A study is conducted on the impact of a Sepsis Code Hospital Protocol upon antibiotic use, hospital stay and mortality.
DesignA quasi-experimental, retrospective observational study was carried out.
SettingA polyvalent ICU with 11 beds belonging to a tertiary hospital.
PatientsPatients admitted to the ICU with severe sepsis or septic shock.
InterventionsA post-intervention group (POST-SC) (September 2012–August 2013) was compared with a historical control group (PRE-SC) (January–December 2010).
VariablesType of antibiotic treatment, antibiotic therapeutic strategy and clinical outcomes. Antibiotic use was expressed as defined daily doses/100 stays.
ResultsA total of 42 patients with severe sepsis or septic shock were included in the POST-SC group and 50 in the PRE-SC group. Total antibiotic consumption (defined daily doses) was similar in both groups. In the POST-SC group the gradual reduction rate was significantly greater (75% vs 30.8%; p<0.005), while the restricted use antibiotic prescription rate was significantly lower (74% vs 52%; p=0.031). Lastly, the POST-SC group presented significantly lower mortality both in hospital and after 28 days (23% vs 44% [p=0.035] and 31% vs 56% [p=0.01], respectively), as well as a decrease in ICU stay at the limit of statistical significance (5 vs 10.5 days; p=0.05).
ConclusionThe implementation of a Sepsis Code Hospital Protocol was associated with improved antibiotic use, with a significant increase in gradual therapeutic reduction, a lower use of restricted use antibiotics, a significant reduction in mortality, and a tendency towards a shorter ICU stay.
Se analiza el impacto de un Código Sepsis intrahospitalario sobre el uso y consumo de antibióticos, la estancia hospitalaria y la mortalidad.
DiseñoEstudio retrospectivo cuasiexperimental observacional.
ÁmbitosUCI polivalente de 11 camas en un hospital de tercer nivel.
PacientesPacientes ingresados en UCI con diagnóstico de sepsis grave o shock séptico.
IntervencionesUn grupo postintervención (POST-CS) (septiembre 2012-agosto 2013) se comparó con un grupo histórico (PRE-CS) control (enero-diciembre 2010).
VariablesTipo de tratamiento antibiótico, estrategia terapéutica antibiótica y resultados clínicos. El consumo de antibióticos fue expresado en dosis diarias definidas/100 estancias.
ResultadosSe incluyeron 42 pacientes con sepsis grave/shock séptico en el grupo POST-CS y 50 en el grupo PRE-CS. El consumo total de antibióticos (dosis diarias definidas) fue similar en ambos grupos. En el grupo POST-CS la tasa de desescalamiento fue significativamente mayor (75 vs 30,8%, p<0,005), mientras que la prescripción de antibióticos de uso restringido fue significativamente menor (74 vs 52%, p=0,031). Finalmente, el grupo de pacientes POST-CS presentó una mortalidad intrahospitalaria y a 28 días significativamente menor (23 vs 44% [p=0,035] y 31 vs 56% [p=0,01]), así como una disminución de la estancia en UCI en el límite de la significación estadística (5 vs 10,5 días, p=0,05).
ConclusiónLa implantación de un programa de Código Sepsis intrahospitalario se asoció a una mejor utilización del tratamiento antibiótico, incrementándose significativamente el desescalamiento terapéutico y disminuyendo el uso de antibióticos de uso restringido, así como a una significativa disminución de la mortalidad y una tendencia hacia una menor estancia en UCI.
