To determine the prevalence of persistent COVID-19 symptoms in SARS-CoV-2 patients 15 months after ICU discharge,their impact on physical, psychological, and neurocognitive domains, and the burden on primary caregivers.
DesignDescriptive, ambispective observational study.
SettingIntensive Care Unit from a tertiary-level hospital.
PatientsSARS-CoV-2 patients discharged from ICU.
Main variables of interestdemographics and hospitalization data. Questionnaires assesing persistent COVID symptoms, functional tests (6-Minute Walk Test), anxiety (Beck Anxiety Inventory), PTSD and Zarit Caregiver Burden scales. Statistical analysis was performed using Stata for Mac, version 14.2.
Results85 patients were evaluated, with a median age of 60.3 years (IQR 54.0–68.9), 70.6% males. A high percentage of patients reported musculoskeletal disorders such as arthralgia (44.7%) and myalgia (38.2%), cognitive impairments (52.9%), sleep disturbances (34.1%), asthenia (44.5%) and anxiety (34.5%). The overall BAI score was 2 (0–9), with paraesthesia being the most common symptom. Additionally, 29.4% of patients reported “fear of the worst”, 35% had unpleasant or recurrent memories of their ICU stay, and 16.4% were unable to relax (moderate/severe degree). Interviews with primary caregivers revealed that 22.2% reported caregiving as a significant burden.
Conclusionspersistent COVID affects three primary functional domains: physical, cognitive and psychological, as well as on primary caregivers concerns and burdens.
Determinar la prevalencia de síntomas persistentes de COVID en pacientes con SARS-CoV-2, 15 meses tras el alta de UCI, su impacto en los dominios físico, psicológico y neurocognitivo, y la carga en los cuidadores principales.
DiseñoEstudio observacional descriptivo, ambispectivo.
ÁmbitoUnidad de Cuidados Intensivos de un hospital de tercer nivel.
PacientesPacientes con SARS-CoV-2 dados de alta de UCI.
Variables de interés principalesDatos demográficos y de hospitalización. Se aplicaron cuestionarios de síntomas persistentes de COVID, pruebas funcionales (Test de la Marcha de 6 Minutos), ansiedad (Inventario de Ansiedad de Beck (IAB)), Escala de TEPT y de Zarit. Análisis estadístico mediante Stata para Mac, versión 14.2.
ResultadosSe valoraron 85 pacientes, mediana de edad 60,3 años (RIC 54,0-68,9) y 70,6% hombres. Alto porcentaje de pacientes refirió trastornos musculoesqueléticos, artralgias (44,7%) y mialgias (38,2%), alteraciones cognitivas (52,9%), trastornos del sueño (34,1%), astenia (44,5%) y ansiedad (34,5%). La puntuación global en el IAB fue de 2 (0-9), siendo la parestesia el síntoma más común. El 29,4% de los pacientes manifestó temor a lo peor, el 35% recuerdos desagradables o recurrentes de su estancia en la UCI y el 16,4% no poder relajarse (grado moderado/severo). El 22,2% de los cuidadores refirió el cuidado como una carga significativa.
ConclusionesEl COVID prolongado afecta a tres dominios funcionales principales: físico, cognitivo y psicológico, así como a las preocupaciones y cargas de los cuidadores principales.
Post-Intensive Care Syndrome (PICS) is an escalating concern that affects up to 80% of patients following an ICU stay due to severe illness. This syndrome is characterized by clinical manifestations across three primary domains: physical, neurological, and cognitive. These symptoms can persist for months, or even up to a year, significantly diminishing patients' quality of life.1–3 The disease caused by SARS-COV-2 has led to considerable short- and long-term sequelae, commonly referred to as post-COVID syndrome or long COVID. These sequelae continue after hospital discharge4–6 and may coincide with, exacerbate, or overlap with symptoms of PICS. While the long-term effects of coronavirus disease have been extensively studied in recent years, few investigations have specifically focused on the persistence of these sequelae in ICU-treated patients7 and their impact on the three key domains: physical, neurological, and cognitive.
The aim of this study was to assess the prevalence of persistent COVID-19 symptoms in a cohort of SARS-CoV-2 patients fifteen months after ICU discharge and to evaluate their effects on the physical, psychological, and neurocognitive domains, as well as the burden placed on their primary caregivers
Patients and methodsStudy Design: This ambispective observational study was conducted on a cohort of patients who were evaluated at a post-COVID clinic 15 months after their discharge from the ICU due to severe COVID-19 respiratory infection. The research was carried out between August 2020 and April 2021 at a tertiary-level hospital with 31 ICU beds during the pandemic. Inclusion Criteria: Patients over 18 years old who required invasive mechanical ventilation (IMV) or non-invasive mechanical ventilation (NIMV) for more than 5 days were included. Patients who required ventilation for more than 4 days were also eligible if they had comorbidities such as morbid obesity, chronic obstructive pulmonary disease (COPD), or ischemic heart disease. Individuals unable to participate in the interview, either in person or by phone, due to cognitive or functional impairment were excluded from the study.
