Patients with cirrhosis present a highly vulnerable and rebalanced hemostasis state. Assessing the bleeding risk in these patients is complex. It is essential to recognize that conventional coagulation tests do not adequately reflect the true risk of bleeding or thrombosis.
The detailed understanding of this balance and the application of more precise diagnostic tools, such as viscoelastic tests that can more accurately evaluate their coagulation status, facilitate clinical management and can improve the results in these patients.
The haemorrhagic risk of this group of patients is conditioned by specific factors of liver disease, such as portal hypertension and altered haemostatic status, and by systemic factors like the presence of infections and kidney disease, which are independent predictors of bleeding during high-risk procedures. These concepts and the recommendations from the most recent clinical practice guidelines are reviewed in this article.
Los pacientes con cirrosis presentan un estado hemostático reequilibrado y altamente vulnerable. Valorar el riesgo hemorrágico en estos pacientes es complejo. Es esencial reconocer que las pruebas de coagulación convencionales no reflejan adecuadamente el riesgo real de sangrado o trombosis.
La comprensión detallada de este equilibrio y la aplicación de herramientas diagnósticas más precisas, como los test viscoelásticos, que pueden evaluar de manera más certera su estado de coagulación, facilitan el manejo clínico y pueden mejorar los resultados en estos pacientes.
El riesgo hemorrágico en estos pacientes está condicionado por factores propios de la enfermedad hepática, como la hipertensión portal, el estado hemostático alterado y por factores sistémicos como la presencia de infecciones y enfermedad renal, que son predictores independientes de riesgo de sangrado en procedimientos de alto riesgo. A continuación, se revisan estos conceptos y las recomendaciones de las guías de práctica clínica más recientes.
Liver diseases have traditionally been associated with bleeding disorders; however, current evidence suggests a significant risk of thrombotic complications.1 Historically, cirrhotic patients were thought to be “auto-anticoagulated” due to deficiencies in coagulation factors and abnormalities in conventional laboratory tests.2 Nonetheless, several arguments challenge this assumption.
The balance between procoagulant and anticoagulant factors in these patients is significantly altered, creating a complex hemostatic environment,3,4 which affects both the intrinsic and extrinsic coagulation pathways.
Both primary and secondary hemostasis are compromised by the decreased hepatic synthesis of several coagulation factors—including factors II, V, VII, IX, X, and XI. Despite this reduction, levels of von Willebrand factor (vWF) and factor VIII are typically elevated, contributing to a procoagulant state.2
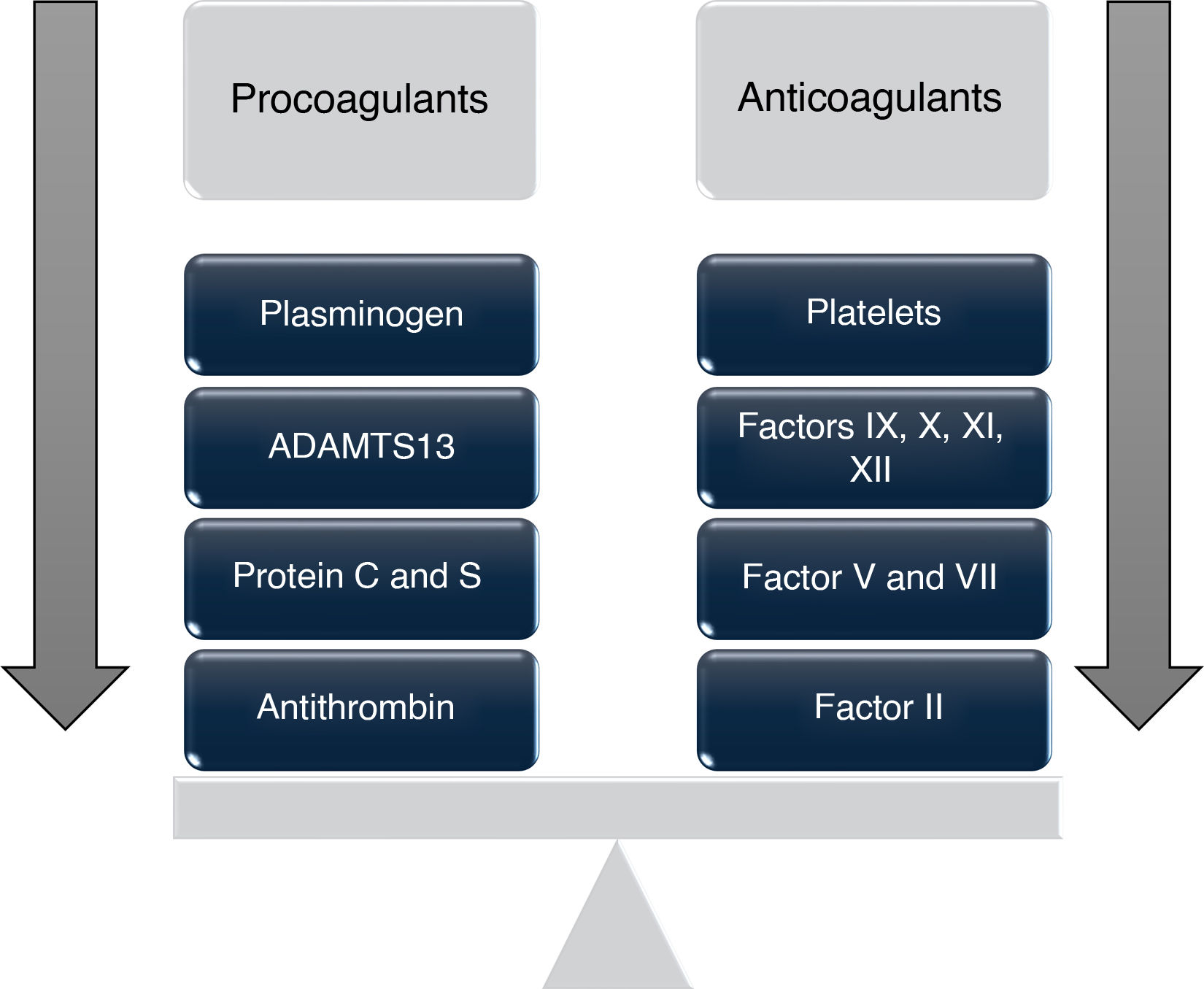
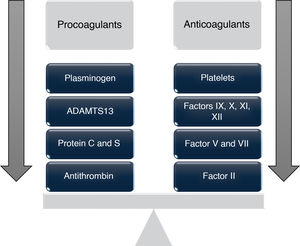
