Edited by: Rosario Amaya Villar - Unidad de Cuidados Intensivos, Hospital Universitario Virgen del Rocio, Sevilla, España
Last update: January 2025
More infoTo determine which method of Positive End-expiratory Pressure (PEEP) titration is more useful, and to establish an evidence base for the clinical impact of Electrical Impedance Tomography (EIT) based individual PEEP setting which appears to be a promising method to optimize PEEP in Acute Respiratory Distress Syndrome (ARDS) patients.
DesignA systematic review and meta-analysis.
Setting4 databases (PUBMED, EMBASE, Web Of Science, and the Cochrane Library) from 1980 to December 2020 were performed.
ParticipantsRandomized clinical trials patients with ARDS.
Main variablesPaO2/FiO2-ratio and respiratory system compliance.
IntervensionThe quality of the studies was assessed with the Cochrane risk and bias tool.
Results8 trials, including a total of 222 participants, were eligible for analysis. Meta-analysis demonstrates a significantly EIT-based individual PEEP setting for patients receiving higher PaO2/FiO2 ratio as compared to other PEEP titration strategies [5 trials, 202 patients, SMD 0.636, (95% CI 0.364−0.908)]. EIT-drived PEEP titration strategy did not significantly increase respiratory system compliance when compared to other peep titration strategies, [7 trials, 202 patients, SMD −0.085, (95% CI −0.342 to 0.172)].
ConclusionsThe benefits of PEEP titration with EIT on clinical outcomes of ARDS in placebo-controlled trials probably result from the visible regional ventilation of EIT. These findings offer clinicians and stakeholders a comprehensive assessment and high-quality evidence for the safety and efficacy of the EIT-based individual PEEP setting as a superior option for patients who undergo ARDS.
Para determinar qué método de valoración de la presión espirfinal positiva (PEEP) es más útil, y para establecer una base de evidencia para el impacto clínico de la tomode impedeléctrica (EIT) basada en el ajuste individual de PEEP que parece ser un método prometedor para optimizar la PEEP en pacientes con síndrome de dificultad respiraguda (ARDS).
DiseñoUna revisión sistemática y metanálisis.
ÁmbitoSe realizaron 4 bases de datos (PUBMED, EMBASE, Web Of Science y Cochrane Library) de 1980 a diciembre de 2020.
ParticipantesEnsayos clínicos aleatorizados de pacientes con SDRA.
Variables principalsPaO2/FiO2 ratio y compatibilidad respiratoria.
IntervenciónLa calidad de los estudios se evaluó con la Cochrane risk and bias tool.
ResultadosOcho ensayos, incluyendo un total de 222 participantes, fueron elegibles para el análisis. El análisis de ≥ eta demuestra una configuración individual significativamente basada en MEITPpara pacientes que reciben una mayor proporción EE2/P PiO2en comparación con otras estrategias de titulación FOPEEP SMD CI. La estrategia de titulación de PEEP derivada del tie no aumentó significativamente el cumplimiento del sistema respiren comparación con otras estrategias de titulación de PEEP, [7 ensayos, 202 pacientes, DME -0,085, (IC del 95%: −0,342−0,172)].
ConclusionesLos beneficios de la valoración de la PEEP con EIT en los resultados clínicos de SDRA en ensayos controlados con placebo probablemente sean el resultado de la ventilación regional visible del EIT. Estos hallazgos ofrecen a los médicos y a las partes interesadas una evaluación integral y evidencia de alta calidad para la seguridad y eficacia de la configuración individual de PEEP basada en EIT como una opción superior para los pacientes que se someten a SDRA.
