To assess how antibiotic administration delay and inadequacy influence survival in septic shock patients.
DesignA prospective, observational cohort study was carried out between September 2005 and September 2010.
ScopePatients admitted to the ICU of a third level hospital.
PatientsA total of 342 septic shock patients.
InterventionsNone.
Variables of interestThe time to antibiotic administration (difference between septic shock presentation and first administered dose of antibiotic) and its adequacy (in vitro susceptibility testing of isolated pathogens) were determined.
ResultsICU and hospital mortality were 26.4% and 33.5%, respectively. The median delay to administration of the first antibiotic dose was 1.7h. Deceased patients received antibiotics significantly later than survivors (1.3±14.5h vs. 5.8±18.02h; p=0.001). Percentage drug inadequacy was 12%. Those patients who received inadequate antibiotics had significantly higher mortality rates (33.8% vs. 51.2%; p=0.03). The coexistence of treatment delay and inadequacy was associated to lower survival rates.
ConclusionsBoth antibiotic administration delay and inadequacy exert deleterious effects upon the survival of septic shock patients, independently of their characteristics or severity.
Evaluar cómo influye el retraso en la administración de la primera dosis de antibiótico y la inadecuación de la pauta seleccionada en la supervivencia de los pacientes en shock séptico.
DiseñoEstudio prospectivo de cohortes observacional realizado entre septiembre de 2005 y septiembre de 2010.
ÁmbitoPacientes hospitalizados en la UCI de un hospital de tercer nivel.
PacientesTrescientos cuarenta y dos pacientes con cuadro de shock séptico.
IntervencionesNinguna.
Variables de interés principalesSe determinó el tiempo hasta la administración del antibiótico (diferencia entre la presentación del shock séptico y la primera dosis de antibiótico) y la adecuación del mismo (susceptibilidad in vitro de los microorganismos aislados).
ResultadosLa mortalidad en UCI fue del 26,4% y a nivel hospitalario del 33,5%. La mediana de retraso en la administración de la primera dosis de tratamiento antibiótico fue de 1,7h. Los pacientes fallecidos recibieron el antibiótico significativamente más tarde (1,3±14,5h frente a 5,8±18,02; p=0,001) que los supervivientes. El porcentaje de inadecuación del tratamiento antibiótico fue del 12%. Los pacientes tratados inadecuadamente presentaron cifras de mortalidad hospitalaria significativamente más altas (33,8% frente a 51,2%; p=0,03) respecto a los que recibieron una pauta antibiótica adecuada. La coexistencia de retraso e inadecuación en el tratamiento antibiótico se asoció a una menor supervivencia de los pacientes.
ConclusionesTanto el retraso como la inadecuación del tratamiento antibiótico tienen efectos negativos sobre la supervivencia de los pacientes en shock séptico independientemente de las características de estos o de su estado de gravedad.
Although the mortality rate associated to sepsis remains high, its prognosis has improved in recent years.1 The development and subsequent adoption in clinical practice of the Surviving Sepsis Campaign (SSC) are largely responsible for this change. A number of initiatives have demonstrated at both multicenter level and on an isolated basis that adoption of the SSC is not only feasible but is associated to important improvements in patient survival and sociosanitary cost savings.2–5
The basic instrument of the mentioned Campaign is a series of “bundles”, consisting of groups of interventions and treatments which when applied jointly and within a given (and usually short) time period are expected to exert a synergic effect and thus afford better outcomes than when such measures are applied individually. However, the level of evidence supporting each measure varies and is subject to change as further experience is gradually gained. An example of this is hemodynamic resuscitation, fundamented upon a randomized study6 which nevertheless has not been corroborated by a recently published study.7 For obvious reasons, the administration of antibiotics is not regulated by the same criteria, and the indication of such drugs is exclusively based on observational studies. Such studies have evidenced the deleterious effects of delays in antibiotic administration and inadequate antibiotic treatment choice upon septic patient survival.8–11 Nonetheless, it must be mentioned that the studies are often heterogeneous–some focusing on specific disorders such as community-acquired pneumonia, ventilator associated pneumonia or bacteremia–and do not necessarily address the development of severe sepsis or septic shock associated to such disorders.
The present study evaluates the influence of delays in administering the first dose of antibiotic and inadequacy of the selected antibiotic treatment regimen upon the survival of patients with septic shock.
Patients and methodsStudy populationA prospective, observational cohort study was made, involving all the patients over 18 years of age admitted to the Intensive Care Unit (ICU) with septic shock according to the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS12 Consensus Conference during the period between September 2005 and September 2010 (both months included).
Infection was suspected from the presence of an infectious focus documented by radiological or laboratory test data consistent with infection or a clinical syndrome associated to a high probability of infection or shock not explainable by other causes.
The severity of the patients was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE II) score and the Sepsis-related Organ Failure Assessment (SOFA) score. The APACHE II was applied following the first 24h of admission to the ICU, while the SOFA was applied at the time of admission to the Unit.