Severe sepsis is one of the most common diseases in hospitals, especially in intensive care units (ICU).1 Recent epidemiological studies demonstrate that its incidence is increasing,2 surpassing other diseases such as stroke, cancer, or myocardial infarction. In addition to resulting in a significant consumption of resources, this disease has a high mortality rate, which in the case of septic shock can reach figures around 50%.3,4 Although until just a few years ago antibiotics were the cornerstone of sepsis treatment, and bearing in mind their great importance, it has been seen that the chances of surviving this condition depend in large part on getting an early diagnosis and starting an appropriate treatment approach early, since it is a time-dependent disease.5,6 Since Rivers et al.7 published their paper in 2001, which demonstrated how an early and “aggressive” intervention in the first 6h consisting of following an action protocol guided by specific clinical targets decreased mortality by 16%, successive guidelines and recommendations for the clinical management and treatment of patients with severe sepsis or septic shock have been published worldwide; the last main example was the project known as the Surviving Sepsis Campaign,8 with the aim of decreasing sepsis mortality by 25%.
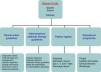
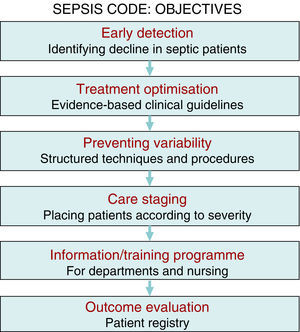
There is sufficient scientific evidence to assert that the early and targeted application of these diagnostic/therapeutic measures (bundles) significantly increase survival and decrease both the hospital stay and healthcare costs.9 However, despite publicity and educational campaigns, the degree of compliance with these measures continues to be low in most hospital settings. A recent paper by Ferrer et al.10 demonstrated how the management and treatment measures in the first 6 and 24h recommended by the guidelines were only taken in 10% and 15.7% of cases, respectively. This fact translated to a slight decrease in the mortality and resource consumption figures, far from the expectations put forth by the Surviving Sepsis Campaign. Since delays in diagnosis, initial resuscitation, administering the right antibiotic therapy, and controlling the focus of infection raise the incidence of organ failure, mortality, hospital stay, and resource consumption,11,12 and also knowing that the degree of compliance with the recommended measures for the initial management of sepsis is low, in recent years different hospitals have started organising multidisciplinary work groups for comprehensive severe sepsis management. In our centre, the Sepsis Code Working Group (SC-WG) was created in 2011. It is a multidisciplinary group with the aim of recognising and treating severe sepsis and septic shock early by implementing a series of interventions: training and information sessions, drafting action protocols and software help tools, as well as creating records in a database (Fig. 1).
This paper analysed the effects of implementing a Sepsis Code Hospital Protocol (SCHP) for the critical patients admitted to the ICU with a diagnosis of severe sepsis or septic shock. The primary objective is to analyse the changes in the profile and appropriateness of the antibiotic therapy used and, secondly, to analyse the differences in the clinical outcomes between the two study groups.
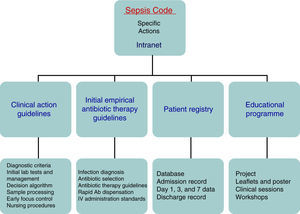
Patients and methodsThe Hospital Clínico Universitario de Valladolid SC-WG was established in May 2011 with the aim of creating, spreading, and implementing a hospital improvement plan for providing comprehensive care for patients with severe sepsis and/or septic shock (Fig. 1). The SC-WG is formed by a select group of physicians from different specialties and nursing staff. The actions that were carried out included implementing an educational and information programme with several training sessions in medical and nursing departments, creating clinical action guidelines on septic patients in both a paper and electronic format (diagnosis algorithms, severity staging, decision and treatment trees on leaflets and posters, etc.), initial antibiotic therapy guidelines in severe sepsis and septic shock, infection focus control guidelines, collection, handling, and transport guidelines for biological samples for culture, drug preparation and administration guidelines, as well as a rapid dispensation system for antibiotics. Moreover, a software application integrated into the hospital intranet and a digital registry for including and following patients included in the SCHP were designed (Fig. 2).