Methodology: Patients who met the inclusion criteria were contacted by phone, and the study objectives were explained. Those who agreed to participate were scheduled for an in-person post-ICU consultation, where a comprehensive questionnaire was administered. This questionnaire included sections on anamnesis to evaluate dysautonomia, neurological and musculoskeletal manifestations, and motor functional capacity. Symptoms were collected using a checklist and rated on a dichotomous scale (yes-no). Anxiety levels were assessed using the Beck Anxiety Inventory (BAI),8 which classifies anxiety into the following categories: 0−7 (normal), 8−15 (mild), 16−25 (moderate), and 26−63 (severe). Post-traumatic stress was evaluated using the PTSD Symptom Severity Scale,9 which measures three dimensions: re-experiencing, avoidance, and hyperarousal. Physical capacity and functional status were assessed using the 6-Minute Walk Test,10 recording heart rate, transcutaneous oxygen saturation, and dyspnea and fatigue using the Borg scale. Balance and fall risk were evaluated with the Timed Up and Go (TUG) test,11 performed twice and recording the best result: <10 s (low fall risk), 10−20 s (frailty), and >20 s (high fall risk). For patients residing outside the province, a telephone survey was conducted. Primary caregivers were also assessed using the Zarit Caregiver Burden Scale,12 either in person or by phone. Retrospective data on demographic, analytical, epidemiological, and clinical variables were collected from the electronic medical record. This included information on administered medications (corticosteroids, vasopressors, muscle relaxants, hypnotics, analgesics), the presence of delirium or agitation, and the duration of mechanical ventilation. Delirium was assessed based on the primary nurse’s observations of agitation, hallucinations, or dangerous behavior. The descriptive analysis was performed using Stata 14.2 software for Mac. Quantitative variables are expressed as means and standard deviations (SD) or medians and interquartile ranges (IQR), as appropriate. Qualitative variables were reported as frequencies and percentages. The study was approved by the Drug Research Ethics Committee of the General University Hospital of Castellon (Castellon, Spain) and conducted in compliance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all study participants.
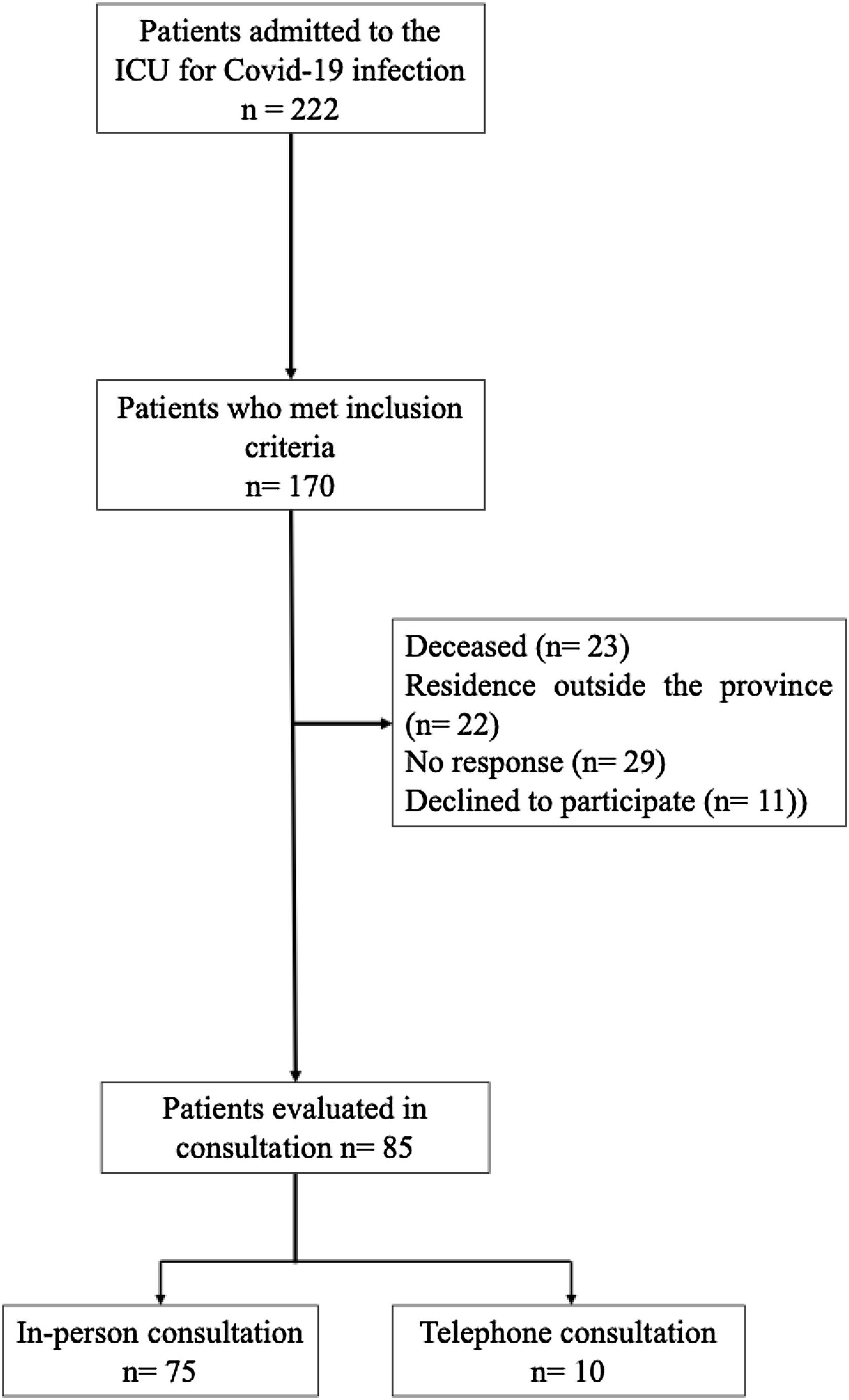
ResultsFrom August 2020 to March 31, 2021, a total of 222 patients were admitted to the ICU. Of these, 170 patients (76.6%) met the inclusion criteria for clinical evaluation in the post-ICU follow-up consultation. However, 23 patients (13.5%) had died before the evaluation, 22 patients (12.9%) resided outside the province, 29 patients (17.05%) did not respond to phone calls, and 11 patients (6.5%) declined to participate. Finally 85 patients were included in the study, with 75 (88.23%) attending the in-person consultation and 10 being evaluated by phone. A flow diagram illustrating the study population is presented in Fig. 1.