These changes can lead to a condition in which the risk of bleeding or thrombosis increases depending on individual circumstances,2,4 giving rise to the concept of "rebalanced coagulation," which suggests that the decrease in procoagulant factors is offset by a concomitant reduction in anticoagulant proteins such as protein C, protein S, antithrombin, heparin cofactor II, and α2-macroglobulin, all synthesized in the liver and found to be decreased in patients with liver disease.5
This rebalancing is further affected by thrombocytopenia and platelet function defects, which may be compensated by elevated vWF levels (300% in stable cirrhosis, 360% in acute decompensated cirrhosis, and up to 700% in acute-on-chronic liver failure) and decreased levels of its regulator, ADAMTS13 (which can drop to 90% below normal), impacting the overall coagulation profile.2,6
Thrombocytopenia is multifactorial: firstly, due to splenic sequestration (a congestive spleen related to portal hypertension); however, not all cirrhotic patients with thrombocytopenia exhibit splenomegaly, and reducing portal pressure does not always normalize platelet counts.6 Secondly, due to "hypersplenism," resulting from increased immunomediated macrophage activity leading to platelet destruction, especially relevant in patients with hepatitis C and primary biliary cirrhosis.3 Lastly, due to insufficient hepatic production of thrombopoietin (TPO), whose levels vary depending on the stage of liver disease. Additionally, bone marrow suppression caused by antiviral therapy, alcohol, or folate deficiency also contributes to thrombocytopenia in these patients. Regardless of cause or disease severity, this thrombocytopenia is compensated by an increase in the release of reticulated platelets (RePLT), which have greater prothrombotic and procoagulant potential than mature platelets, sometimes exceeding 2%, a value that appears to predict higher risk of hepatic decompensation.7
Changes in platelet function and vWF are useful for stratifying the prognosis of cirrhosis, with increased platelet aggregation associated with a higher risk of portal vein thrombosis and hepatic decompensation.8
Regarding fibrinolysis, all the proteins involved are synthesized by the liver, except for tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1). Cirrhotic patients show reduced levels of plasminogen, plasmin inhibitor, thrombin-activatable fibrinolysis inhibitor (TAFI), and factor XIII in both acute and chronic liver failure. In contrast, plasma levels of profibrinolytic and antifibrinolytic proteins are often elevated, likely due to increased endothelial cell activation or reduced clearance.5 Consequently, these patients may exhibit either hyperfibrinolysis or hypofibrinolysis, with sepsis being one of the main causes of the latter.9,10