Study design and variablesThe study was conducted in the 11-bed medical/surgical ICU at the Hospital Clínico Universitario de Valladolid. It was a quasi-experimental, retrospective, observational study in which patients diagnosed with severe sepsis/septic shock upon admission to the ICU were included, according to the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.13
A consecutive population of septic patients cared for during the 12 months after the implementation of the SCHP (September 2012–August 2013) (POST-SC) were compared against a historic cohort of septic patients, also collected consecutively in 2010 in the same centre, prior to the implementation of the abovementioned SC (PRE-SC).
The patients’ severity was estimated using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score at the time of admission to the ICU.
Patients under 18 and those who developed severe sepsis/septic shock during their stay in the ICU were excluded from the study, because as there was another reason for admission. Patients admitted to the ICU for under 24h were not considered for antibiotic consumption since in that situation it was not possible to calculate the defined daily doses (DDD), as no hospital stays were generated. Similarly, patients for whom it was decided to limit therapeutic efforts were excluded from the study. In the PRE-SC patient group, prescriptions were issued based on the treating physician's judgement. In the POST-SC group, the empirical antibiotic therapy in patients with severe sepsis or septic shock was chosen following the Initial Empirical Antibiotic Therapy Guidelines created for that purpose by the SC-WG.
Lastly, the microbiology and antibiotic prescription results were systematically reviewed on days 3 and 7, and the antibiotic therapy re-assessed in each case, as well as the progress and final clinical outcome.
The study was evaluated and approved by the Hospital Clínico Universitario de Valladolid Ethics and Research Committee. All the clinical and demographic data were collected and analysed in an anonymised form according to the Data Protection Act by using our centre's Research Data Management System.
Recording variablesEpidemiological, demographic, and clinical variables were recorded for the patients included in the study: age, sex, APACHE II, infection type (community-acquired or nosocomial), focus of infection, type of microorganism causing the infection, sepsis severity level (severe sepsis or septic shock), ICU and hospital stay and mortality in both cohorts.
Regarding the antibiotic therapy, the microbiology results from the cultures were reviewed and the appropriateness of the empirical treatment started in the ICU was assessed. The antibiotic group that was empirically prescribed was recorded, with a special mention for restricted use antibiotic prescriptions and the type of treatment strategy followed. The following antibiotics were considered to be restricted use: carbapenems, linezolid, tigecycline, and daptomycin. These antibiotics were considered equally restricted in both periods, under the control of the Pharmacy Department.
The appropriateness of the antibiotic therapy was determined according to the in vitro sensitivity of the isolated microorganisms (agents causing the infection). An empirical treatment was considered appropriate when at least one drug was effective according to the in vitro results.14 The antibiotic de-escalation was analysed, to understand how the antibiotic therapy was re-evaluated once the microbiology results were available, substituting the initially chosen empirical antibiotic with other narrower spectrum antibiotics.15 Based on this, the treatment strategy was classified into three groups:
- (a)
“De-escalated” treatment: interrupting an antibiotic or changing to a narrower-spectrum antibiotic, based on the microbiology, antibiogram, and focus of infection results.
- (b)
“Non-de-escalated” treatment: despite the availability of the microbiology results and benefit of changing to a different, more appropriate, narrower-spectrum antimicrobial, this was not done. Cases were not considered a “non-de-escalated” treatment if, despite having microbiological cultures available, changing to a narrower-spectrum antibiotic was not considered beneficial (e.g. in situations such as drug allergies, no indication for an antibiotic for a specific focus of infection, etc.).
- (c)
Change in treatment: adding a new antibiotic or changing to one with a broader spectrum.
The time (days) that a change in treatment was delayed in groups a and c was analysed, as well as the days of antibiotic treatment in the ICU and the total days of antibiotic treatment during their hospital stay. Likewise, the antibiotic consumption per treatment group and/or primary indication was measured.