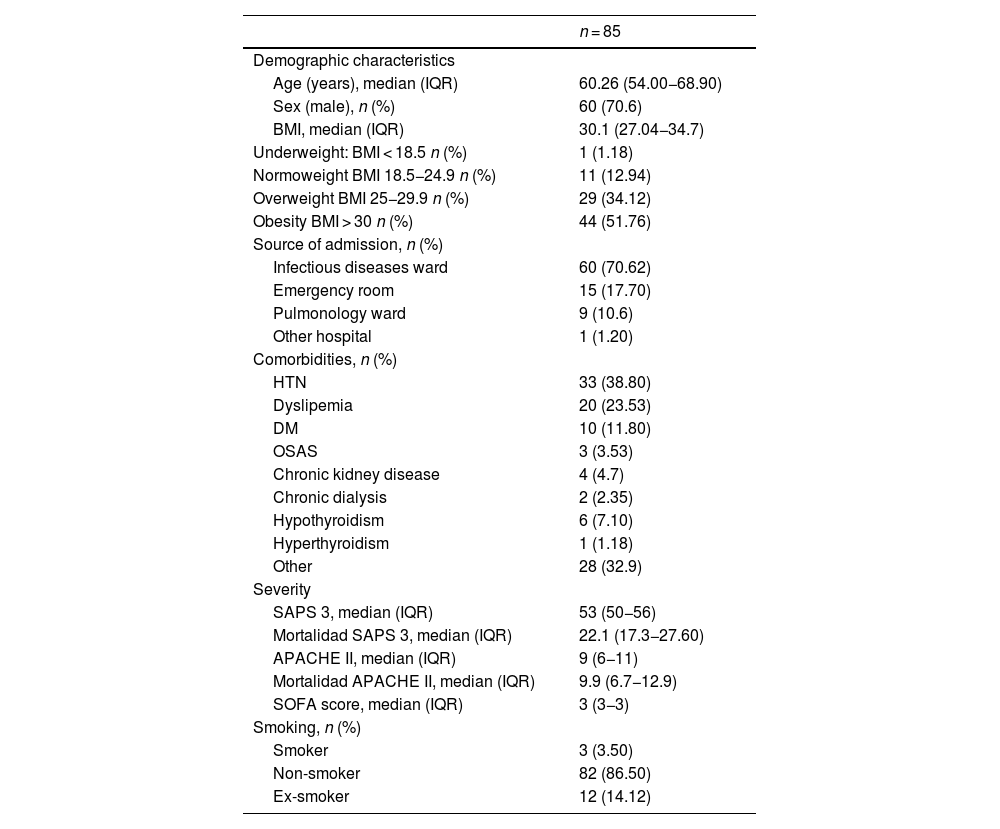
The median age of the participants was 60.26 years (IQR 54.0–68.9), with 70.60% (60 patients) being men. The majority of patients (70.62%) were admitted to the ICU from the infectious diseases ward, while 17.7% came from the emergency department. Hypertension was the most common comorbidity, affecting 38.80% of patients, followed by dyslipidemia (23.53%), diabetes mellitus (11.82%), and hypothyroidism (7.10%). Additionally, 3.5% of patients were active smokers, and 14.12% were former smokers. The median body mass index (BMI) was 30.1 kg/m2 (27.04-34.7), with 51.76% classified as having class I obesity and 34.12% as overweight. The severity index measured by the SAPS 3 score was 53 points (50−56), indicating a predicted mortality rate of 22.1% (17.3−27.6%). The median SOFA score punctuation was 3 points (3–3), and 14.12% of patients required vasopressor support. Details of the sociodemographic characteristics and severity indices of the population are provided in Table 1.