These antithrombotic changes, "compensated" by prothrombotic alterations, occur simultaneously, resulting in a "new" hemostatic state characterized by a delicate rebalancing that displays both hypo- and hypercoagulable phenomena (Fig. 1), which is extremely fragile and may become destabilized by situations such as infections, renal failure, or acute episodes of decompensation.6,10,11
In addition to all of the above, there are special situations that pose a greater thrombotic risk and may be common in these patients, such as malignancy (hepatocellular carcinoma), sedentary lifestyle (ascites, sarcopenia, encephalopathy), advanced age, or elevated estrogen levels.
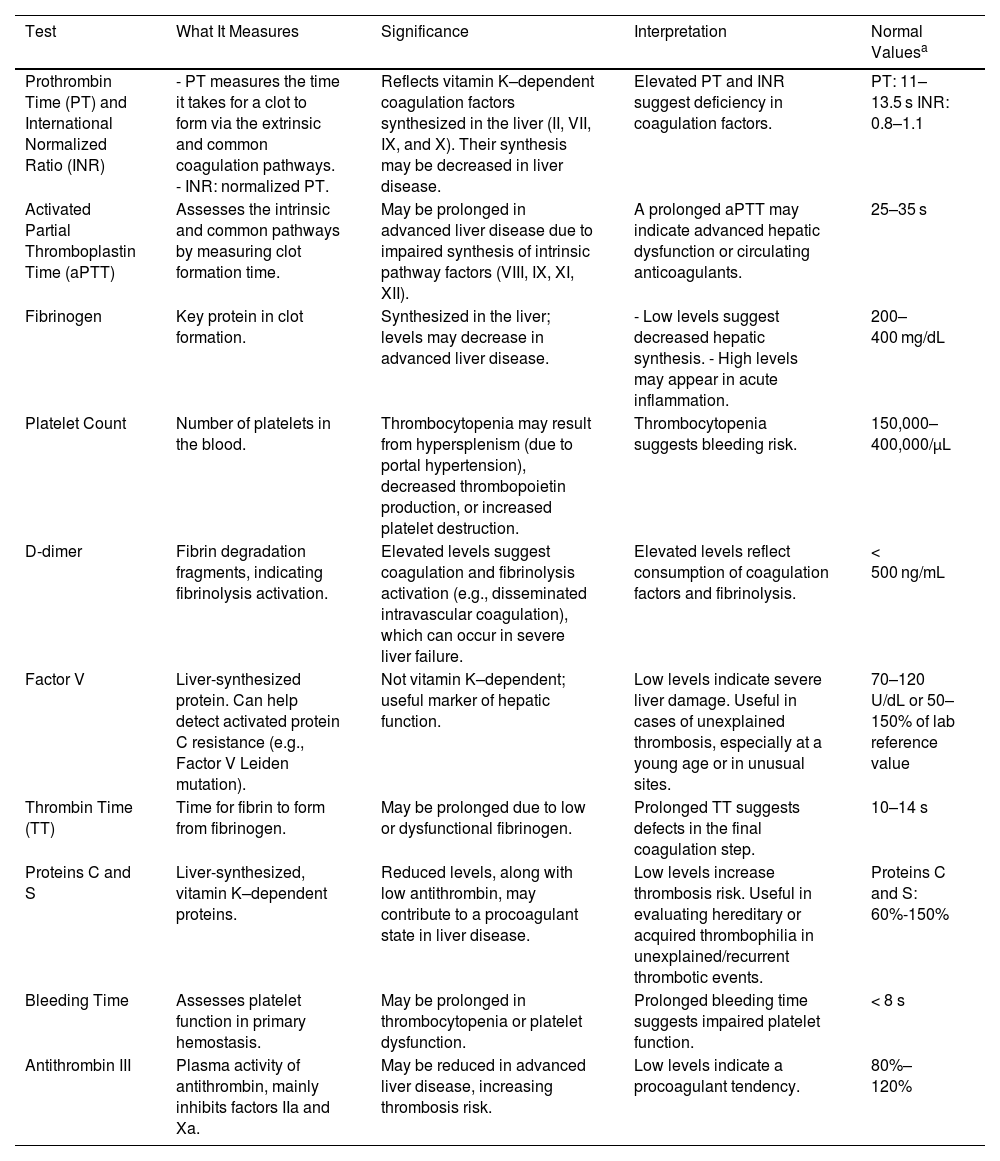
Coagulation testsCoagulation test results vary and are intrinsically linked to the degree of hepatic dysfunction. Patients may develop either a hypocoagulable or hypercoagulable state.12 Therefore, constant monitoring and evaluation of coagulation is essential in the clinical management of these patients, as the liver plays a central role in coagulation factor synthesis, and its compromised function can impair the body’s ability to maintain adequate hemostasis. This makes it necessary to assess coagulation not only with classical tests (Table 1) but also with advanced tests such as viscoelastic testing.13
Classic coagulation tests.
| Test | What It Measures | Significance | Interpretation | Normal Valuesa |
|---|---|---|---|---|
| Prothrombin Time (PT) and International Normalized Ratio (INR) | - PT measures the time it takes for a clot to form via the extrinsic and common coagulation pathways. - INR: normalized PT. | Reflects vitamin K–dependent coagulation factors synthesized in the liver (II, VII, IX, and X). Their synthesis may be decreased in liver disease. | Elevated PT and INR suggest deficiency in coagulation factors. | PT: 11–13.5 s INR: 0.8–1.1 |
| Activated Partial Thromboplastin Time (aPTT) | Assesses the intrinsic and common pathways by measuring clot formation time. | May be prolonged in advanced liver disease due to impaired synthesis of intrinsic pathway factors (VIII, IX, XI, XII). | A prolonged aPTT may indicate advanced hepatic dysfunction or circulating anticoagulants. | 25–35 s |
| Fibrinogen | Key protein in clot formation. | Synthesized in the liver; levels may decrease in advanced liver disease. | - Low levels suggest decreased hepatic synthesis. - High levels may appear in acute inflammation. | 200–400 mg/dL |
| Platelet Count | Number of platelets in the blood. | Thrombocytopenia may result from hypersplenism (due to portal hypertension), decreased thrombopoietin production, or increased platelet destruction. | Thrombocytopenia suggests bleeding risk. | 150,000–400,000/µL |
| D-dimer | Fibrin degradation fragments, indicating fibrinolysis activation. | Elevated levels suggest coagulation and fibrinolysis activation (e.g., disseminated intravascular coagulation), which can occur in severe liver failure. | Elevated levels reflect consumption of coagulation factors and fibrinolysis. | < 500 ng/mL |