We excluded patients under 18 years of age, as well as individuals with recent cardiac arrest or with a case history containing instructions referred to the limitation of therapeutic effort; patients without severe sepsis or septic shock at the time of admission to the ICU but who developed such problems during admission; and all individuals lacking positive microbiological test results in which the adequacy of the antibiotic treatment therefore could not be established.
The Ethics Committee of the hospital evaluated the study, and given its observational nature, the obtainment of informed consent was not considered necessary.
Recording of variablesThe following patient variables were recorded: age, gender, Department of origin, type of patient (medical or surgical), type of infection (community-acquired or nosocomial), comorbidities, immune depression and cause (AIDS, neutropenia [neutrophil count<1×109/l], treatment with glucocorticoids [>0.5mg/kg during>30days] and/or immune suppressor or cytotoxic drugs, solid organ transplantation, allogenic or autogenic bone marrow transplantation, hematological malignancies or solid tumors), microbiological results of the cultures, final main diagnosis, and patient condition at discharge from the ICU and hospital. The existence of organ dysfunction was determined based on the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS Consensus Conference.12
The time to administration of the antibiotic was defined as the difference in hours between the moment of first documented evidence of septic shock according to the criteria of the SCCM/ESICM/ACCP/ATS/SIS Consensus Conference12 and administration of the first dose of antibiotic. Calculation of this parameter was based on the information contained in the clinical evolution notes, medical instructions, nursing notes, vital sign recordings and results of the tests requested during the hours or days prior to admission to the ICU.
The adequacy of antibiotic treatment was established according to the in vitro susceptibility of the isolated microorganisms. In the case of polymicrobial isolates we considered the in vitro susceptibility findings of all the identified microorganisms. Inadequacy was defined as: (1) the absence of antimicrobials with activity against the isolated microorganism; or (2) the administration of an antibiotic to which the microorganism was specifically resistant.
Statistical analysisContinuous variables were reported as the mean±standard deviation (SD) or median and interquartile range (IQR) in the event of a non-normal distribution. Qualitative variables in turn were expressed as absolute values and percentages.
The comparison of means of continuous variables of two groups was carried out using analysis of variance (ANOVA) or the nonparametric Kruskal–Wallis test where indicated. The comparison of proportions in turn was made using the chi-squared test, with the Yates correction where indicated. An alpha risk with p<0.05 was considered for defining statistically significant relationships. A univariate analysis was performed to determine the association of the different variables to patient mortality.
Logistic regression analyses were used to estimate the mortality predicting capacity of the different factors analyzed. All those clinical and laboratory test parameters found to be associated to mortality risk (p<0.15) in the univariate analysis were entered in the model. Adjustment for the following covariables was carried out: age, gender, immune depression, APACHE II, SOFA and other possible identified confounding factors. A survival analysis based on the Kaplan–Meier curves was performed.
The SPSS version 19.0 statistical package (SPSS, Inc., Chicago, IL, USA) was used throughout.
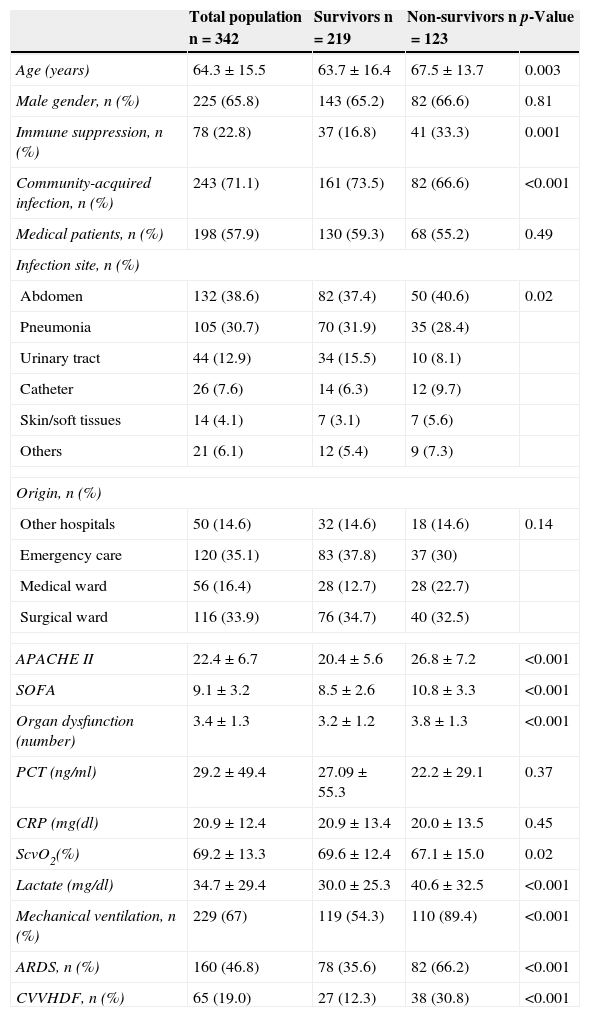
ResultsIn the course of the study period we included 342 cases in which positive cultures were obtained, allowing assessment of the adequacy of the selected antibiotic treatment. The mean patient age was 64.3±15.5 years, and 225 (65.8%) were males. The mean APACHE II and SOFA scores were 22.4±6.7 and 9.1±3.2, respectively. Most of the patients (71.1%) had community-acquired infections. The mortality rate in the ICU was 26.9%, with an in-hospital mortality rate of 36%. The patients who died (in hospital) were comparatively older and were more often affected by immune suppression and nosocomial infections than the survivors (Table 1). Regarding the severity markers, the patients who died had significantly higher severity scores upon admission to the ICU and a larger number of dysfunctional organs. The ScvO2 values were lower in those who died, and the venous lactate levels were higher. There were no significant differences in C-reactive protein or procalcitonin concentrations between the two groups.