Sociodemographic characteristics and severity indices.
| n = 85 | |
|---|---|
| Demographic characteristics | |
| Age (years), median (IQR) | 60.26 (54.00−68.90) |
| Sex (male), n (%) | 60 (70.6) |
| BMI, median (IQR) | 30.1 (27.04−34.7) |
| Underweight: BMI < 18.5 n (%) | 1 (1.18) |
| Normoweight BMI 18.5−24.9 n (%) | 11 (12.94) |
| Overweight BMI 25−29.9 n (%) | 29 (34.12) |
| Obesity BMI > 30 n (%) | 44 (51.76) |
| Source of admission, n (%) | |
| Infectious diseases ward | 60 (70.62) |
| Emergency room | 15 (17.70) |
| Pulmonology ward | 9 (10.6) |
| Other hospital | 1 (1.20) |
| Comorbidities, n (%) | |
| HTN | 33 (38.80) |
| Dyslipemia | 20 (23.53) |
| DM | 10 (11.80) |
| OSAS | 3 (3.53) |
| Chronic kidney disease | 4 (4.7) |
| Chronic dialysis | 2 (2.35) |
| Hypothyroidism | 6 (7.10) |
| Hyperthyroidism | 1 (1.18) |
| Other | 28 (32.9) |
| Severity | |
| SAPS 3, median (IQR) | 53 (50−56) |
| Mortalidad SAPS 3, median (IQR) | 22.1 (17.3−27.60) |
| APACHE II, median (IQR) | 9 (6−11) |
| Mortalidad APACHE II, median (IQR) | 9.9 (6.7−12.9) |
| SOFA score, median (IQR) | 3 (3−3) |
| Smoking, n (%) | |
| Smoker | 3 (3.50) |
| Non-smoker | 82 (86.50) |
| Ex-smoker | 12 (14.12) |
Abbreviations: IQRinterquartile range; BMIBody Mass Index; HTNHypertension; DMDiabetes Mellitus; OSASObstructive Sleep Apnea Syndrome; SAPS 3Simplified Acute Physiology Score; APACHE IIAcute Physiology and Chronic Health Evaluation; SOFASepsis-related Organ Failure Assessment.
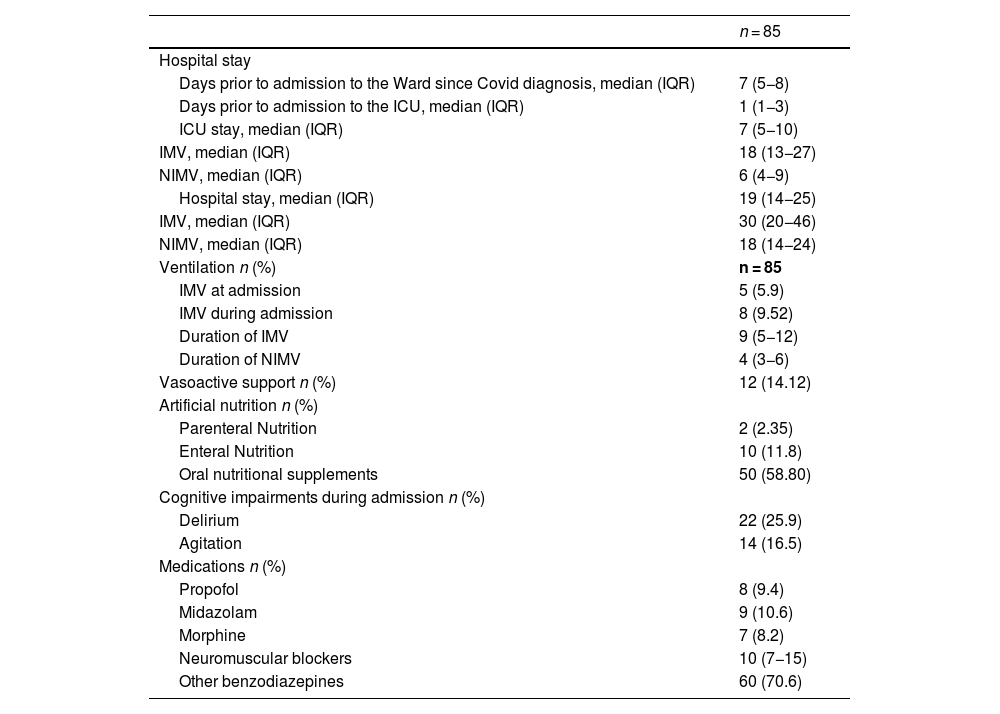
The median time from symptom onset to hospital admission and hospital stay before ICU admission were 7 (5–8) and 1 day (1–3) respectively. The median ICU stay was 7 days (5–10), and the total hospital stay 19 days (14–25).
According to hospital protocol, all patients received an initial regimen of 3 days of 250 mg of methylprednisolone (30 mg/kg/day), followed by a tapering dose of 1 mg/kg/day, with gradual reduction until discontinuation.
All patients on invasive mechanical ventilation (IMV) (15.42%) also received neuromuscular relaxants and hypnotics. The median duration of neuromuscular blockers was 10 days (IQR 7–15 days) and the median duration of IMV was 9 days (5–12). The rest of the patients (84.58%) were ventilated non-invasively using a helmet interface. None of the patients included in the study received high-flow nasal oxygen therapy as a non-invasive mechanical ventilation (NIMV) strategy during their hospital stay, nor after extubation in those managed with invasive mechanical ventilation (IMV).