| Factor V | Liver-synthesized protein. Can help detect activated protein C resistance (e.g., Factor V Leiden mutation). | Not vitamin K–dependent; useful marker of hepatic function. | Low levels indicate severe liver damage. Useful in cases of unexplained thrombosis, especially at a young age or in unusual sites. | 70–120 U/dL or 50–150% of lab reference value |
| Thrombin Time (TT) | Time for fibrin to form from fibrinogen. | May be prolonged due to low or dysfunctional fibrinogen. | Prolonged TT suggests defects in the final coagulation step. | 10–14 s |
| Proteins C and S | Liver-synthesized, vitamin K–dependent proteins. | Reduced levels, along with low antithrombin, may contribute to a procoagulant state in liver disease. | Low levels increase thrombosis risk. Useful in evaluating hereditary or acquired thrombophilia in unexplained/recurrent thrombotic events. | Proteins C and S: 60%-150% |
| Bleeding Time | Assesses platelet function in primary hemostasis. | May be prolonged in thrombocytopenia or platelet dysfunction. | Prolonged bleeding time suggests impaired platelet function. | < 8 s |
| Antithrombin III | Plasma activity of antithrombin, mainly inhibits factors IIa and Xa. | May be reduced in advanced liver disease, increasing thrombosis risk. | Low levels indicate a procoagulant tendency. | 80%–120% |
Viscoelastic tests (VET)—thromboelastography (TEG) and rotational Thromboelastometry (ROTEM)—are specialized tools that provide a more detailed and accurate view of the hemostatic and thrombotic systems. These analyses are especially useful when classical coagulation tests do not fully explain the patient's clinical picture. In fact, one of their main advantages is their ability to integrate coagulation testing with platelet function analysis, offering a more global understanding of hemostatic physiology. They are dynamic tests that assess clot formation and stability, as well as fibrinolysis, analyzing various aspects of coagulation, platelet function, and the fibrinolytic system. Although not useful in predicting bleeding or reducing mortality in cirrhotic patients, their pre- or perioperative application may reduce the need for plasma and platelet transfusions.12,13
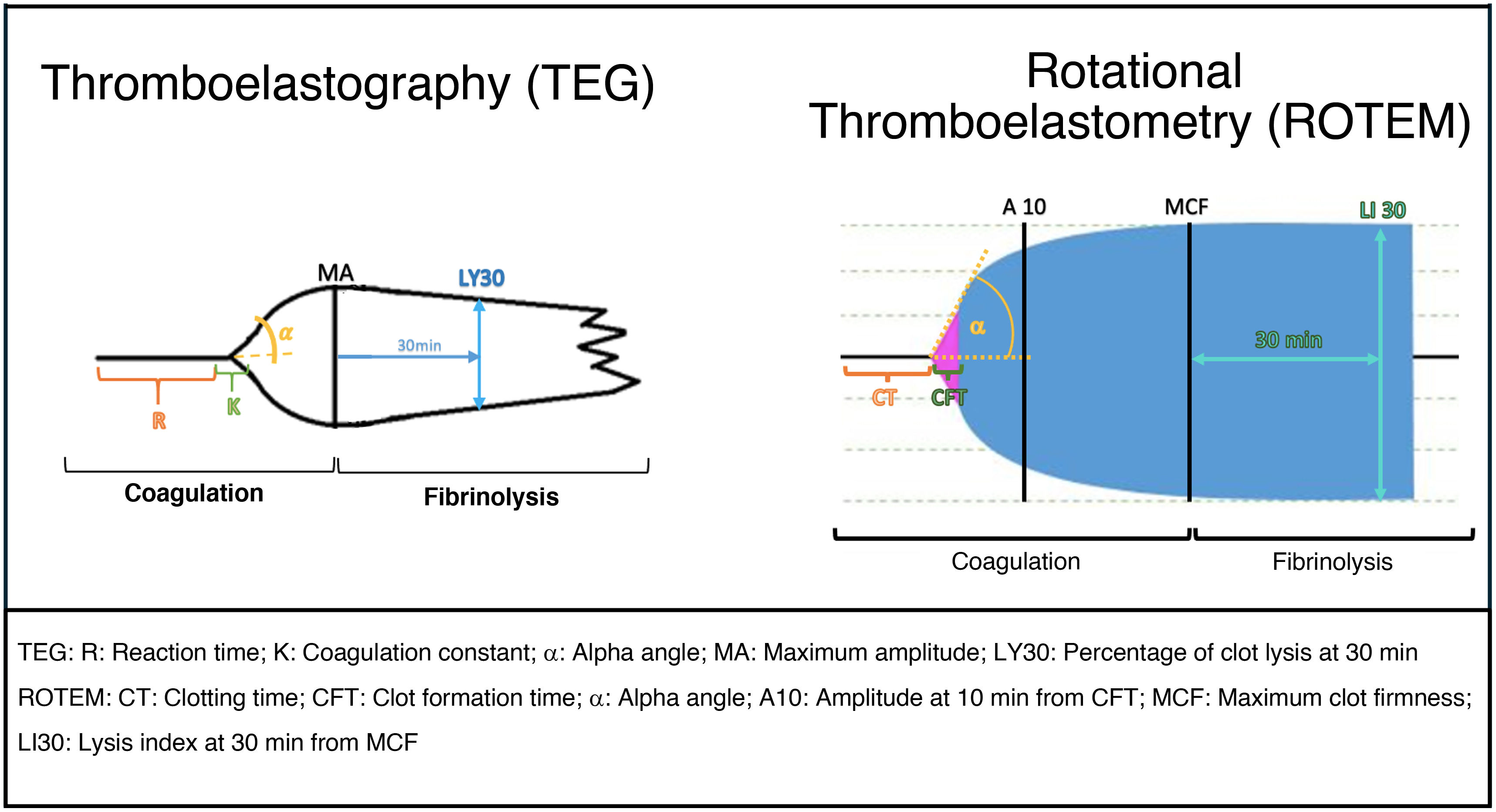
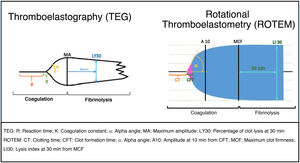
The procedure involves introducing a whole blood sample into a cuvette containing a cylindrical pin, with a processor analyzing the rotational movements between them. Coagulation and lysis cause torsional changes that are analyzed by the processor and reflected in a graph (Table 2, Fig. 2, and Supplementary data).14–16
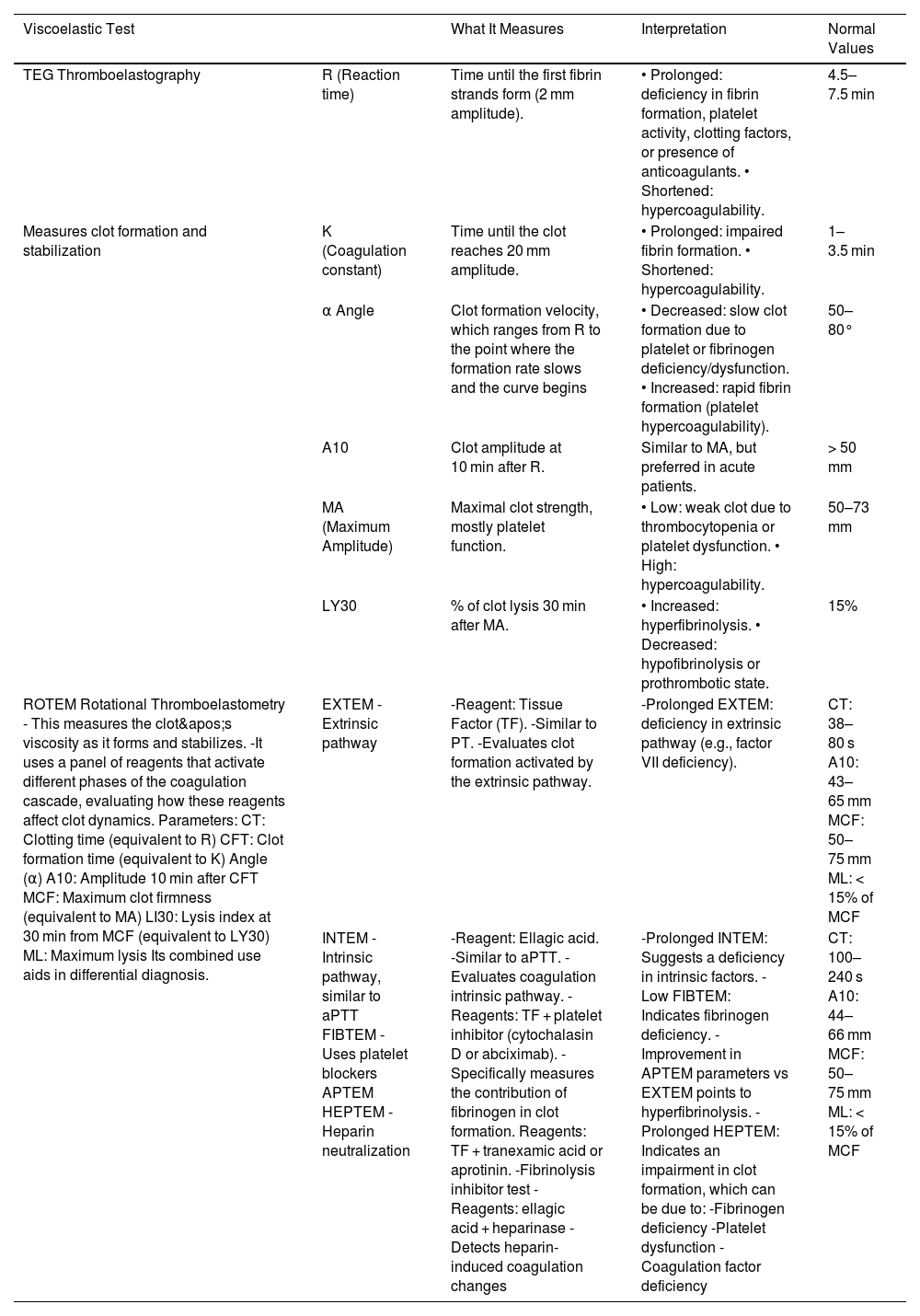
Types of viscoelastic tests, characteristics, and reference values.14–17,20
| Viscoelastic Test | What It Measures | Interpretation | Normal Values | |
|---|---|---|---|---|
| TEG Thromboelastography | R (Reaction time) | Time until the first fibrin strands form (2 mm amplitude). | • Prolonged: deficiency in fibrin formation, platelet activity, clotting factors, or presence of anticoagulants. • Shortened: hypercoagulability. | 4.5–7.5 min |
