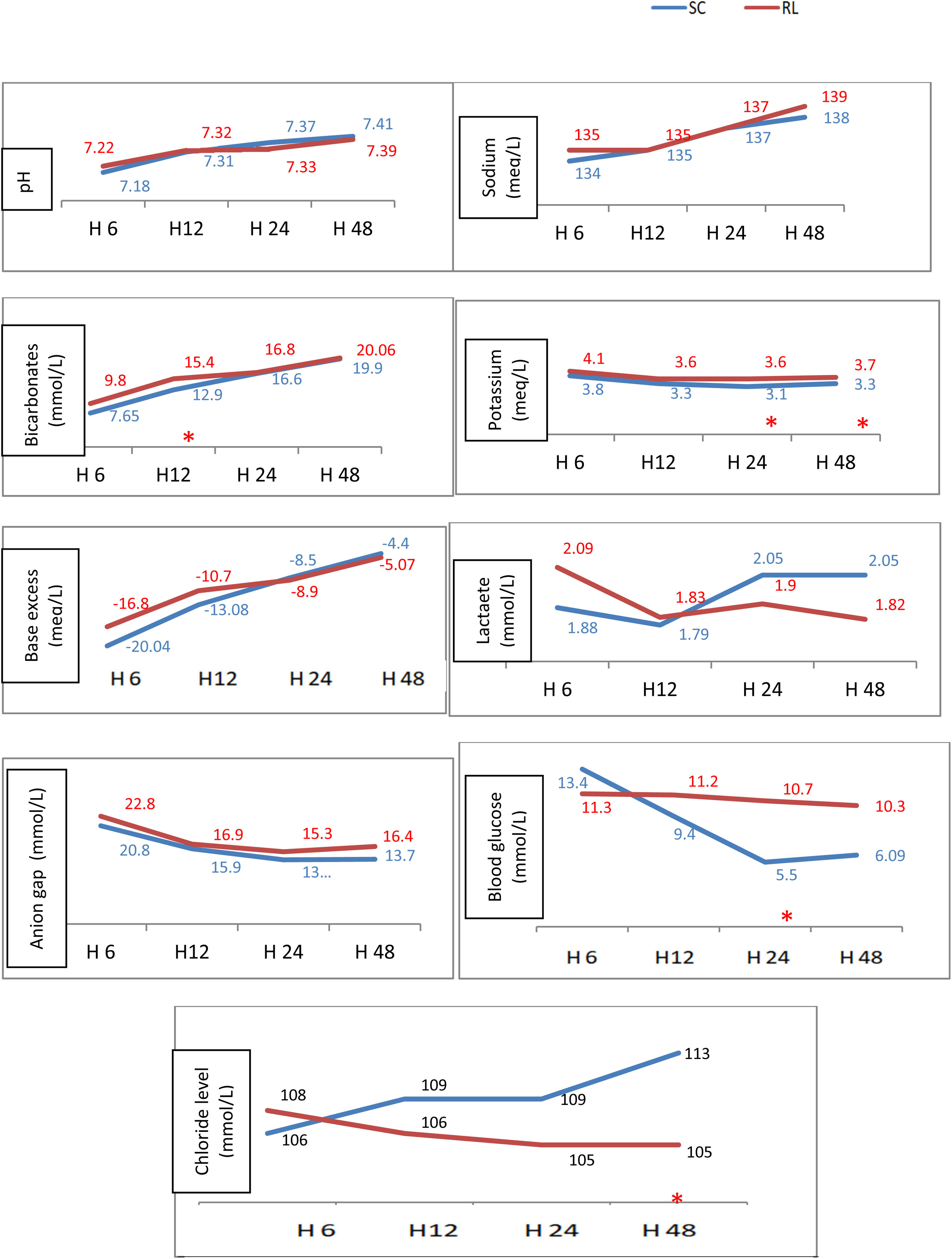
The biomarkers monitoring over the 4-study times showed that in RL group: bicarbonate (mmol/l) was higher at H12 (154 vs 129), serum potassium (mmol/l) was higher closer to normal at H24 (3.6 vs 3.1) and H48 (3.7 vs 3.3) and serum chloride was lower at H48 (105 vs 113 mmol//l). Note that serum lactate did not change during the 4 monitoring times. The variation of different biomarker medians is shown in Fig. 3 and the detailed presentation of trends in key biomarkers is illustrated in box plots in the additional graphs in the Supplementary material 2.