Antibiotic consumption in the hospital setting was expressed as the number of DDD per 100 stays. This is an estimate of the number of DDD for every 100 patient stays, and reflects the pressure or use of a certain antibiotic on the patients who received care. Antibiotic consumption was taken into account during the days the infection for which they were admitted to the ICU lasted, as long as the prescription was a consequence of the initial severe sepsis/septic shock approach. Antibiotics that were prescribed for reinfections and/or colonisations during their stay in the ICU were not analysed, since that was not the study objective. Antifungal drug consumption was not analysed given the rare incidence of fungal infection in both cohorts: 3 patients in the PRE-SC group and 5 in the POST-SC group.
Lastly, the gross intra-ICU mortality, 28-day mortality, in-hospital mortality, and days of stay in the hospital and ICU were also recorded.
Statistical analysisContinuous variables were expressed as a median and interquartile range as the distribution was not normal. Qualitative variables were expressed as they absolute value accompanied by a percentage. The median values for the continuous variables from both groups were compared using the Mann–Whitney U test. Proportions were compared using the chi-squared test. To analyse the difference in mortality between the two periods, a univariate and multivariate binary logistic regression was used, creating a dichotomous variable called sepsis code (SC): yes=post-SC/no=pre-SC. The risk of in-hospital mortality was expressed as an odds ratio (OR) and 95% confidence interval (95% CI). The multivariate analysis included the variables that demonstrated differences with a p<0.1 in the univariate analysis. Similarly, a Kaplan–Meier curve survival analysis was performed. An alpha risk of p<0.05 was used to consider a correlation statistically significant. SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis.
ResultsPatient characteristicsThe PRE-SC study period included 50 consecutive patients diagnosed with severe sepsis/septic shock. The POST-SC group included 42 patients with severe sepsis/septic shock. Table 1 summarises the demographic and clinical characteristics of the two patient groups. There were no differences between the two groups in terms of age and sex. Likewise, there were no significant differences in the APACHE II score on the day of admission to the ICU. Although a higher tendency to be admitted for septic shock was observed in the pre-SC period, it was not statistically significant. Six patients from the PRE-SC group and 4 from the POST-SC group died within the first 24h of admission to the ICU. These patients were only excluded for the antibiotic consumption calculation, but they were taken into account when the rest of the variables were analysed.
Descriptive characteristics of the patients in both groups.
| Pre-SC, n=50 | Post-SC, n=42 | p | |
|---|---|---|---|
| Age in years, median (IQR) | 68.5 (57–75) | 65.0 (60–75) | 0.898 |
| Males, n (%) | 31 (62) | 24 (57) | 0.39 |
| APACHE II, median (IQR) | 21 (16–28) | 20 (15–27) | 0.8 |
| Community-acquired infection n (%) | 42 (88.7) | 39 (95.1) | 0.173 |
| Hospital-acquired infection n (%) | 8 (11.3) | 3 (4.9) | |
| Diagnosis at admission to the ICU, n (%) | |||
| Severe sepsis | 16 (32) | 17 (40.5) | 0.398 |
| Septic shock | 34 (68) | 25 (59.5) | |
| Focus of infection, n (%) | |||
| Abdomen | 8 (16) | 9 (21.4) | 0.1 |
| Respiratory | 23 (46) | 18 (42.8) | 0.8 |
| Urinary tract | 6 (12) | 10 (23.8) | 0.17 |
| Catheter | 3 (6) | 0 (0) | 0.24 |
| Other/unknown | 10 (20) | 5 (11.9) | 0.39 |
| Positive microbiology results, n (%) | 30 (68.2) | 28 (72) | 0.4 |
| Microorganism documented, n (%) | |||
| Gram-positive | 15 (46.9) | 7 (26.9) | 0.32 |
| Gram-negative | 10 (31.3) | 13 (50) | |
| Polymicrobial | 5 (15.6) | 5 (19.2) | |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; IQR, interquartile range; Post-SD, period after the Sepsis Code; Pre-SC, period before the Sepsis Code.