| Measures clot formation and stabilization | K (Coagulation constant) | Time until the clot reaches 20 mm amplitude. | • Prolonged: impaired fibrin formation. • Shortened: hypercoagulability. | 1–3.5 min |
| α Angle | Clot formation velocity, which ranges from R to the point where the formation rate slows and the curve begins | • Decreased: slow clot formation due to platelet or fibrinogen deficiency/dysfunction. • Increased: rapid fibrin formation (platelet hypercoagulability). | 50–80° | |
| A10 | Clot amplitude at 10 min after R. | Similar to MA, but preferred in acute patients. | > 50 mm | |
| MA (Maximum Amplitude) | Maximal clot strength, mostly platelet function. | • Low: weak clot due to thrombocytopenia or platelet dysfunction. • High: hypercoagulability. | 50–73 mm | |
| LY30 | % of clot lysis 30 min after MA. | • Increased: hyperfibrinolysis. • Decreased: hypofibrinolysis or prothrombotic state. | 15% | |
| ROTEM Rotational Thromboelastometry - This measures the clot's viscosity as it forms and stabilizes. -It uses a panel of reagents that activate different phases of the coagulation cascade, evaluating how these reagents affect clot dynamics. Parameters: CT: Clotting time (equivalent to R) CFT: Clot formation time (equivalent to K) Angle (α) A10: Amplitude 10 min after CFT MCF: Maximum clot firmness (equivalent to MA) LI30: Lysis index at 30 min from MCF (equivalent to LY30) ML: Maximum lysis Its combined use aids in differential diagnosis. | EXTEM -Extrinsic pathway | -Reagent: Tissue Factor (TF). -Similar to PT. -Evaluates clot formation activated by the extrinsic pathway. | -Prolonged EXTEM: deficiency in extrinsic pathway (e.g., factor VII deficiency). | CT: 38–80 s A10: 43–65 mm MCF: 50–75 mm ML: < 15% of MCF |
| INTEM -Intrinsic pathway, similar to aPTT FIBTEM -Uses platelet blockers APTEM HEPTEM -Heparin neutralization | -Reagent: Ellagic acid. -Similar to aPTT. -Evaluates coagulation intrinsic pathway. - Reagents: TF + platelet inhibitor (cytochalasin D or abciximab). -Specifically measures the contribution of fibrinogen in clot formation. Reagents: TF + tranexamic acid or aprotinin. -Fibrinolysis inhibitor test -Reagents: ellagic acid + heparinase -Detects heparin-induced coagulation changes | -Prolonged INTEM: Suggests a deficiency in intrinsic factors. -Low FIBTEM: Indicates fibrinogen deficiency. -Improvement in APTEM parameters vs EXTEM points to hyperfibrinolysis. -Prolonged HEPTEM: Indicates an impairment in clot formation, which can be due to: -Fibrinogen deficiency -Platelet dysfunction -Coagulation factor deficiency | CT: 100–240 s A10: 44–66 mm MCF: 50–75 mm ML: < 15% of MCF | |
- •
Do not diagnose hemostatic abnormalities due to hypocalcemia, acidosis, von Willebrand deficiency, protein C and S disorders, or platelet adhesion to the endothelium.15,17
- •
Do not detect coagulation abnormalities caused by hypothermia.