Variation of bio markers throughout the 4 study times.
RL: ringer lactate, SC: Sodium Chloride, *: significant difference.
Legends: The monitoring over the 4 study times (H6, H12, H24, and H48) of acid-base parameters (pH, bicarbonates, base excess), electrolytes (sodium, potassium, chloride, anion gap), blood glucose, and lactate levels showed that in the RL group: bicarbonate improved better at H12 (p = 0.05), potassium was higher at H24 (p = 0.008) and H48 (0.041), lower serum chloride at H48 (p = 0.002) and higher blood sugar at H24.
Our present trial including a double cohort of 88 ICU patients with similar baseline characteristicsdid not reach statistical significancein theresolution of DKA when treated with RL compared with 0.9% SC as fluid therapy. Nevertheless, RL administration was superior in terms of potassium level which was better at H24 and H48 and a lower chloride level at H48 suggesting better control of acidemia. The subgroups analysis according to age, diabetes kind, DKA severity, and SAPS II not found any difference.
Fluid resuscitation is a crucial step in managing DKA because hyperglycemia induces osmotic diuresis, resulting in significant fluid and electrolyte losses. Fluid resuscitation aims to reverse these detrimental effects with replenishing intravascular volume and improving tissue perfusion and oxygen delivery. It also dilutes the concentration of glucose and ketones in the blood, helping to correct hyperglycemia, acidosis and electrolyte disorders.19 Initially, isotonic saline is favored to reestablish circulation but expose to the risk of hyperchloremia associated with cerebral edema.19 The comparison between balanced solutions and serum chloride in terms of efficacy in treating DKA was subject to multiple analyses and incorporating various populations and fixing a several outcome measures.
Our results adhere to those of several studies in this topic. There was no significant difference between balanced crystalloids and normal saline group for the time to resolution of DKA (MD = −1.49, 95%CI: −4.29 to 1.31, p = 0.30, I2 = 65%).12 Similarly, Gupta et al. in their recent updated systematic review and meta-analysis didn’t observe a significant difference between balanced electrolyte solution and normal saline in the time to DKA resolution (MD: −1.63; 95% CI: −7.66 to 4.41; p = 0.60) or length of hospital stay (mean difference (MD): −0.07; 95% CI: −0.44 to 0.31; p = 0.73).13 Tamzil et al.15 not reported, also, in their meta-analysis (8 RCTs, 595 patients) any difference in the duration of DKA resolution. (MD: −4.73, 95%CI −2.72 to 4.92; I2 = 92%; P = 0.180). The time to reach overall DKA endpoints was comparable in both groups in the systematic review of clinical trials of Jahangir et al.20 The absence of effect on insulin intake and mortality was also reported in the meta-analysis of Szabó et al.14 (MD: 0.16 [−3.03, 3.35] h) and (OR: −0.67 [0.12, 3.68] respectively). No difference was also demonstrated on the ICU stay or mortality in a second report.15
Conversely, another relevant meta-analysis (7 RCTs and 3 observational studies with 1006 participants) showed superiority for balanced solutions which resolved DKA faster than 0.9% saline with a mean difference (MD) of −5.36 [95% CI: −10.46, −0.26] h.14 Considering the evidence from pooled small randomized trials with moderate overall certainty of evidence, the use of balanced electrolytes solutions in DKA was associated with faster rates of DKA resolution compared to SC in the systematic review of Catahay et al.21 that showed a pooled hazard ratio at 1.46 [1.10–1.94] (p = 0.009) with 12% heterogeneity while MD was −3.02 (95% CI −6.78 to 0.74; p = 0.12) with heterogeneity of 85%.