Among those non-invasively ventilated, 31.1% experienced episodes of delirium or agitation during their stay. None of these patients received sedation with propofol, midazolam, or dexmedetomidine. Furthermore, 58.9% of patients were treated with benzodiazepines, while 4.1% required morphine infusion to facilitate adaptation to the ventilation and helmet interface. Regarding nutritional support, 72.95% of patients received some form of artificial nutrition during their hospital stay, with 58.8% receiving oral nutritional supplements, 11.8% receiving enteral nutrition, and 2.4% requiring parenteral nutrition. Clinical variables and treatments administered during the course of the illness are summarized in Table 2.
Hospital stay and treatments administered.
| n = 85 | |
|---|---|
| Hospital stay | |
| Days prior to admission to the Ward since Covid diagnosis, median (IQR) | 7 (5−8) |
| Days prior to admission to the ICU, median (IQR) | 1 (1−3) |
| ICU stay, median (IQR) | 7 (5−10) |
| IMV, median (IQR) | 18 (13−27) |
| NIMV, median (IQR) | 6 (4−9) |
| Hospital stay, median (IQR) | 19 (14−25) |
| IMV, median (IQR) | 30 (20−46) |
| NIMV, median (IQR) | 18 (14−24) |
| Ventilation n (%) | n = 85 |
| IMV at admission | 5 (5.9) |
| IMV during admission | 8 (9.52) |
| Duration of IMV | 9 (5−12) |
| Duration of NIMV | 4 (3−6) |
| Vasoactive support n (%) | 12 (14.12) |
| Artificial nutrition n (%) | |
| Parenteral Nutrition | 2 (2.35) |
| Enteral Nutrition | 10 (11.8) |
| Oral nutritional supplements | 50 (58.80) |
| Cognitive impairments during admission n (%) | |
| Delirium | 22 (25.9) |
| Agitation | 14 (16.5) |
| Medications n (%) | |
| Propofol | 8 (9.4) |
| Midazolam | 9 (10.6) |
| Morphine | 7 (8.2) |
| Neuromuscular blockers | 10 (7−15) |
| Other benzodiazepines | 60 (70.6) |
Abbreviations: IQR: Interquartile range; IMV: Invasive Mechanical Ventilation; NIMV: Non-Invasive Mechanical Ventilation.
The most common physical symptoms reported by patients in the post-ICU follow-up consultation were arthralgia (44.71%) and fatigue (43.53%). Notable neurological and cognitive symptoms included headaches (40%), cognitive impairments such as memory issues or "brain fog" (52.9%), anxiety (34.5%), sleep disturbances (34.1%), and gait instability (27.1%). In terms of mobility, 8.33% of patients required technical assistance to move, and 2.38% needed a wheelchair. Additionally, 5.95% required help with basic activities, such as personal hygiene. Table 3 shows the symptomatology and quality of life of patients at 15 months post-ICU discharge
Symptoms and quality of life at 15 months post-ICU discharge.
| n (%) | |
|---|---|
| Dysautonomia manifestations | |
| Exercise intolerance | 11 (12.94) |
| Chest discomfort | 5 (5.9) |
| Palpitations | 4 (4.71) |
| Dysautonomia | 5 (5.88) |
| Orthostatic tachycardia | 1 (1.2) |
| Neurologic manifestations | |
| Headache | 12 (40) |
| Asthenia | 8 (26.7) |
| Anosmia | 8 (9.4) |
| Ageusia/dysgeusia | 9 (10.5) |
| Cognitive impairments: memory disturbances, mental fog… | 45 (52.9) |
| Gait instability | 23 (27.1) |
| Stress | 27 (31.8) |
| Depression | 22 (26.5) |
| Anxiety | 29 (34.5) |
| Sleep disturbances | 29 (34.1) |
| Sexual dysfunction | 14 (16.5) |
| Musculoeskeletal manifestations | |
| Arthralgia | 38 (44.7) |
| Myalgia | 33 (38.2) |
| Muscle contractures | 25 (29.41) |
| Fatigue | 37 (43.53) |
| Muscle weakness | 33 (38.82) |
| Mobility (n = 84): | |
| 74 (88.1) |
| 1 (1.2) |
| 7 (8.33) |
| 2 (2.38) |
| Activities for daily living (n = 84): | |
| 79 (94.05) |
| 2 (2.38) |
| 3 (3.57) |
| Return to work (n = 84): | |
| 29 (42.65) |
| 5 (7.35) |
| 26 (38.24) |
| 8 (11.76) |
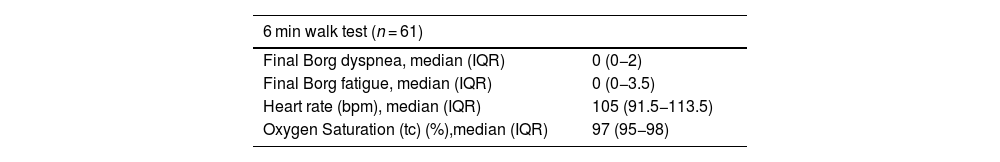
Out of the 75 in-person visits, 61 patients completed the six-minute walk test. Ten patients were not tested due to reduced mobility, while the remaining four, who walked to the appointment, did not undergo the additional test. The test was completed without interruption in any case. At the conclusion of the test, the median Borg scale scores for dyspnea and fatigue were 0 points (0−2) and 0 points (0−3.5), respectively. In the TUG test, 11.5% (7 patients) scored above 14 s, indicating frailty and risk of falls (Table 4).