- •
Do not assess the microcirculation, where hemostatic alterations likely originate.17
- •
Require a learning curve for execution and interpretation.
- •
Can be performed either in the lab or at the patient’s bedside, providing rapid test execution and immediate results, allowing early transfusion therapy targeted to specific disorders such as reduced coagulation factors or quantitative/functional platelet defects.
- •
Use whole blood.
- •
Help optimize the use of blood products, reducing the need for blood component transfusions.
The literature describes the utility of viscoelastic tests in cirrhotic patients with esophageal variceal bleeding, outlining the following sequence for their use in this setting18–20:
- 1
Initial Evaluation (ROTEM/TEG):
- •
Perform ROTEM or TEG to evaluate real-time hemostasis.
- •
Possible tests: EXTEM, INTEM, FIBTEM, APTEM (ROTEM) or full TEG.
- 2
Evaluation Phases:
- •
Clot Formation Analysis: EXTEM or TEG:
- o
R (reaction time):
▪Prolonged: Coagulation factor deficiency.
▪Causes: Chronic liver disease, vitamin K deficiency, heparin.
- o
K (clot formation time) and α-angle:
▪Prolonged or small α: Fibrinogen or platelet deficiency.
▪Causes: Severe liver dysfunction, thrombopathy, hypofibrinogenemia.
- o
- •
Clot Stability Evaluation (Maximum Amplitude [MA]):
- o
MA:
▪Low: Weak clot (fibrinogen or platelet deficiency).
▪Causes: Hypofibrinogenemia or platelet dysfunction due to cirrhosis or cirrhosis-associated coagulopathy.
- o
- •
Fibrinolysis Evaluation:
- o
APTEM:
▪No lysis expected in APTEM (LY30 near 0%) due to inhibited fibrinolysis.
▪High LY30 in APTEM may indicate residual fibrinolysis, possibly due to disseminated intravascular coagulation or sepsis.
- o
- 3
Management (Table 3)
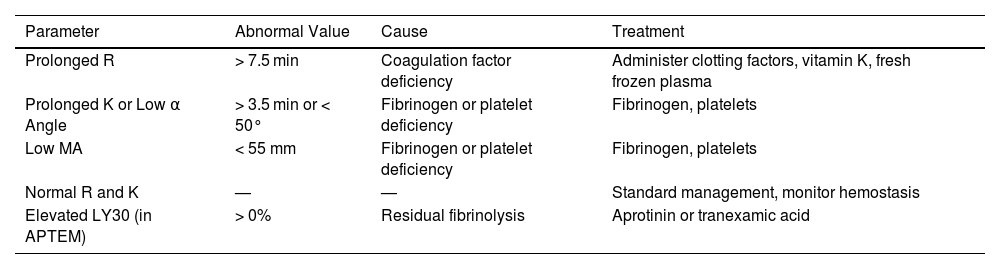
Table 3.Management of variceal bleeding in a cirrhotic patient.18–20
Parameter Abnormal Value Cause Treatment Prolonged R > 7.5 min Coagulation factor deficiency Administer clotting factors, vitamin K, fresh frozen plasma Prolonged K or Low α Angle > 3.5 min or < 50° Fibrinogen or platelet deficiency Fibrinogen, platelets Low MA < 55 mm Fibrinogen or platelet deficiency Fibrinogen, platelets Normal R and K — — Standard management, monitor hemostasis Elevated LY30 (in APTEM) > 0% Residual fibrinolysis Aprotinin or tranexamic acid
In upper GI bleeding due to esophageal varices in cirrhotic patients, hyperfibrinolysis plays a key role, justifying the use of APTEM to assess clot stability without fibrinolytic interference.
Treatment may include a combination of transfusions and drugs to stabilize coagulation and prevent recurrent bleeding, as outlined in subsequent sections. Viscoelastic tests allow more precise treatment guidance, optimizing transfusion use based on patient-specific needs.
Bleeding in patients with chronic liver disease: recommendations for prevention and treatmentAccording to the clinical scenario, the risk of bleeding varies varies, as described below21:
- •
Patients with stable cirrhosis without decompensation: Coagulation tests are not recommended prior to most low-risk procedures. Prophylactic and routine correction of INR22 is also not indicated, as no direct relationship has been demonstrated between elevated INR values and bleeding episodes. Depending on the risk associated with the procedure, platelet level correction may be necessary.
- •
Patients with decompensated cirrhosis: These patients present with hyperfibrinolysis and coagulopathy due to platelet dysfunction. However, the key factor in bleeding events is increased portal hypertension.21 Therefore, a restrictive transfusion strategy is required, which includes avoiding fresh frozen plasma and red blood cell concentrates.
- •
Patients undergoing liver transplantation: These patients typically present a hypercoagulable state, generally due to tissue factor exposure and the progressive recovery of hepatic synthesis in the liver graft, which gradually increases procoagulant factors23 except in cases of overt surgical bleeding, which contributes to consumptive or dilutional coagulopathy.