Concerning the change in key biomarkers, our main findings are summarized in the better serum potassium levels, less hyperchloremia and better bicarbonate levels at H12 without risking hyperlactatemia with the administration of RL. Our findings go in the trends of the main recent meta-analyses.12–15 There was a significant reduction in the post-resuscitation chloride (MD −3.16, 95%CI −5.82 to −0.49, p = 0.02, I2 = 73%) among patients received balanced crystalloids.12 Balanced solutions resulted in significantly higher post-resuscitation bicarbonate levels (MD: 1.63; 95% CI: 0.86–2.39; p < 0.001) and lower post-resuscitation chloride levels (MD: −2.37; 95 % CI: −3.56 to −1.19; p < 0.001).13 Post-resuscitation chloride (MD: −4.26 [−6.97, −1.54] mmol/l) and sodium (MD: −1.38 [−2.14, −0.62] mmol/l) levels were significantly lower. In contrast, levels of post-resuscitation bicarbonate (MD: 1.82 [0.75, 2.89] mmol/l) were significantly elevated in the balanced solutions-group compared to the saline-group.14 There was a significantly lower post resuscitation chloride concentration in the balanced electrolytes solutions (MD = 2.96, 95%CI −4.86 to −1.06; I2 = 59 %; p = 0.002) and a reduction in duration for normalization of bicarbonate (MD = 3.11, 95% CI −3.98 to 2.23; I2 = 5%; p = 0.0004).15 Aditianingsih et al.22 in their randomized, single blind controlled trial showed that fluid resuscitation of DKA patients with RL resulted in slightly but not significantly higher baseexcess and anion gap than normal saline. Thus, balanced electrolyte solution as an alternative fluid resuscitation may prevents hyperchloremic acidosis in diabetic ketoacidosis patients. In our series, despite pH didn’t differ, chloremia was reduced by 8 mmol/l at average after 48 H of managing DKA with RL.
The effect of balanced crystalloids was more beneficial in the largest American trial designed as secondary analysis of cluster trials (n = 172, 94 were assigned to balanced crystalloids and 78 to saline).23 Indeed, the time to DKA resolution in the balanced crystalloids group was shorter by 4 h on average.23 Also, the same investigators revealed shorter time to insulin infusion discontinuation in the balanced crystalloids group (9.8 versus 13.4 h as medians; p = 0.03).23 The Phase 2 trial of Ramanan et al.18 found that plasmalyte (PL) resulted in faster resolution of metabolic acidosis without increasing ketosis (at 24H, DKA resolution occurred in 69% (PL) and 36% (SC) of patients; OR = 4.24, 95% CI [1.68–10.72], p = 0.002). Also, the length of ICU stay was slightly shorter for the PL group (49 h vs 55 h).18 The authors recommend confirming this hypothesis in a larger phase 3 trial.
In our series, the taken fluid volume and the cumulative received dose of insulin within 48 h (IU) were less in the RL group but without reaching statistical significance (respectively 6200 ml vs 6440 ml, p = 0.36 and 205 vs 225 IU, p = 0.2). In the Emergency department with a different primary outcome which was the rate of ICU admission, Attokaran et al.24 showed a higher rate in the SC group (50% vs 39.1%). However,this differencewas not found after adjustment for pH at presentation and diabetes type (p = 0.71).
Additionally, we found that serum potassium was higher with RL without exceeding the standards while it was low with SC at H24 and H48. Conversely, in the above cited meta-analysis of Liu et al.,12 balanced crystalloids as compared to normal saline has no effect on the incidence of hypokalemia. This result can be explained by the type of balance crystalloid used, and therefore the potassium content, which differed from one trial to another.We indicate here that the RL we used provided 0.40 g per 1000 ml of solution.
