Sedation plays a fundamental role in the management of critically ill patients. However, available intravenous sedatives are limited and associated with known risks and challenges. Volatile anesthetics (VA) have recently emerged as an attractive alternative for intensive care unit (ICU) sedation. Due to their favorable pharmaco-kinetic and dynamic profile and well-documented potential advantages,1 these agents have been progressively introduced into clinical practice. Currently, two specific devices, the Sedaconda Anesthetic Conserving Device (Sedaconda-ACD®, Sedana Medical, Sweden) and the MIRUS® system (Pall Medical, Germany) are in clinical use and the Sedaconda-ACD®, has been approved in 16 European countries, including Spain, for ICU sedation with Isoflurane.1 Reduced wake-up times and lack of accumulation but also potential benefits beyond hypnosis have increased the interest in Isoflurane for ICU sedation. These include: (1) a lung protective effect by reducing the pulmonary release of pro-inflammatory cytokines,2 which could be particularly beneficial for acute respiratory distress syndrome (ARDS) patients. (2) selective bronchodilation, related to the combination of direct airway smooth muscle relaxation, reduced inflammatory activity and suppression of vagally mediated reflexes.3 As a result, Isoflurane has been proposed for first line treatment of severe bronchospasm complicating mechanical ventilation and in patients with ARDS.4 (3) due to its antiepileptic effect, Isoflurane has been explored as a rescue therapy in refractory and super-refractory status epilepticus. It is thought to suppress seizure activity by inhibiting N-methyl-D-aspartic acid excitotoxicity and activating γ-Aminobutyric acid receptors.5 (4) promotion of a lung-diaphragmatic protective ventilation. By better preserving the respiratory drive than intravenous sedatives6 VA could foster an easier and safer transition to spontaneous breathing. Careful adjustment of VA could modulate the intensity of the respiratory drive reducing lung and diaphragmatic stress and promoting early diaphragmatic activity could prevent disuse atrophy.
However, and despite these potential benefits, the expectations generated must be carefully balanced against still existing uncertainties and insufficient evidence about efficacy, safety, implementation barriers, and effects beyond hypnosis.
Isoflurane has proven to be non-inferior to propofol in terms of efficacy and safety in short sedation periods and it has been suggested that it may facilitate ventilator weaning.7,8 A reduced median wake-up time on day 2 of isoflurane as compared with propofol was observed. However, this difference was 10 min in a trial where the maximum sedation time was 54 h, making it difficult to extrapolate these results to critically ill patients subjected to prolonged ventilation, where an alternative to intravenous sedation is more necessary. No differences in length of mechanical ventilation were found,7 even in the post hoc analyses between patients who continued on isoflurane versus those on propofol.9 Despite the promising results observed in the short term, long-term effects and outcomes associated with Isoflurane for ICU sedation are not yet fully understood. Currently, comparative studies with propofol are underway, focusing on outcomes such as length of mechanical ventilation, delirium or spontaneous breathing effort (NCT05312385, NCT05327296, NCT04341350).