Acute Respiratory Distress Syndrome (ARDS) was first described in 1967 and is characterized by the abrupt onset of clinically significant hypoxemia with the presence of diffuse pulmonary infiltrates.1 The criteria were updated in 2012 in the so-called Berlin definition of ARDS in adults based on the severity of hypoxemia represented by the ratio of the partial pressure of oxygen in arterial blood to inspired oxygen.2 ARDS is a life-threatening condition of seriously ill patients and is a prevalent cause of acute respiratory failure, carrying high mortality of 30–40%.3 The pathophysiological features were the ratio of ventilation to perfusion mismatch, decreased thoracic compliance, increased dead space, and elevated pulmonary arterial pressure. Therefore, early recognition of ARDS modifiable risk factors and the avoidance of aggravating factors during the patient's hospital stay can help decrease its development.4 Of all adjunctive therapies, lung-protective ventilation is still the key to a better outcome in ARDS. Recent guidelines on mechanical ventilation in ARDS provide evidence-based recommendations related to 6 interventions, including low tidal volume and inspiratory pressure ventilation, prone positioning, high-frequency oscillatory ventilation, higher vs. lower Positive End-Expiratory Pressure (PEEP), lung recruitment maneuvers, and extracorporeal membrane oxygenation.5
Setting PEEP appropriately is now recognized as an important aspect of a lung-protective ventilation strategy and not just a strategy to improve oxygenation. Setting PEEP levels 5 cm H2O may be harmful in the acute phase of ARDS. Setting PEEP appropriately is a balance between maintaining alveolar recruitment and avoiding alveolar over distention.6 Several methods have been proposed for PEEP titration in an individual patient with ARDS, including gas exchange, compliance, pressure-volume curve, stress index, esophageal manometry, lung volume, imaging.7 Of the imaging method, Electrical Impedance Tomography (EIT) plays an important role.
EIT is a bedside monitoring tool that non-invasively visualizes local ventilation and arguably lung perfusion distribution. EIT images possess a high temporal and functional resolution allowing the visualization of dynamic physiological and pathological changes on a breath-by-breath basis.8 Current studies focus mainly on its clinical applications to quantify lung collapse, tidal recruitment, and lung over distension to titrate PEEP, which is new in the PEEP setting.9–12
Previous studies compared the methods of conventional PEEP titration strategies but not EIT-based individual PEEP setting. Thus, to assess the effect of EIT-based individual PEEP setting, we undertook a systematic review and meta-analysis investigating the difference between two methods (EIT and other method) in the final P/F ratio or the final compliance when PEEP is titrated using those methods.
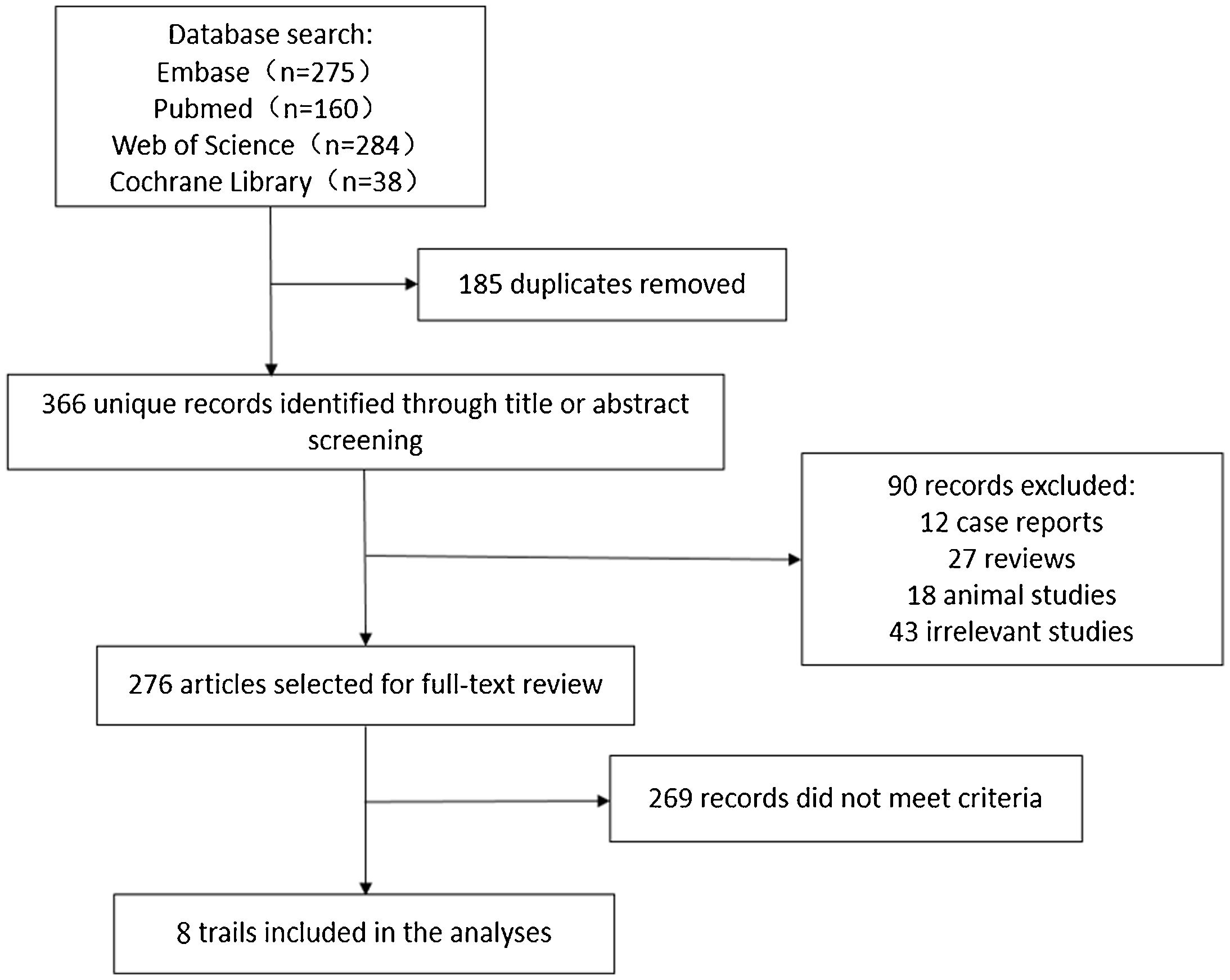