The result we showed about the better bicarbonate level at H12 in the RL group (15.4 vs 12.9 mmol/l, p < 0.005) was similar to the result of the Australian multicenter retrospective examination of patients admitted to ICU for DKA.25 In this study, PL group experienced faster metabolic acidosis resolution (median serum bicarbonate correction was higher in the PL vs NS groups at 4–6 h (8.4 vs 1.7 mmol/l) and 6–12 h (12.8 vs 6.2 mmol/l) from baseline (p < 0.05), reduced hyperchloremia, and improved blood pressure and urine output.25 The authors also reported a result that has not been highlighted in our trial concerning the change in base excess (median standard base excess improved by 10.5 vs 4.2 meq/l at 4–6 h and by 16.0 vs 9.1 meq/l at 6–12 h in the PL and NS groups, respectively (p < 0.05).25
Our results about the possible favorable effect on preventing hyperchloremia and hypokalemia should be of particular interest given the harm of these disorders as found in the meta-analysis of Maharjan et al.26 In fact, hyperchloremia was associated with longer in-hospital length of stay and longer time to resolution of DKA and potassium replacement at < 10 mmol/l was associated with higher mortality.26 Hyperchloremic acidosis also has important clinical implications. Indeed, the compensation of acidosis by tachypnea can lead to respiratory muscle fatigue and, if not appropriatelymanaged, respiratory arrest. That include also the risk of arrhythmias, which can increase mortality if left untreated. A pH below 7.20 can increase the risk of ventricular fibrillation and heart failure by impairing myocardial contraction. This last complication is strengthened by associated hypokalemia as potassium plays a crucial role in regulating cardiac electrical activity. Hypokalemia, grafted on hyperchloremic acidosis, can lead to cardiac membrane potential alterations and repolarization delay, predisposing to atrial fibrillation, and potentially life-threatening ventricular tachycardia or fibrillation. Regarding the topic of diabetic decompensation, hypokalemia can reduce insulin receptor sensitivity, leading to insulin resistance and potentially contributing to the delay in correcting hyperglycemia.27 This is what is being tried to prevent, among other things, by balanced electrolyte solutions.
Finally, we can conclude that in adult patients with DKA, balanced solutions may be superior in preventing hyperchloremia and hypokaliemia compared to 0.9% SC. The clinical recommendation that can be raised is as follows: the use of balanced electrolyte solutions should be considered as the preferred resuscitation fluid in the management of DKA given their lower predisposition to cause hyperchloremic metabolic acidosis and hypokalimia. Nevertheless, we estimated that the unavailability of serum ketone assay, the blinding deficiency (no concealment of group assignment), the restricted sample size, and the absence of renal function assessment were the weaknesses in this trial. Thus, we propose future research directions to address these identified limitations and to reach statistical significance for the hypothesis.
CRediT authorship contribution statementAhlem Trifi: Conceptualization, Methodology, Analysis, Software, Writing – original draft, Supervision, Software, Validation. Ikram Ben Braik: Data curation, Investigation. Hounaida Galai: Data curation, Investigation. Noussair Azzouz: Data curation, Investigation. Badis Tlili: Data curation, Investigation. Asma Mahdi: Visualization, Investigation. Linda Messaoud: Data curation. Eya Seghir: Visualization, Investigation. Asma Ouhibi: Data curation. Sami Abdellatif: Writing – review & editing, Validation.
Declaration of Generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the author(s) used ChatGPT in order to translate the abstract into Spanish. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Not applicable.





![Analysis of Outcomes (DKA resolution of at H48 and Change in base excess ≥ −3 meq/l at H48) by subgroups. RL: ringer lactate, SC: sodium chloride, DKA: diabetic ketoacidosis SAPS: Simplified Acute Physiology Score, BE: bas excess. Legends: Comparison of subgroups according to age (< versus ≥25 years), diabetes kind (type 1 vs 2 vs inaugural), Severity of DKA (assessed by baseline pH: <7.20 vs >7.20), and severity illness (assessed by SAPS II severity score <18 vs >18) showed a similarity in the effect of RL and SC for the studied endpoints. However, there was a trend towards significance of SC superiority on the change in base excess ≥ −3 meq/l at H48 for type 2 diabetes strata (OR = 1.39 [0.88–6.45]) and SAPS II > 18 (OR = 1.40 [0.9–6.2]). Analysis of Outcomes (DKA resolution of at H48 and Change in base excess ≥ −3 meq/l at H48) by subgroups. RL: ringer lactate, SC: sodium chloride, DKA: diabetic ketoacidosis SAPS: Simplified Acute Physiology Score, BE: bas excess. Legends: Comparison of subgroups according to age (< versus ≥25 years), diabetes kind (type 1 vs 2 vs inaugural), Severity of DKA (assessed by baseline pH: <7.20 vs >7.20), and severity illness (assessed by SAPS II severity score <18 vs >18) showed a similarity in the effect of RL and SC for the studied endpoints. However, there was a trend towards significance of SC superiority on the change in base excess ≥ −3 meq/l at H48 for type 2 diabetes strata (OR = 1.39 [0.88–6.45]) and SAPS II > 18 (OR = 1.40 [0.9–6.2]).](https://static.elsevier.es/multimedia/21735727/unassign/S2173572725000852/v1_202504150419/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