In both periods, most of the patients had community-acquired infections. There were no statistically significant differences in terms of the sepsis focus of origin. A higher trend towards isolating gram-negative bacteria as the causative agents of the infection was also observed in the post-SC period, but it did not reach statistical significance. It should be noted that the pathogen isolation rate was high and similar between both groups (68% vs 72%).
Antibiotic prescriptionsThe antibiotic treatment results are shown in Table 2. The percentage of patients who received appropriate empirical treatment was very high, and although no significant differences were found between the two groups, it was higher in the POST-SC group (86.7% vs 96.2%, p=0.45). Regarding empirical treatment, the most prescribed combination was carbapenem+linezolid. It was used more often in the pre-SC period than in the post-SC period, although it did not reach statistical significance (56.0% vs 39.3%, p=0.37).
Influence of implementing the Sepsis Code Hospital Protocol on the antibiotic therapy used, the treatment strategy, and the clinical outcomes.
| Pre-SC, n=50 | Post-SC, n=42 | p | |
|---|---|---|---|
| Total days of antibiotic therapy, median (IQR) | 18 (22) | 13 (9) | 0.267 |
| Days of antibiotic therapy in the ICU, median (IQR) | 11 (16) | 5 (8) | 0.016 |
| Appropriate empirical treatment, n (%) | 26 (86.7) | 25 (96.2) | 0.45 |
| Antibiotic groups used empirically, n (%) | |||
| Monotherapy | 7 (14) | 9 (21) | 0.81 |
| Carbapenem+linezolid | 18 (36) | 13 (31) | |
| Cephalosporin+macrolides | 6 (12) | 7 (17) | |
| Restricted antibiotics used empirically (carbapenem, linezolid, tigecycline, daptomycin), n (%) | 37 (74) | 22 (52) | 0.031 |
| Treatment strategy, n (%) | |||
| De-escalated | 8 (30.8) | 15 (75.0) | 0.006 |
| Non-de-escalated | 8 (30.8) | 4 (20.0) | |
| Change in treatment | 10 (38.5) | 1 (5) | |
| Days until de-escalated, median (IQR) | 4 (4) | 3 (2) | 0.280 |
| Clinical outcomes | |||
| Intra-ICU mortality, n (%) | 22 (44) | 12 (28) | 0.09 |
| In-hospital mortality, n (%) | 28 (56) | 13 (31) | 0.014 |
| 28-day mortality, n (%) | 22 (44) | 10 (23) | 0.035 |
| ICU stay in days, median (IQR) | 10.5 (10) | 5 (9) | 0.05 |
| Hospital stay in days, median (IQR) | 26.5 (31) | 16.5 (24) | 0.3 |
ICU, intensive care unit; IQR, interquartile range; Post-SD, period after the Sepsis Code; Pre-SC, period before the Sepsis Code.
Fewer days of antibiotic treatment were observed in the POST-CS group, with the antibiotic therapy duration being significant shorter during the ICU stay: OR 11 (95% CI 4–20) vs OR 5 (95% CI 2–10); p=0.016. Furthermore, it should be noted that in the POST-SC group, the use of restricted use antibiotics as an empirical treatment was significantly lower vs the PRE-SC group (74% vs 52%, p=0.031).
Once the microbiology results were available, antibiotic de-escalation was carried out significantly more in the POST-SC group than in the PRE-SC group (75% vs 30.8%, p=0.006). It should be noted that in the PRE-SC group, there were 4 patients who despite having microbiology results available could not be classified into any treatment strategy group: in one “de-escalating” was inappropriate, and therefore the patient was not classified into any group. The other 3 patients died before the definitive microbiology results were issued.
Similarly, there were 8 patients in the POST-SC group with microbiology isolations who also could not be classified into any treatment strategy: in 4 patients “de-escalating” was inappropriate and the remaining patients died before the definitive microbiology results were issued.