Six minute walk test and Timed Up and Go test.
| 6 min walk test (n = 61) | |
|---|---|
| Final Borg dyspnea, median (IQR) | 0 (0−2) |
| Final Borg fatigue, median (IQR) | 0 (0−3.5) |
| Heart rate (bpm), median (IQR) | 105 (91.5−113.5) |
| Oxygen Saturation (tc) (%),median (IQR) | 97 (95−98) |
| Timed up and go test | N (%) |
|---|---|
| < 10 s | 54 (88.5) |
| < 20 s | 3 (4.9) |
| < 30 s | 4 (6.6) |
Abbreviations: IQR: Interquartile range.
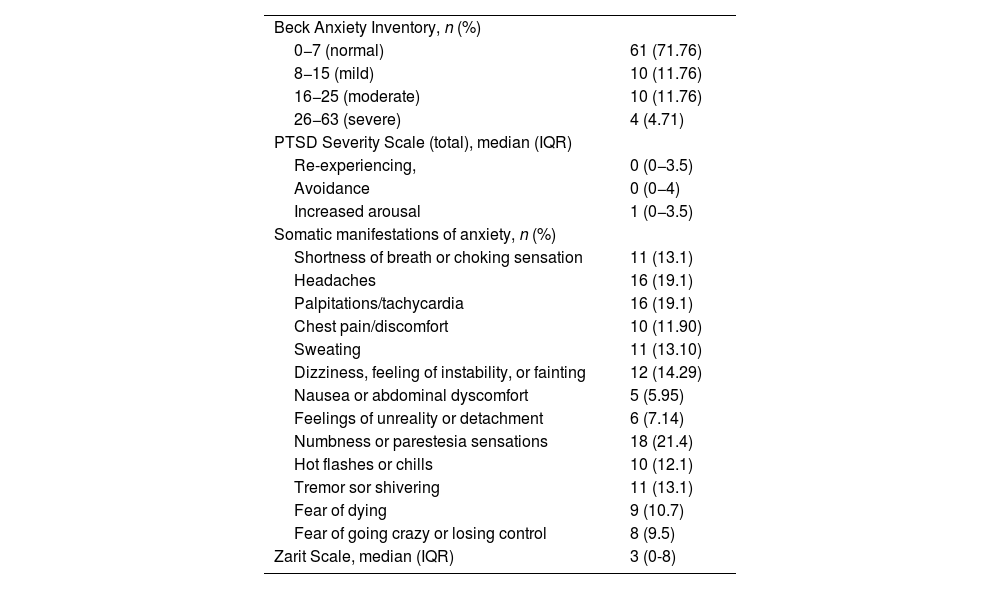
According to the Beck Anxiety Inventory (BAI), 28.23% of patients reported some level of anxiety, with 11.76% experiencing mild to moderate anxiety and 4.71% experiencing severe anxiety. Overall, 32.47% reported feeling nervous to varying degrees during the consultation, with 18.18% experiencing mild fear and 9.09% expressing moderate fear of dying, although the intensity was moderate. Additionally, 14.29% felt mild discomfort, and 10.29% were concerned about losing control. The Beck questionnaire indicated that 14.29% of patients experienced moderate clumsiness or numbness, 11.69% reported mild flushing, and 12.99% had mild difficulty relaxing. According to the BAI, 9.09% expressed severe fear of the worst happening, while 18.18% felt mildly unstable, and 9.09% and 5.19% experienced moderate and severe fear, respectively (Table 5).
Assessment of the Beck Anxiety Inventory, PTSD, and Somatic Symptoms Related to Anxiety.
| Beck Anxiety Inventory, n (%) | |
| 0−7 (normal) | 61 (71.76) |
| 8−15 (mild) | 10 (11.76) |
| 16−25 (moderate) | 10 (11.76) |
| 26−63 (severe) | 4 (4.71) |
| PTSD Severity Scale (total), median (IQR) | |
| Re-experiencing, | 0 (0−3.5) |
| Avoidance | 0 (0−4) |
| Increased arousal | 1 (0−3.5) |
| Somatic manifestations of anxiety, n (%) | |
| Shortness of breath or choking sensation | 11 (13.1) |
| Headaches | 16 (19.1) |
| Palpitations/tachycardia | 16 (19.1) |
| Chest pain/discomfort | 10 (11.90) |
| Sweating | 11 (13.10) |
| Dizziness, feeling of instability, or fainting | 12 (14.29) |
| Nausea or abdominal dyscomfort | 5 (5.95) |
| Feelings of unreality or detachment | 6 (7.14) |
| Numbness or parestesia sensations | 18 (21.4) |
| Hot flashes or chills | 10 (12.1) |
| Tremor sor shivering | 11 (13.1) |
| Fear of dying | 9 (10.7) |
| Fear of going crazy or losing control | 8 (9.5) |
| Zarit Scale, median (IQR) | 3 (0-8) |
Abbreviations: PTSD: Post-traumatic stress; IQR: Interquartile range.