Characteristics of the study population.
| Total population n=342 | Survivors n=219 | Non-survivors n=123 | p-Value | |
|---|---|---|---|---|
| Age (years) | 64.3±15.5 | 63.7±16.4 | 67.5±13.7 | 0.003 |
| Male gender, n (%) | 225 (65.8) | 143 (65.2) | 82 (66.6) | 0.81 |
| Immune suppression, n (%) | 78 (22.8) | 37 (16.8) | 41 (33.3) | 0.001 |
| Community-acquired infection, n (%) | 243 (71.1) | 161 (73.5) | 82 (66.6) | <0.001 |
| Medical patients, n (%) | 198 (57.9) | 130 (59.3) | 68 (55.2) | 0.49 |
| Infection site, n (%) | ||||
| Abdomen | 132 (38.6) | 82 (37.4) | 50 (40.6) | 0.02 |
| Pneumonia | 105 (30.7) | 70 (31.9) | 35 (28.4) | |
| Urinary tract | 44 (12.9) | 34 (15.5) | 10 (8.1) | |
| Catheter | 26 (7.6) | 14 (6.3) | 12 (9.7) | |
| Skin/soft tissues | 14 (4.1) | 7 (3.1) | 7 (5.6) | |
| Others | 21 (6.1) | 12 (5.4) | 9 (7.3) | |
| Origin, n (%) | ||||
| Other hospitals | 50 (14.6) | 32 (14.6) | 18 (14.6) | 0.14 |
| Emergency care | 120 (35.1) | 83 (37.8) | 37 (30) | |
| Medical ward | 56 (16.4) | 28 (12.7) | 28 (22.7) | |
| Surgical ward | 116 (33.9) | 76 (34.7) | 40 (32.5) | |
| APACHE II | 22.4±6.7 | 20.4±5.6 | 26.8±7.2 | <0.001 |
| SOFA | 9.1±3.2 | 8.5±2.6 | 10.8±3.3 | <0.001 |
| Organ dysfunction (number) | 3.4±1.3 | 3.2±1.2 | 3.8±1.3 | <0.001 |
| PCT (ng/ml) | 29.2±49.4 | 27.09±55.3 | 22.2±29.1 | 0.37 |
| CRP (mg(dl) | 20.9±12.4 | 20.9±13.4 | 20.0±13.5 | 0.45 |
| ScvO2(%) | 69.2±13.3 | 69.6±12.4 | 67.1±15.0 | 0.02 |
| Lactate (mg/dl) | 34.7±29.4 | 30.0±25.3 | 40.6±32.5 | <0.001 |
| Mechanical ventilation, n (%) | 229 (67) | 119 (54.3) | 110 (89.4) | <0.001 |
| ARDS, n (%) | 160 (46.8) | 78 (35.6) | 82 (66.2) | <0.001 |
| CVVHDF, n (%) | 65 (19.0) | 27 (12.3) | 38 (30.8) | <0.001 |
APACHE II, Acute Physiology and Chronic Health Evaluation; CVVHDF, continuous venovenous hemodiafiltration; CRP, C-reactive protein; PCT, procalcitonin; ARDS, adult respiratory distress syndrome; SOFA, Sepsis-related Organ Failure Assessment; ScvO2, central venous oxygen saturation.