Antibiotic consumptionThe antibiotic consumption results are expressed as the number DDD/100 stays comparing both periods: pre- and post-SC. 6 and 4 patients were excluded in the PRE-SC and POST-SC groups, respectively, as they were admitted to the ICU for under 24h and there was no hospital stay.
The mean antimicrobial consumption in the PRE-SC group was 148.04 DDD/100 stays and 154.2 in the POST-SC group. Restricted use antibiotic consumption (carbapenem, linezolid, tigecycline) was higher in the PRE-SC group (104.1 vs 86.1 DDD/100 stays). The largest difference was observed with linezolid (30.4 vs 22.6 DDD/100 stays). In contrast, the consumption of third-generation cephalosporins and piperacillin-tazobactam was higher in the POST-SC group (Table 3).
Difference between the number of daily defined doses of antibiotics per 100 stays in patients with severe sepsis/septic shock admitted to the intensive care unit between the two periods.
| Antibiotics | Pre-SC period | Post-SC period | Difference | Percentage |
|---|---|---|---|---|
| Carbapenems | ||||
| Imipenem | 18.8 | 0.6 | ||
| Meropenem | 51 | 62.9 | ||
| Total | 69.8 | 63.5 | −6.3 | −9 |
| Third-generation cephalosporins | ||||
| Ceftriaxone | 12.70 | 28.17 | ||
| Cefotaxime | 3.1 | 0 | ||
| Total | 15.8 | 28.17 | +12.37 | +78 |
| Macrolides | ||||
| Azithromycin | 10.5 | 8.1 | −2.4 | −22.8 |
| Anti-pseudomonas penicillins and cephalosporins | ||||
| Piperacillin-tazobactam | 8.6 | 26.8 | ||
| Ceftazidime | 2.83 | 0.2 | ||
| Cefepime | 1.61 | 0 | ||
| Total | 13.04 | 27 | +13.96 | +107 |
| Antibiotics against resistant gram-positive bacteria | ||||
| Linezolid | 30.40 | 22.6 | ||
| Vancomycin | 0.2 | 2.7 | ||
| Total | 30.6 | 25.3 | −5.3 | −17.3 |
| Tetracyclines | ||||
| Tigecycline | 3.9 | 0 | −3.9 | −100 |
| Quinolones | 4.4 | 2.1 | −2.3 | −52.3 |
| Total DDD/100 stays | 148.04 | 154.2 | ||
DDD, daily defined dose; Post-SC, period after the Sepsis Code; Pre-SC, period before the Sepsis Code.
The clinical outcomes are shown in Table 2. In-hospital, intra-ICU, and 28-day mortality were higher in the PRE-SC group. Statistically significant differences were found in in-hospital mortality (56% vs 31% [95% CI 3.2–46.9], p=0.014) and in 28-day mortality (44% vs 23% [95% CI – 0.8 and 41.2], p=0.035). The intra-ICU stay was longer in the PRE-SC group than in the POST-SC group, with borderline statistical significance (10.5 vs 5 days, p=0.05).
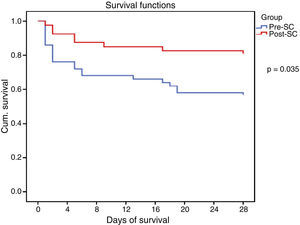
All the collected variables were included in a binary logistic regression model (BLR) for the mortality analysis. In the univariate analysis, only the SCHP, septic shock, and APACHE II variables showed statistically significant differences for in-hospital mortality and 28-day mortality. By including all the variables in the multivariate BLR model, they all continued to show an independent association with in-hospital mortality and 28-day mortality. Thus, the pre-SC variable presented an OR of 3.08 (95% CI 1.03–9.21, p=0.03) for 28-day mortality and 3.51 (95% CI 1.24–9.90, p=0.01) for in-hospital mortality (Table 4). Lastly, the Kaplan–Meier survival curve analysis demonstrated statistically significant higher 28-day mortality in the PRE-SC patient group (Fig. 3).