On the PTSD Symptom Severity Scale, 6.49% of patients reported experiencing intense psychological distress or physiological reactivity to stimuli related to their ICU admission several times a week. The median score in the re-experiencing section was 1.5 points (0−4). In the avoidance section, 11.69% reported difficulty remembering important aspects of their ICU stay, occurring 2–4 times a week. Furthermore, 12.99% indicated a significant loss of interest in meaningful activities, and 7.79% experienced a decrease in emotional response. The median score in this avoidance section was 3 (0–6). In the hyperarousal section, 14.47% of patients reported difficulty falling asleep 2–4 times a week, while 9.09% experienced irritability or angry outbursts with the same frequency. Additionally, 7.79% had difficulty concentrating more than 5 times a week, and 15.58% were overly alert or easily startled 2–4 times a week. The median score in the hyperarousal section was 2 (0–5). Regarding somatic symptoms evaluated in the PTSD questionnaire, the most frequently reported were headaches and palpitations (19.1%) and sensations of paresthesia (21.4%) (Table 5).
Finally, on the Zarit scale for primary caregivers, 10.39% reported persistent fear for their relative's future, and 22.2% indicated that they sometimes felt a significant burden. The overall score was 3 points (0−8) (Table 5).
DiscussionThis study investigates the presence of symptoms related to long COVID in a cohort of patients 15 months after ICU discharge, focusing on the occurrence of general, neurological, physical, and cardiovascular symptoms which overlap with those described in patients with PICS. Long COVID is a syndrome characterized by the persistence of COVID-19 symptoms for weeks or months following the initial infection, or by the emergence of new symptoms after a symptom-free interval (1–3). Patients discharged from the ICU often experience a decline in physical, neurological, and cognitive health, commonly referred to as post-intensive care syndrome (PICS). The effects of COVID-19 can compound or overlap with those associated with PICS, impacting the various domains that define this condition and significantly affecting patients’ quality of life, as well as their work and social interactions.13–16
To date, numerous studies have documented various manifestations of post-COVID syndrome.6,17,18 In our sample, the most prevalent musculoskeletal symptoms included arthralgia, fatigue, and muscle weakness. These findings align with previously published studies,6,17,19 which identifies fatigue, dyspnea, and muscle weakness as the most common physical symptoms reported. Furthermore, musculoskeletal issues appear to be more pronounced in COVID-19 patients compared to PICS patients without COVID.7,20 Both, fatigue and muscle weakness significantly impact patients’ functional capacity, diminishing their quality of life and hindering their ability to return to work.20 Few studies have specifically assessed functional capacity or physical limitations one year post-ICU discharge. The six-minute walk test, a validated measure for critically ill patients, has consistently demonstrated lower-than-expected values in individuals after ICU discharge.21 In a cohort of 45 patients, Daste et al.16 reported a dyspnea score of 3.5 out of 10 on the Borg scale and an oxygen saturation level of 97% following the six-minute walk test, conducted three months after ICU discharge. In our sample, the reported values for dyspnea and fatigue were low, likely due to the time elapsed since discharge and the fact that most of these patients did not experience risk factors such as invasive mechanical ventilation or the use of neuromuscular relaxants. Moreover, ICU survivors are at an increased risk of falls one year after discharge,16,21 a trend reflected in our study, where over 10% of patients scored more than 14 s on the Timed Up and Go (TUG) test.
Cognitive impairments, including delirium, memory loss, and executive function disorders, can persist for months after discharge, with many patients failing to achieve full recovery.16 While this study did not employ a specific scale to assess cognitive status, the reported symptoms of memory loss and "brain fog" indicate cognitive impairment. Some authors suggest that it remains unclear whether these impairments arise from the direct effects of the virus or as indirect sequelae of the viral illness.16 Psychiatric disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD) are prevalent in post-intensive care syndrome (PICS)3 and significantly impact quality of life after ICU stays, sometimes persisting for up to a year post-discharge. Anxiety affects between 32% and 40% of patients one year after discharge, making it one of the most common disorders associated with post-ICU syndrome.7,13 In our study, varying degrees of anxiety were observed in patients, as assessed by the Beck Anxiety Inventory, with headache, dizziness, and gait instability being the most frequently reported somatic manifestations.
PTSD symptoms persisted in 17% of our patients one year after ICU discharge. Although the severity scale indicated low overall scores in each section, 6.49% of patients reported experiencing intrusive memories related to their ICU stay, while 12.9% reported a loss of interest in significant activities. Additionally, between 7.79% and 14.47% experienced varying degrees of hyperactivity. Some researchers propose that confronting ICU-related stress, rather than merely preventing it, may alleviate symptoms, especially in patients receiving psychological support during their hospitalization.22 Several risk factors have been associated with the development of PICS and long COVID or post-COVID syndrome, with some linked directly to patient characteristics and others related to the necessity of ICU admission.3 A recent meta-analysis found that female sex, advanced age, high BMI, and active smoking are associated with an increased risk of developing long COVID.23 Comorbidities such as hypertension, diabetes mellitus, and dyslipidemia have been shown to correlate with both long COVID and PICS.3,13 In our sample, these three comorbidities were the most prevalent, consistent with the findings of Nanwani et al.7 They predispose individuals to chronic inflammatory states and vascular damage,24 potentially explaining their role in prolonging symptoms and complicating full recovery. Although no statistically significant differences were identified between levels of obesity and symptoms, most patients in our study had class I obesity or were overweight. This may relate to the shared characteristic of obesity, long COVID, and PICS, all of which involve persistent chronic inflammatory states that favour prolonged symptomatology.