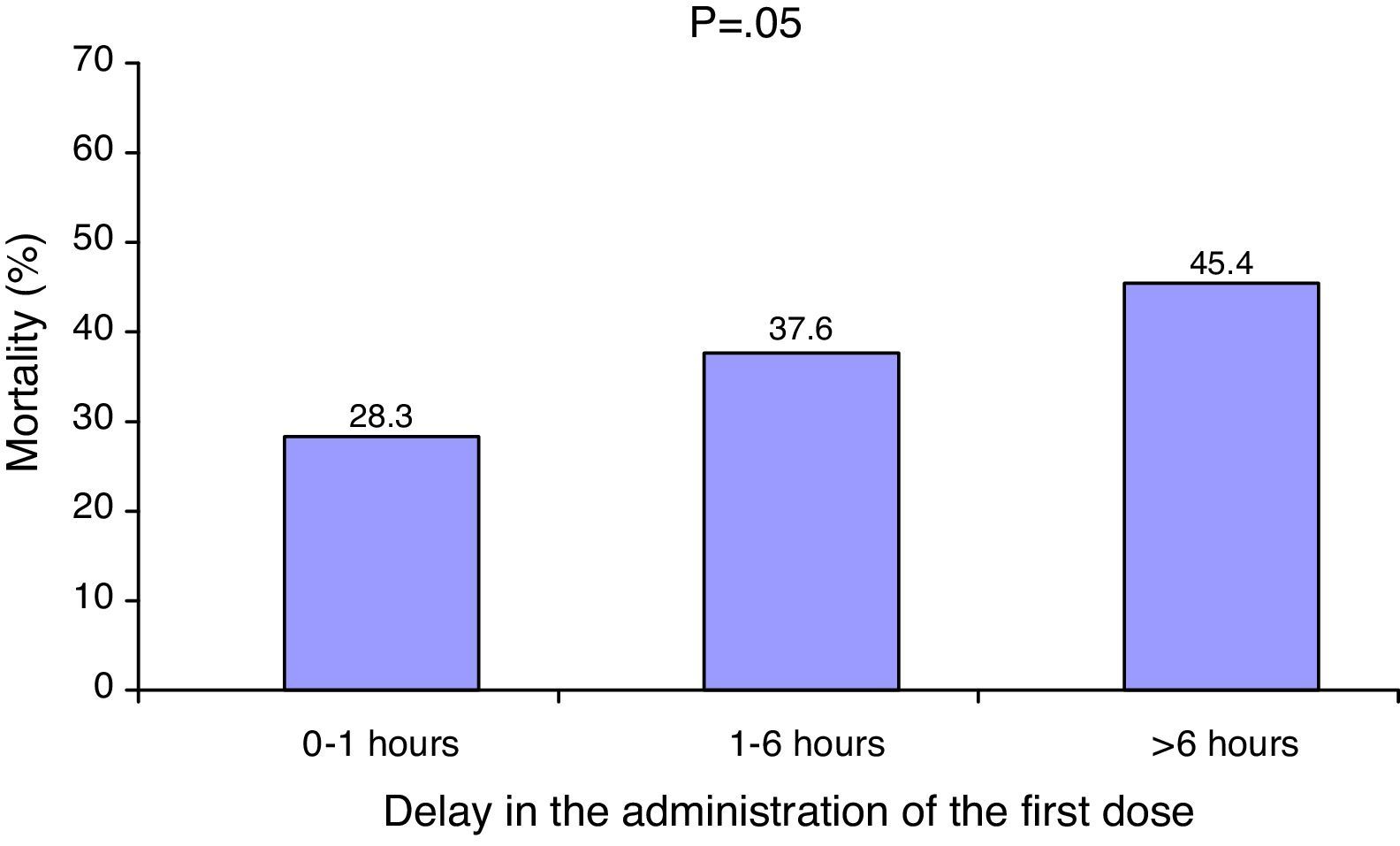
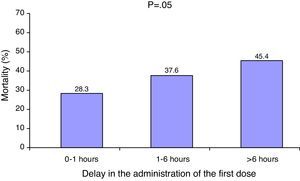
The median delay in administration of the first dose of antibiotic was 1.7h (interquartile range 0–5.5h). A total of 155 patients (43.5%) received the antibiotic treatment within the first hour. The patients who died received the antibiotic significantly later than the survivors (2.7h [interquartile range 0.3–6.2h] vs. 1h [interquartile range 0–4.1h]; p=0.001). There was a significant correlation between increased mortality and a delayed start of antibiotic treatment (Fig. 1).
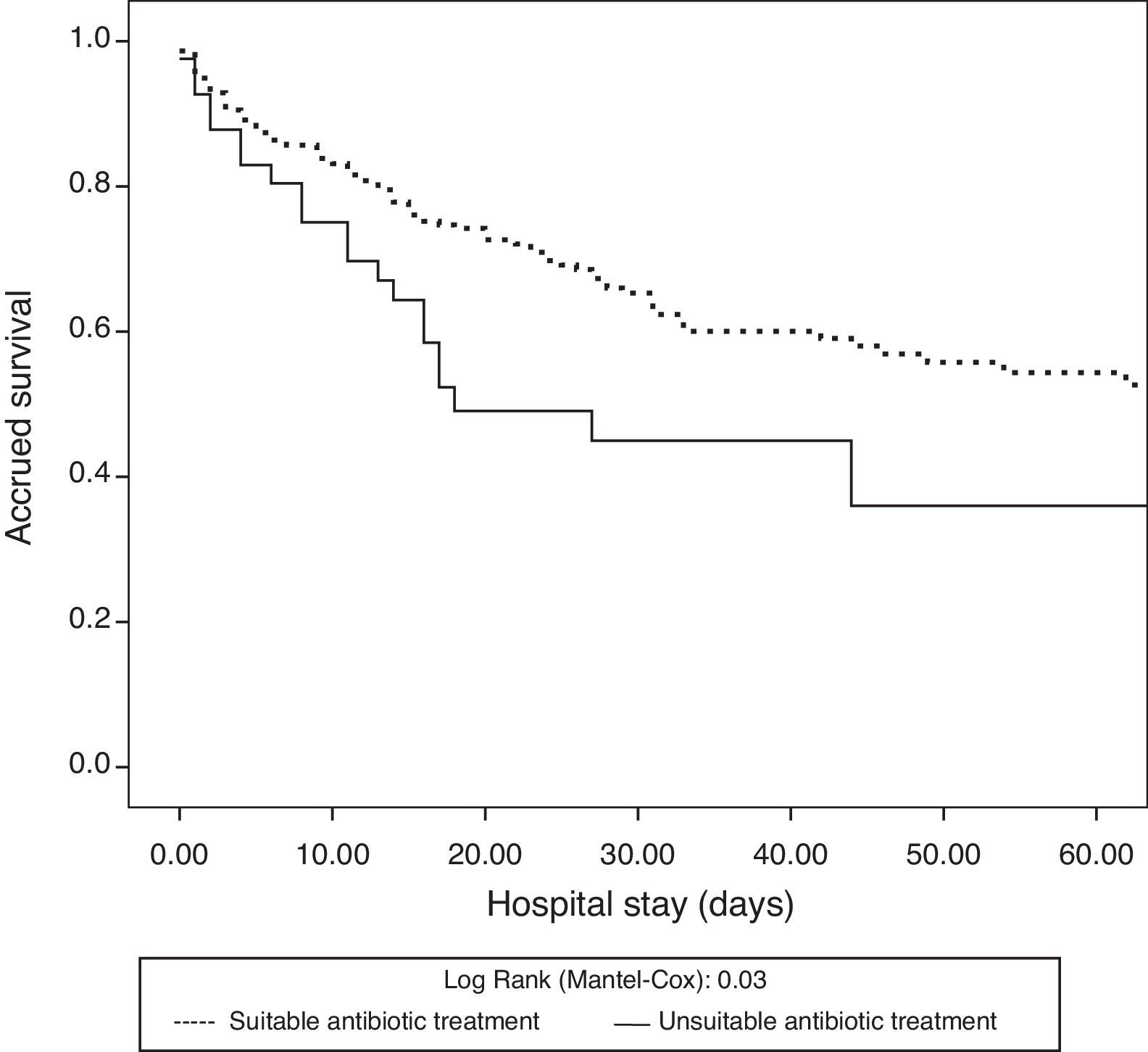
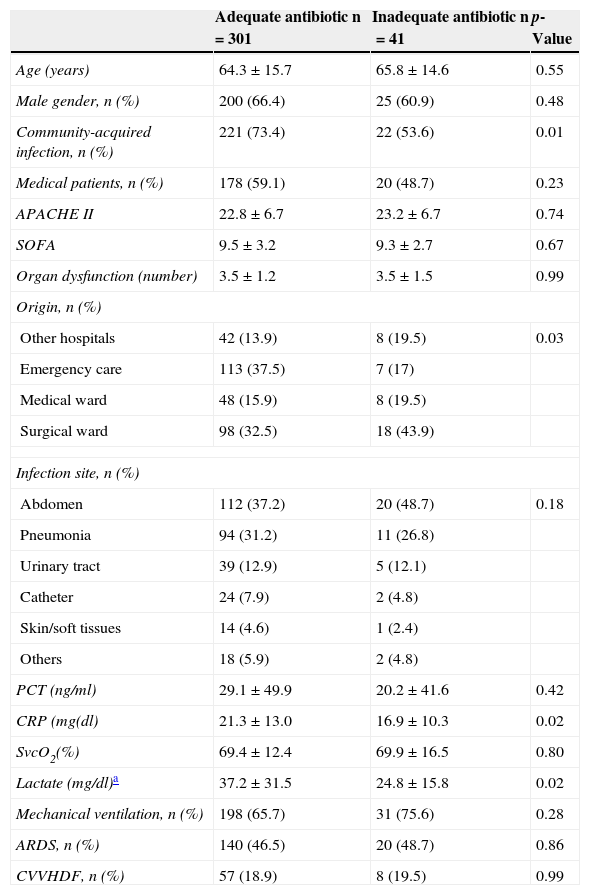
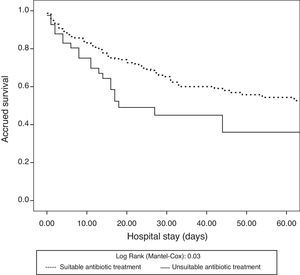
The antibiotic treatment inadequacy rate was 12% (41/342). The patients who received inadequate antibiotic treatment showed a higher frequency of nosocomial infections and came from hospital admission wards. There were no significant differences in demographic characteristics, severity scores, type of patient (medical or surgical) or infection site responsible for septic shock between these patients and the individuals who received adequate treatment (Table 2). The patients who received inadequate therapy had a significantly higher in-hospital mortality rate (33.8% vs. 51.2%; p=0.03) than those who received adequate treatment. Likewise, there were significant differences in the survival analysis between the patients who received adequate treatment and those who did not (log rank test: 0.03) (Fig. 2).