Multivariate binary logistic regression for 28-day and in-hospital mortality.
| 28-day mortality | In-hospital mortality | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| APACHE II | 1.17 (1.08–1.28) | 0.001 | 1.16 (1.07–1.26) | 0.001 |
| Shock | 6.34 (1.71–23.48) | 0.006 | 4.73 (1.50–14.89) | 0.008 |
| Pre-SC | 3.08 (1.03–9.21) | 0.039 | 3.51 (1.24–9.90) | 0.017 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; 95% CI, 95% confidence interval; OR, odds ratio; Pre-CS, period before the Sepsis Code.
This work demonstrates how a relatively economic intervention, albeit with significant human effort, such as implementing actions and tools aimed at improving the diagnosis and treatment of severe sepsis and septic shock by creating the SC-WG not only promoted a direct decrease in the mortality figures, but also better optimised antibiotic prescriptions, and interestingly a trend towards a shorter stay in the ICU.
The multidisciplinary nature of a project with this characteristics poses significant challenges concerning efficient teamwork, the dynamic, and group relations, cooperation, communication, and information exchange, and entails a significant endeavour for in-hospital training and information sessions. The primary objective for creating multidisciplinary SCHP teams is not only to apply the best evidence-based treatments (fluid therapy, sample collection for culture, antibiotic therapy, and early focus control, etc.), but to also optimise resources, avoid interprocedure variability, promote the spread of best practices, improve care and patient safety, as well as establish suitable care staging (placing the patients in the most appropriate place based on their severity at any given time) to, in the end, create a registry of the outcomes and analyse the programme's efficiency (Fig. 1). Therefore, the primary purpose is to decrease the high mortality rate associated with this disease, while other objectives, such as better optimisation of resource consumption resulting from this disease, are also achievable, as demonstrated in this study.
Some studies in the medical literature have already demonstrated the possibility of achieving the objective of decreasing mortality by implementing the use of bundles.16,17 However, the repercussions of implementing the SCHP programmes on antibiotic treatment optimisation had not been analysed until now. This optimisation would consist of improving and rationalising the antibiotic consumption and prescriptions through measures such as establishing more appropriate and rational antibiotic coverage, early antibiotic de-escalation, selecting the appropriate drug, and personalised treatment adjustments.
In this sense, this paper is innovative, since it analyses this relationship and shows satisfactory results. So it demonstrates a decrease in the use of the linezolid/carbapenem combination as an empirical treatment in the post-intervention period on the one hand, while a very significant increase in the antibiotic de-escalation rate was observed during the post-SC period on the other. The first studies on antibiotic “de-escalation” were conducted in critical patients with nosocomial pneumonia. One of these studies, conducted in 24 Spanish ICUs prospectively analysed 244 pneumonia patients treated empirically with imipenem plus aminoglycoside or vancomycin, and no influence from the de-escalation was observed on mortality.18 However, in a recent study by Garnacho-Montero et al., antibiotic therapy de-escalation in a population of 628 patients with severe sepsis or septic shock was independently correlated with a lower in-hospital 28-day mortality rate.19 In our case, the BLR analysis did not demonstrate that de-escalation was a variable that was correlated with mortality. The limited sample size could have affected this finding.
In terms of antibiotic consumption, a statistically significant decrease in the number of days under antibiotic therapy in the ICU was observed after implementing the SC, likely in relation to the shorter intra-ICU stay. Nevertheless, no differences in the total number of DDD/100 stays were observed for severe sepsis/septic shock patients in the ICU between the two periods. This means that the number of patients who were exposed to antimicrobial treatment for every 100 stays was similar after the SC was implemented. This value is similar to that presented by other centres in this type of patient.20 In contrast, differences were observed in the consumption of certain antibiotic groups after implementing the SC. The use of antibiotics against resistant gram-positive bacteria decreased in 17.3% of the DDD/100 stays, as did the use of macrolides, which decreased in 22% of the DDD/100 stays. The more than two-fold increase in the consumption of piperacillin-tazobactam vs the PRE-SC group is also noteworthy. These data reflect a reduction in the antibiotic spectrum used after implementing the SC. It is worth pointing out that the DDD is a unit of measurement that does not necessarily reflect the daily recommended or prescribed dose, since these are based on individual characteristics (age, weight) and on pharmacokinetic considerations, especially in critical patients. The consumption data expressed in DDD provide an approximate estimate and not an accurate picture of their real use.