The use of corticosteroids is identified in the literature as one of the modifiable risk factors for ICU-acquired muscle weakness. However, since all patients were treated with the same corticosteroid regimen, this factor was not considered a differential risk factor between the study groups.
On the other hand, prolonged mechanical ventilation is a recognized risk factor for long-term physical impairments, which could account for some of the functional deficits observed in certain patients.15 In our sample, most patients received non-invasive ventilatory support. This lower percentage is attributed to the widespread implementation of non-invasive mechanical ventilation (NIMV) using a helmet interface in our unit, a practice that has been standard since the H1N1 influenza pandemic, during which it demonstrated highly satisfactory outcomes.25 This approach may explain the lower incidence of functional and cognitive impairments since they did not require deep sedation; however, some still experienced delirium, a factor closely related to cognitive impairment.7,23 The use of non-invasive ventilation (NIV) in these patients underscores the high prevalence of persistent symptoms, even in the absence of certain risk factors such as deep sedation and neuromuscular relaxants. This finding suggests that intrinsic patient characteristics significantly contribute to the development of PICS or long COVID and highlights the necessity for a personalized approach that considers both individual patient traits and treatment strategies to mitigate the risk of long-term sequelae.
We have not identified factors that could differentiate the pathology specific to the persistent COVID entity from the post-ICU syndrome. What we observed in our sample is that patients with shorter stays exhibited neurological and musculoskeletal symptoms, which may be more related to persistent COVID. This could be associated with the inflammation triggered by the infection and the viral persistence in various tissues, as described in the literature. One question that arises from the results is that it is not only patients with specific pathologies, multi-organ failure, need for invasive mechanical ventilation, and prolonged ICU stays who are susceptible to developing post-intensive care syndrome.
The pandemic also contributed to the development of post-intensive care syndrome (PICS) within patients’ families, particularly during the initial wave when caring for an ill relative was often impossible.26 In our sample, we observed an emotional burden on primary caregivers; however, the overall level of caregiver overload was relatively low. These findings align with those reported by Torres et al.,27 who similarly noted that psychological sequelae had a more significant negative impact on caregiver overload than physical sequelae, underscoring the importance of addressing the needs of families.
One might consider that a comparison with a control group would have been highly valuable in helping to differentiate the symptoms specific to post-COVID syndrome and PICS. However, although this was not the primary aim of our study, we did not have a sufficient sample of non-COVID patients during the study period. This limitation prevented us from making a representative comparison of the substantial healthcare and emotional burden experienced at that time.
Several limitations should be acknowledged in our study. First, the small sample size and single-center design may limit the generalizability of our findings. Moreover, patients admitted during the first wave of the pandemic were not included, potentially leading to an underestimation of symptom prevalence. Additionally, the study was conducted solely by ICU physicians, and logistical constraints prevented consultations from occurring before the 12-month mark due to the high clinical workload.
One of the strengths of our study is that, unlike many others,2 most of the interviews (88.23%) were conducted in person rather than by phone. Furthermore, in addition to assessing PICS, we also evaluated clinical manifestations associated with post-COVID syndrome and measured physical activity using validated scales tailored for this purpose.
ConclusionsThe assessment of patients 15 months post-ICU discharge due to COVID-19 demonstrated a significant prevalence of persistent symptoms associated with the disease, which overlapped with the hallmark symptoms of post-intensive care syndrome (PICS). These symptoms adversely affected the three primary functional domains: physical, cognitive, and psychological. The findings underscore the necessity for ongoing evaluations of patients following ICU discharge, advocating for a personalized approach to mitigate long-term sequelae. There is also a deep impact on primary caregivers concerns and burdens, relevant secondary characters frequently forgotten from the sequelae of the PICS and post-COVID syndrome.
CRediT authorship contribution statementConceptualisation, M.L.M.-C. and F.S.-M.; methodology, M.L.M.-C., S.A.-T., and F.S.-M.; data curation, M.L.M.-C., B.B-M., J.C-F., C.V-M., A.B.G-N., A.R.V-L. and R.N-A.; writing—original draft preparation, M.L.M.-C.; writing—review and editing, F.S.-M.; visualisation, S.A.-T., B.B-M, J.C-F., C.V-M., A.B.G-N, A.R.V-L. and R.N-A.; project administration, M.L.M.-C. All authors have read and agreed to the published version of the manuscript.
Declaration of Generative AI and AI-assisted technologies in the writing processNo AI-assisted writing tools were employed in the drafting or revision of this manuscript.
FundingThis research received no external funding.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.