Differences between patients with and without adequate antibiotic treatment.
| Adequate antibiotic n=301 | Inadequate antibiotic n=41 | p-Value | |
|---|---|---|---|
| Age (years) | 64.3±15.7 | 65.8±14.6 | 0.55 |
| Male gender, n (%) | 200 (66.4) | 25 (60.9) | 0.48 |
| Community-acquired infection, n (%) | 221 (73.4) | 22 (53.6) | 0.01 |
| Medical patients, n (%) | 178 (59.1) | 20 (48.7) | 0.23 |
| APACHE II | 22.8±6.7 | 23.2±6.7 | 0.74 |
| SOFA | 9.5±3.2 | 9.3±2.7 | 0.67 |
| Organ dysfunction (number) | 3.5±1.2 | 3.5±1.5 | 0.99 |
| Origin, n (%) | |||
| Other hospitals | 42 (13.9) | 8 (19.5) | 0.03 |
| Emergency care | 113 (37.5) | 7 (17) | |
| Medical ward | 48 (15.9) | 8 (19.5) | |
| Surgical ward | 98 (32.5) | 18 (43.9) | |
| Infection site, n (%) | |||
| Abdomen | 112 (37.2) | 20 (48.7) | 0.18 |
| Pneumonia | 94 (31.2) | 11 (26.8) | |
| Urinary tract | 39 (12.9) | 5 (12.1) | |
| Catheter | 24 (7.9) | 2 (4.8) | |
| Skin/soft tissues | 14 (4.6) | 1 (2.4) | |
| Others | 18 (5.9) | 2 (4.8) | |
| PCT (ng/ml) | 29.1±49.9 | 20.2±41.6 | 0.42 |
| CRP (mg(dl) | 21.3±13.0 | 16.9±10.3 | 0.02 |
| SvcO2(%) | 69.4±12.4 | 69.9±16.5 | 0.80 |
| Lactate (mg/dl)a | 37.2±31.5 | 24.8±15.8 | 0.02 |
| Mechanical ventilation, n (%) | 198 (65.7) | 31 (75.6) | 0.28 |
| ARDS, n (%) | 140 (46.5) | 20 (48.7) | 0.86 |
| CVVHDF, n (%) | 57 (18.9) | 8 (19.5) | 0.99 |
APACHE II, Acute Physiology and Chronic Health Evaluation; CVVHDF, continuous venovenous hemodiafiltration; CRP, C-reactive protein; PCT, procalcitonin; ARDS, adult respiratory distress syndrome; SOFA, Sepsis-related Organ Failure Assessment; ScvO2, central venous oxygen saturation.
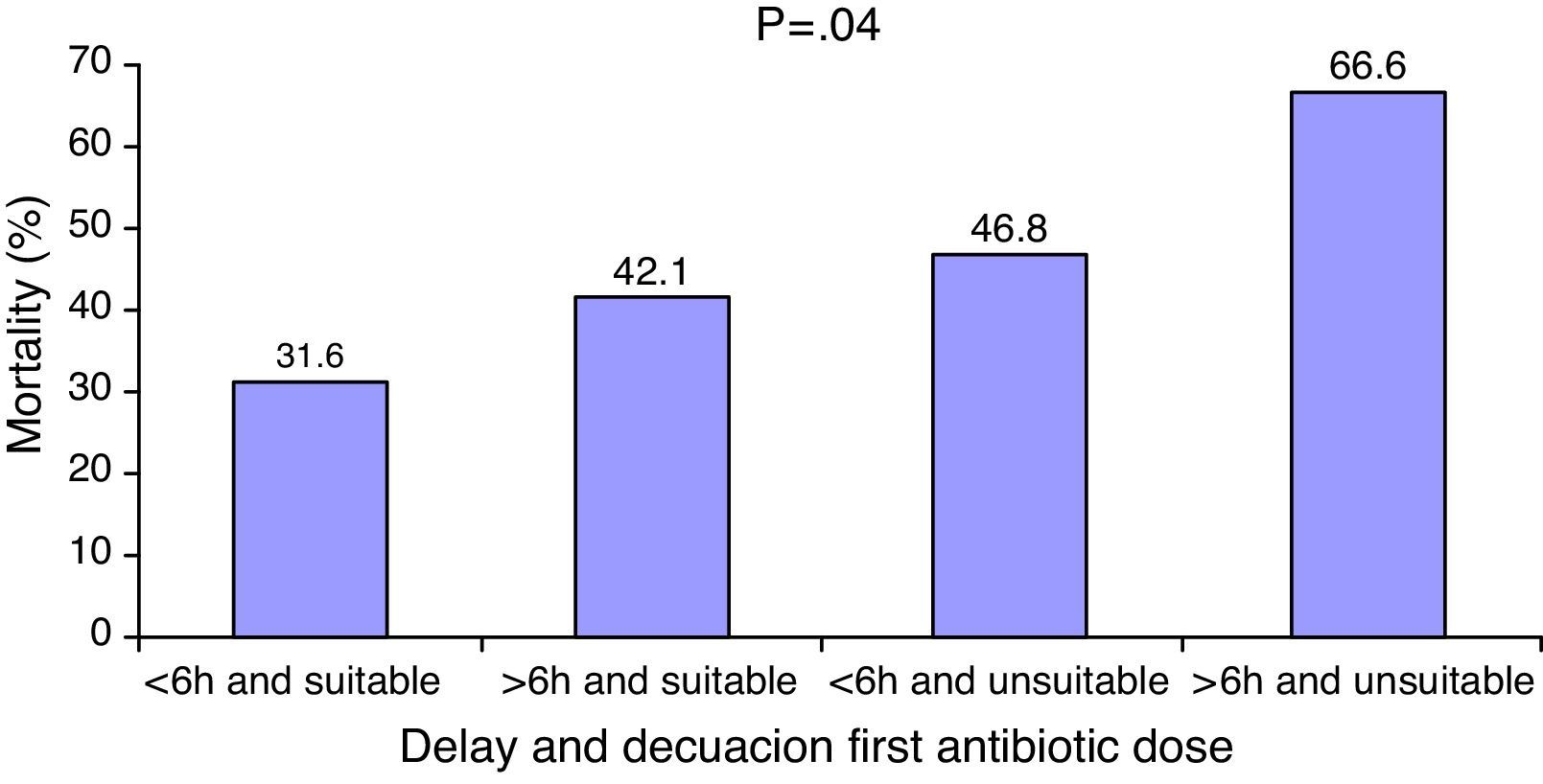