- •
Patients with acute liver failure: Despite significant alterations in conventional coagulation tests, these patients are characterized by a hypercoagulable state. In general, bleeding episodes are infrequent in this context, and prophylactic correction of coagulation parameters is not necessary.24,25
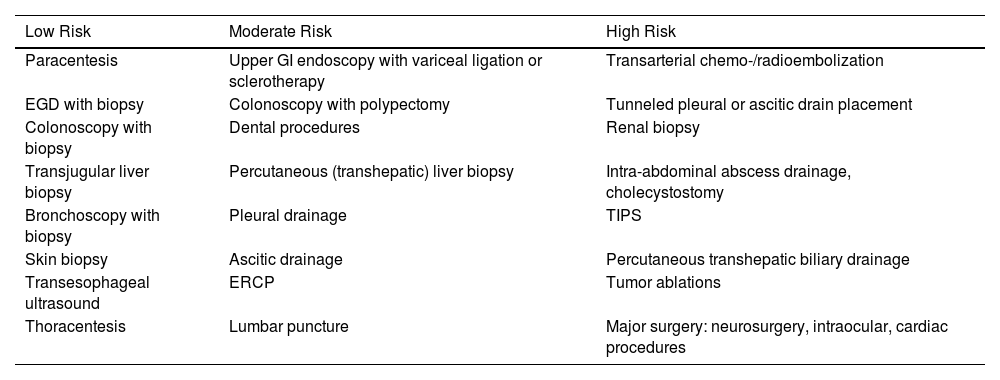
Another important element is the stratification of bleeding risk²¹ in patients undergoing invasive procedures. Procedures are considered high-risk if bleeding occurs in >1.5% of recorded cases. Table 4 stratifies the most common procedures in critically ill and liver disease patients. Prophylactic use of blood products does not lead to a reduction of hemorrhagic complications in invasive procedures, especially in those classified as low-risk.25–31
Bleeding Risk Stratification of Common Procedures in Critically Ill and Hepatic Patients.
| Low Risk | Moderate Risk | High Risk |
|---|---|---|
| Paracentesis | Upper GI endoscopy with variceal ligation or sclerotherapy | Transarterial chemo-/radioembolization |
| EGD with biopsy | Colonoscopy with polypectomy | Tunneled pleural or ascitic drain placement |
| Colonoscopy with biopsy | Dental procedures | Renal biopsy |
| Transjugular liver biopsy | Percutaneous (transhepatic) liver biopsy | Intra-abdominal abscess drainage, cholecystostomy |
| Bronchoscopy with biopsy | Pleural drainage | TIPS |
| Skin biopsy | Ascitic drainage | Percutaneous transhepatic biliary drainage |
| Transesophageal ultrasound | ERCP | Tumor ablations |
| Thoracentesis | Lumbar puncture | Major surgery: neurosurgery, intraocular, cardiac procedures |
In case of discrepancies, procedures were classified as moderate risk.
AGA: American Gastroenterology Association; AMG: Mexican Association of Gastroenterology; BSH: British Society for Haematology; ERCP: endoscopic retrograde cholangiopancreatography; EASL: European Association for the Study of the Liver; EGD, esophagogastroduodenoscopy; ISTH: International Society on Thrombosis and Haemostasis; SETH: Spanish Society for Liver Transplantation; SIR: Society of Interventional Radiology; TIPS: transjugular intrahepatic portosystemic shunt.
In the presence of active bleeding or a high-risk procedure, it is essential to maintain the patient in optimal condition, ensuring a normothermic body temperature (>36 °C), a pH > 7.35, and ionized calcium levels > 1 mmol/L.33 Additionally, whenever possible, viscoelastic testing is recommended to guide the optimization of the coagulation process, as shown in Table 3 and Fig. 2.
In order of priority, platelets, followed by fibrinogen, and to a lesser extent coagulation factors, are the elements to optimize—especially in patients with acute liver failure.24 Restrictive transfusion strategies have shown better outcomes in liver transplant, GI bleeding, and bleeding due to other causes in cirrhotic patients.25,28,30
However, since viscoelastic tests are not available in all centers, the following are reference values for conventional tests, along with therapeutic recommendations for patients with chronic liver disease, as suggested by various scientific societies and research groups21,25,26,29,30,32–36:
- •
Platelets: Prophylactic transfusion is recommended in hospitalized adult patients with counts <10 × 10⁹/L37 according to the EASL and AABB guidelines.28,36 For liver disease patients undergoing high-risk procedures or in whom local hemostasis is not possible, platelet transfusion or thrombopoietin receptor agonists should not be used routinely but may be individualized.28 Target levels vary between 20 × 10⁹/L and 50 × 10⁹/L depending on the procedure (Table 1, Supplementary data).
During liver transplantation, the SETH-SETH consensus recommends intraoperative levels >30 × 10⁹/L and >50 × 10⁹/L if there is active bleeding²⁶.
- •
Thrombopoietin receptor agonists (TPO-R): Avatrombopag and lusutrombopag are good alternatives to platelet transfusion, requiring 5–7 days to reach peak platelet count,27 making them unsuitable for acute disease. They are not recommended when platelet count is >50 × 10⁹/L37 or when bleeding can be controlled with local hemostasis.28 Current data come from studies in Child-Pugh stages A and B cirrhosis; there is limited evidence in stage C and acute-on-chronic liver failure.38
- •
Fibrinogen: The 2022 EASL guideline strongly discourages routine prophylactic correction28 and recommends maintaining serum fibrinogen >1.2 g/dL using synthetic fibrinogen or cryoprecipitates36 in cases of bleeding or before high-risk invasive procedures.21 According to Budnick et al., prophylactic cryoprecipitate transfusion for fibrinogen <1.5 g/dL did not alter bleeding or mortality risk.37
- •
INR: A target INR < 2 is only recommended in cases of active bleeding, using prothrombin complex concentrate (PCC) instead of fresh frozen plasma (FFP)39 due to the increase in portal hypertension from large transfused volumes (1.4 mmHg per 100 mL of FFP),40 which favors variceal and surgical site bleeding.25,41,42 In cirrhotic patients undergoing invasive procedures, prophylactic INR correction is not recommended as it is not associated with bleeding.28,38
Regarding PCC, both 3-factor (II, IX, X) and 4-factor (II, VII, IX, X with proteins C and S) formulations are available. These have 25 times higher coagulation factor concentration than FFP, enabling rapid INR correction—complete infusion takes approximately 10 min.28 Since INR is not a reliable measure in cirrhotic patients, and dosing is based on INR and weight, further studies are needed to optimize dosing.27 An in vitro study showed an exaggerated procoagulant response in cirrhotic patients after PCC administration, compared to healthy subjects: 150% in decompensated cirrhosis, 270% in acute-on-chronic liver failure, and 97% in healthy controls.43
- •
Hemoglobin: Maintain levels between 7–8 g/dL; do not exceed 9 g/dL to avoid increasing portal hypertension.