The anti-inflammatory and lung-protective effects of VA in ARDS are only supported by preclinical experimental models and studies,2 and there is currently no evidence to support that these findings are applicable to ICU patients. Regarding the bronchodilation properties, most data are also based on experimental studies.10,11 The scarce available clinical evidence is either derived from non-asthmatic patients12 or limited to case reports13 and the most extensive series focus on pediatric patients.14–16 While the underlying mechanisms and outcomes observed in these studies could potentially be extrapolated to adults, robust evidence is still lacking.3
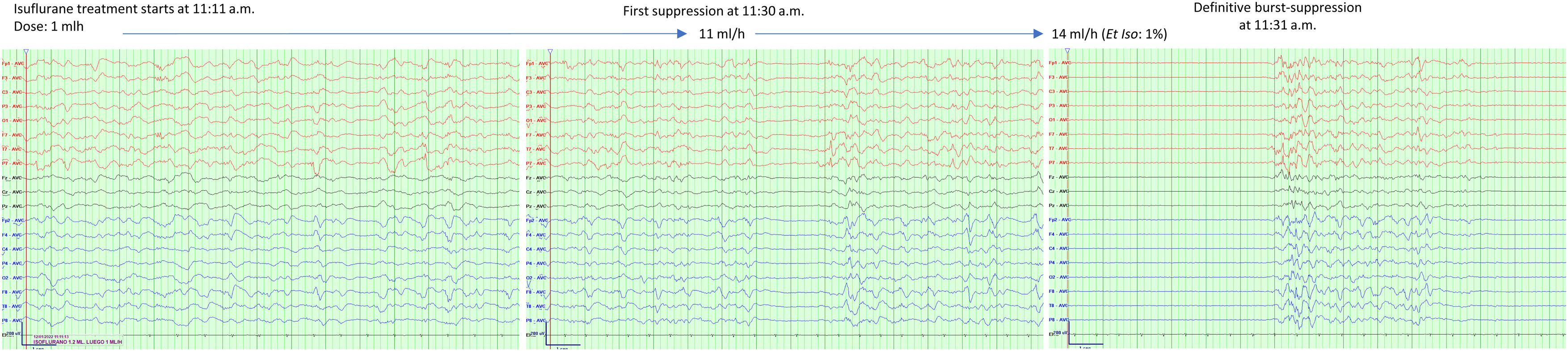
Among VAs, Isoflurane has the most substantial evidence, consistently and reliably inducing a rapid almost immediate electroencephalographic burst suppression response. Fig. 1 presents an example of the induction of a burst suppression pattern in one of our patients. Isoflurane has been associated with sustained termination of status epilepticus in up to 51% of patients, and in 29% of them were terminated without any need for additional therapy.17 However, the treatment is associated with side effects. Hypotension (related to high required doses), is reported in 89% of patients,17 and relapse in 41% of patients.18 Additionally, observed hippocampal changes on Magnetic Resonance Imaging raise questions about its neuroprotective efficacy.19
Inhaled sedation is delivered using reflectors (anesthetic-conserving devices) that increase instrumental dead space. Although currently used reflectors have reduced their volume to 50 ml, there is still a concern especially during low tidal volume ventilation where it can add up to 10% of the dead-space fraction. This requires careful monitoring of arterial and end-tidal partial pressure of CO2, strict avoidance of any unnecessary additional instrumental dead space (i.e., connectors, etc) and proper training of ICU professionals to minimize potential associated risks. Neglecting these precautions could easily offset the alleged benefits on the respiratory drive, breathing pattern and diaphragmatic protective ventilation, benefits that remain yet to be proven clinically. A still unsolved problem is the combination of current devices with active humidification systems. In the case of Mirus®, such a combination is not possible. In the case of Sedaconda®, the distal configuration can be used, but this configuration implies the need for higher doses of VA, since reflection of the drug is not possible.
In conclusion, reliable and fast-acting hypnotics are needed to expand the narrow range of currently available options for ICU sedation. Isoflurane represents a very interesting and useful addition to the therapeutic armamentarium, offering new attractive possibilities, although remaining uncertainties related to long-term sedation periods and other potential benefits beyond hypnosis need to be addressed and supported by more consistent evidence in ICU patients. We believe that future randomized clinical trials will better establish the indications and target population of VA in ICU improving the options for critically ill patient’s sedation. Nevertheless, the inherent complexity in the design and execution of such trials impose important challenges for the generation of future robust evidence.
CRediT authorship contribution statementJMA, MPE, JON, APL and FSS contributed to the initial concept and design. JMA and FSS participated in the final draft of the manuscript. All authors read and approved the final manuscript.
Declaration of Generative AI and AI-assisted technologies in the writing processNon-use of some form of AI.
FundingNo funding.