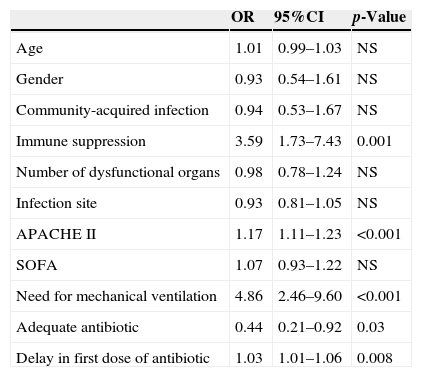
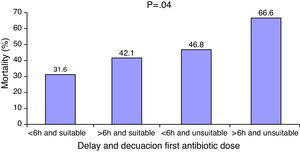
The coexistence of antibiotic treatment delay and inadequacy was associated to poorer patient survival (Fig. 3). In the logistic regression analysis, after adjusting for potential confounding factors, the inadequacy of treatment (OR: 0.44 [95%CI: 0.21–0.92]; p=0.03) and delays in treatment administration (OR: 1.01 [95%CI: 1.01–1.06]; p=0.008) were identified as independent survival markers (Table 3).
Logistic regression analysis referred to in-hospital mortality.
| OR | 95%CI | p-Value | |
|---|---|---|---|
| Age | 1.01 | 0.99–1.03 | NS |
| Gender | 0.93 | 0.54–1.61 | NS |
| Community-acquired infection | 0.94 | 0.53–1.67 | NS |
| Immune suppression | 3.59 | 1.73–7.43 | 0.001 |
| Number of dysfunctional organs | 0.98 | 0.78–1.24 | NS |
| Infection site | 0.93 | 0.81–1.05 | NS |
| APACHE II | 1.17 | 1.11–1.23 | <0.001 |
| SOFA | 1.07 | 0.93–1.22 | NS |
| Need for mechanical ventilation | 4.86 | 2.46–9.60 | <0.001 |
| Adequate antibiotic | 0.44 | 0.21–0.92 | 0.03 |
| Delay in first dose of antibiotic | 1.03 | 1.01–1.06 | 0.008 |
APACHE II, Acute Physiology and Chronic Health Evaluation; 95%CI, 95% confidence interval; NS: statistically nonsignificant; OR, odds ratio; SOFA, Sepsis-related Organ Failure Assessment; ScvO2, central venous oxygen saturation.
Our results confirm that independently of the infection site involved, the origin of the infection (nosocomial or community-acquired) or the severity of the condition, delays in the start of antibiotic treatment and inadequate antibiotic treatment in septic shock patients exert a cumulative deleterious effect upon in-hospital survival.