- •
Factor XIII: If < 50%, correct in the absence of other abnormalities.
- •
Other Treatments:
- o
Tranexamic acid: Although widely studied for massive bleeding in trauma and postpartum hemorrhage, its routine use is discouraged in cirrhotic patients,28 based on the HALT-IT trial which included patients with variceal bleeding44 and showed no mortality benefit at 5 days (RR, 0.99, 95%CI, 0,82–1,8). According to the 2022 EASL guideline.28 in cirrhotic patients with active variceal bleeding controlled with portal hypotensive drugs and endoscopic treatment, correction of hemostatic abnormalities or tranexamic acid use is not indicated. Therapeutic administration should be considered if there is clinical suspicion of hyperfibrinolysis (bleeding coagulopathy with decreased fibrinogen) or compatible changes in thromboelastography.26
In liver transplant, prophylactic use is recommended for Child-Pugh class B or C patients26 when viscoelastic testing is unavailable.
- o
Vitamin K: Routine administration does not improve INR values45,46 because coagulation factor deficits are mainly due to liver dysfunction rather than vitamin deficiency.
- o
Desmopressin: Stimulates endothelial release of vWF as a primary hemostatic mechanism. Since vWF levels are usually elevated in cirrhosis, there is no strong scientific basis for its use; however, it may be useful in patients with concomitant renal insufficiency.27
- o
Hospitalized cirrhotic patients have a twofold increased risk of thromboembolic disease vs non-cirrhotic hospitalized patients, and a higher incidence rate of portal vein thrombosis, ranging from 0.6% to 26% among patients with chronic liver disease, increasing in proportion to disease severity.6 Therefore, classic coagulation tests and an elevated INR do not correlate with a greater bleeding tendency nor do they provide protection vs venous thromboembolism.47
In clinical practice, anticoagulation is often avoided in cirrhotic patients due to concerns about inducing serious bleeding, especially in those with prolonged INR or thrombocytopenia.4,12 However, this approach is shifting as the pathophysiology of the hemostatic balance is better understood.48 Current clinical practice guidelines from major hepatology societies, such as the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), recognize the importance of thromboprophylaxis in hospitalized cirrhotic patients who are at high risk of thrombosis and low risk of bleeding.4,28
In its 2022 clinical practice guidelines, the EASL28 states that the estimated risk of deep vein thrombosis (DVT) and pulmonary embolism in cirrhotic patients is at least comparable to that of the general population—particularly in those with nonalcoholic steatohepatitis (NASH), which constitutes an independent risk factor for venous thromboembolism.49 The reported prevalence of DVT ranges from 1.2% to 7%,50–52 and several studies and meta-analyses describe a 1.7-fold increased relative risk (RR) of DVT, especially in relation to the lack of pharmacologic thromboprophylaxis.53–56
The EASL recommends the use of thromboprophylaxis in hospitalized cirrhotic patients with platelet counts >50 × 10⁹/L using low molecular weight heparin (LMWH), considering this a safe practice with no significant increase in bleeding risk.28 This also applies to patients with Child-Pugh class A and B cirrhosis. In patients with Child-Pugh class C or with platelet counts between 20 and 50 × 10⁹/L, evidence is currently insufficient to support its use.
For portal vein thrombosis, the VII Baveno Consensus57 recommends initiating anticoagulation in cirrhotic patients with a diagnosis (confirmed by Doppler ultrasound, contrast-enhanced CT, or MRA) of asymptomatic portal vein thrombosis within the first 6 months—whether total or partial occlusion (>50%)—regardless of extension to the superior mesenteric vein. Anticoagulation is also advised in symptomatic patients and/or liver transplant candidates regardless of the extent of thrombosis. If the diagnosis is made via Doppler ultrasound, confirmation by contrast-enhanced CT or MRA is recommended.44
Systemic heparin infusion is recommended for the treatment of symptomatic portal vein, mesenteric, and deep vein thrombosis, although the optimal laboratory parameter for monitoring remains unresolved; both anti-Xa levels and activated partial thromboplastin time (aPTT) are currently under evaluation.27
ConclusionsThe paradigm surrounding liver disease patients has changed. The long-held belief in a predominant hypocoagulable state has given way to the concept of rebalanced hemostasis, wherein the patient may be in a procoagulant or anticoagulant state depending on the clinical context. Conventional hemostasis tests have limited utility, while viscoelastic tests may provide more precise information. In general, patients with liver disease do not require correction of coagulation abnormalities unless there is active bleeding or a high-risk invasive procedure. These patients should be considered for pharmacological thromboprophylaxis similarly to the general population.
CRediT authorship contribution statementAll authors contributed equally to the development of this manuscript and approved the final version.
Declaration of Generative AI and AI-assisted technologies in the writing processNo generative AI tools were used in the preparation of this manuscript.
FundingNone declared.
None declared.
We thank the coordinators of SEMICYUC Working Group on Critical Digestive Disease, Dr. David Toapanta, as well as SEMICYUC Working Group on Hemotherapy, Hematology, and Critical Oncology, Dr. Kapil Nanwani, for their involvement in the preparation of this joint document.