Regarding the clinical outcomes from the patients studied after implementing the SCHP, it should be noted that although the intra-ICU and in-hospital mortality figures in the post-SC period are similar to those from other recent series,2,21–23 they are significantly lower than the ones in our centre prior to implementing the programme. In fact, the multivariate BLR model demonstrates how implementing the SCHP was, along with the APACHE II score and the presence of septic shock at admission, the only variable independently associated with in-hospital and 28-day mortality, such that the risk of a septic patient dying was more than 3-fold higher in the pre-SC period than in the post-SC period. This is logical, since applying a SC promotes education about diagnosing the disease early as well as implementing measures earlier such as administering antibiotic therapy, vigorous fluid therapy, controlling the focus, extracting cultures, better risk staging, etc., in the early hours of the disease, measures which alone have been demonstrated to improve the prognosis for this condition if they are optimally carried out. It is logical that implementing all these measures also translates to a decrease in the intra-ICU and/or in-hospital stay, as the results from this study suggest. It should be mentioned that during both periods no other intervention was performed on this group of patients that could justify these findings.
This study has some limitations that should be pointed out: the main one is that it is not a prospective trial, but rather a retrospective comparison of the effect of implementing the SC with a historical cohort from before it was implemented in a limited number of patients. No other laboratory data were collected, not even regarding treatments other than the antibiotics (doses of amines, corticosteroids, need for renal support therapy, etc.). Furthermore, the increase in gram-negative infections that occurred in the second study period may have partially influenced the results by decreasing the consumption of linezolid, although it is true that this drug was mainly used empirically, when the microbiology results were not yet available to guide the treatment.
In summary, the SC-WG's implementation of actions and tools aimed at better diagnosis and treatment for severe sepsis and septic shock not only contributed to an optimisation of antibiotic prescriptions, but also to a decrease in-hospital and 28-day mortality figures, and a trend towards a shorter intra-ICU stay.
Although larger, multicentre studies with a higher number of patients are necessary to confirm these findings, undoubtedly the presented results should motivate and bring about the creation of multidisciplinary work groups to promote establishing SCHP programmes in all our healthcare centres.
FundingThis study was conducted within the grant programme to increase research activity awarded by the Castilla and Leon Regional Health Authority for the year 2016.
AuthorshipLGL, SGC, and DAO coordinated the study design. LGL, FBD, RCG, FDG, and DAO participated in collecting and analysing the data. LGL reviewed the patients’ medical records. LGL, SGC, AFS, and TSS performed the pharmacoeconomic analysis. MFM and DAO performed the statistical analysis on the clinical and survival endpoints. Lastly, LGL, SGC, DAO, and FGM drafted and wrote the manuscript.
Conflict of interestsThe authors declare that they have no conflicts of interest.
The authors thank the entire Hospital Clínico Universitario de Valladolid Multidisciplinary Sepsis Code Working Group for their work and dedication, without which this study would not have been possible.
Please cite this article as: García-López L, Grau-Cerrato S, de Frutos-Soto A, Bobillo-De Lamo F, Cítores-Gónzalez R, Diez-Gutierrez F, et al. Impacto de la implantación de un Código Sepsis intrahospitalario en la prescripción de antibióticos y los resultados clínicos en una unidad de cuidados intensivos. Med Intensiva. 2017;41:12–20.