The importance of the early administration of antibiotic treatment in patients with severe sepsis and septic shock is evident. With some exceptions, the studies conducted to date confirm the existence of a strong association between the prompt administration of antibiotic treatment and improved survival rates, shorter hospital stays and increased healthcare cost savings.8–11,13 Indeed, some authors suggest that the beneficial effects of the SSC “bundle” of resuscitation measures may be due to the impact of the early administration of antibiotic treatment.1 Although the results of our study support the importance of early antibiotic treatment, previous experiences of our group have shown that the increase in survival is largely conditioned by the number of measures applied, and not so much by the nature of such measures.2 In our study, the median time to administration of the antibiotic was 1.7h, which is slightly shorter than in many of the studies found in the literature.14–17 Regarding the impact upon mortality, the latter was found to increase 1% for every hour of delay in starting antibiotic treatment, regardless of the origin of the patient, the severity score, or the origin of the infection. The effect was less pronounced than that described by Kumar et al.,8 who in their study published in 2006 reported a decrease in survival of 7.6% for every hour of delay. However, it must be taken into account that the study of Kumar et al. only included patients with adequate antibiotic treatment, and that the delays were quantified from the time of hypotension onset, while in our case the reference used was the time of manifestation of septic shock–excluding patients who had responded to fluid therapy. Our results are similar to those reported by Gaieski et al.14 or more recently by Ferrer et al.13 These latter investigators, in the largest patient population published to date, found the negative impact of a delayed start in antibiotic treatment to be independent of the hospital area in which the patient is located or the severity of the patient condition.13 Although the sample size is not comparable, the characteristics of our series (heterogeneous and derived from different hospital areas) are similar to those of the aforementioned study. Other studies such as those published by Puskarich et al.17 or Bloos et al.16 have been unable to reproduce the results obtained by Kumar et al.–a fact that could be explained by differences in the study populations or in the methodology employed.
The use of an inadequate antibiotic regimen is not uncommon, and occurs in 11%18 to 44%10 of the cases, depending on the series. Treatment inadequacy has been associated to deleterious effects upon the survival of patients with bacteremia, peritonitis, ventilator associated pneumonia and sepsis.9,14,18–22 However, although it seems obvious that a wrong choice of antibiotic can influence the prognosis of septic patients, a number of studies have been unable to demonstrate such an effect.23–26 This could be because the impact of inadequacy upon mortality may be influenced by patient severity,27,28 the infection site producing sepsis28 or the causal microorganism,29 among other factors. The studies published to date show that the more serious the condition of the patient, the greater the potential benefit obtained from adequate antibiotic administration. The study conducted by Kumar et al.29 in over 5000 septic shock patients is the series reporting the greatest differences in mortality according to the suitability of antibiotic treatment (with 5-fold higher mortality in the group receiving inadequate treatment). On the other hand, Garnacho-Montero et al., in a group of 406 septic patients, found the greatest differences in survival in relation to the adequacy of treatment to correspond to the most seriously ill patients–those with organic dysfunction associated to sepsis.28 The results of our own study support this observation. In a population exclusively composed of septic shock patients with high severity scores and an average of three dysfunctional organs, adequate antibiotic treatment was associated to a 50% decrease in mortality risk. On the other hand, the impact of adequate therapy may be attenuated if administration is delayed. The mortality rate we recorded in the patients with adequate treatment administered in the first 6h was 31% vs. 46% in those who received inadequate treatment. These differences in turn decreased to one-third when adequate therapy was provided beyond the first 6h (42% vs. 46%). Bloos et al. recently published similar results, though in their case the recorded differences in mortality were smaller, probably because the patients involved were in less serious condition and therefore (as has been commented) were less sensitive to the impact of inadequate antibiotic treatment.16
In application to septic patients, the SSC recommends the use of broad spectrum antibiotics as soon as possible, and always within the first hour of onset of the condition (in the case of patients already in hospital) or in the first 3h (in the case of patients in the Emergency Care Department).30 Although the adoption of these recommendations in clinical practice has resulted in a shortening of the time to administration of antibiotic treatment, an increase in patient survival, and sociosanitary cost savings, different studies have highlighted the difficulties in transferring these recommendations to daily practice.31,32 This situation is well known and affects not only patients with sepsis but also individuals with disorders as common as ischemic heart disease.33 Thus, considering the benefits obtained, we must favor initiatives designed to overcome the barriers limiting the administration of antibiotic treatment according to the recommendations of the CSS – dedicating special attention to the hospital admission areas, since these are the hospital settings with the longest reaction times and with the highest inadequacy rates.34
Our study has a number of limitations, including the fact that generalization of the results to other patient populations may be limited, since this is a single-center study involving a high proportion of immunosuppressed patients. Furthermore, our analysis was limited to patients with microbiologically confirmed infections; we therefore do not know whether the results are applicable to patients with systemic infections in the absence of microbiological findings. No evaluation was made of either the dose of antibiotic or the administration interval as factors that may influence treatment suitability. We therefore do not know how such factors could have affected the results obtained. Lastly, we have not been able to extend the multivariate analysis to include other treatments such as control of the infectious site or hemodynamic resuscitation. It is therefore not possible to establish whether the decrease in mortality is an effect of antibiotic treatment alone or the summative effect of earlier global patient management.
ConclusionsBoth delays in the start of antibiotic treatment and inadequate antibiotic treatment have negative effects upon the survival of patients with septic shock, independently of the characteristics of the patients or their severity. Measures therefore should be adopted to ensure identification and treatment as early as possible, using broad-spectrum antibiotics for this purpose.
Financial supportThis study received financial support from the Spanish Ministry of Health through FIS grant PI070723.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Suberviola Cañas B, Jáuregui R, Ballesteros MÁ, Leizaola O, González-Castro A, Castellanos-Ortega Á. Efectos del retraso y la inadecuación del tratamiento antibiótico en la supervivencia de los pacientes en shock séptico. Med Intensiva. 2015;39:459–466.