MethodsFour electronic databases (PUBMED, EMBASE, Web Of Science, and the Cochrane Library) were searched from inception to December 2020, for trials investigating any other PEEP titration strategies and EIT based individual PEEP setting of ARDS patients, with MeSH headings and text words (discussed in detail in search strategies, web appendix). We searched for any additional studies in the references of all identified publications, including previous relevant meta-analyses and narrative reviews. This study is registered with the INPLASY website, number INPLASY202160094.
Selection criteriaFor inclusion, any studies except reviews and case report in adults, and examine the effect of any other PEEP titration strategies and EIT based individual PEEP setting on respiratory markers and clinical impacts of ARDS. The outcome was assessed by use of respiratory markers (PaO2/FiO2-ratio and respiratory system compliance). Only studies published as full-length articles or letters in peer-reviewed English-language journals were included.
Data extractionThe following information was extracted and entered into databases by three investigators (MNY, TTZ, CW): study design, type of intervention, patients’ characteristics, and outcomes (web appendix). If relevant information regarding the design or ARDS outcomes was unavailable, or doubt existed about duplicate publications, authors were contacted to obtain the necessary information (web appendix). Uncertainties were resolved by consensus.
This study is reported by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement12–14 and the Cochrane Handbook for Systematic Reviews of Interventions.15 Our detailed study protocol is available online and has been previously published16. No institutional review board approval was required for this meta-analysis because the study included data that had been published previously.
Statistical analysisDichotomous outcomes were reported using relative risks (RR) and 95% confidence intervals (CIs). For continuous outcomes (e.g., PaO2/FiO2-ratio and respiratory system compliance), we evaluated the standardized difference in means (SMD). Studies were weighted using inverse variance and data were pooled using random-effects models. We examined funnel plots of treatment effect versus study precision to assess for publication bias. Baseline characteristics of trial participants were summarized using weighted averages. Subgroup analyses were performed by stratifying studies by characteristics of interest (co-interventions, recruitment maneuvers, methods of setting PEEP) and assessing for quantitative interaction using Chi-square tests for heterogeneity between subgroups. We used Stata 15.0 to conduct statistical analyses.
Role of the fundingsourceFunding source of Health special talents program of Suzhou high tech Zone had a role in study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and all took full responsibility for the decision to submit for publication.
ResultsIncluded studies and study qualityEight trials met the inclusion criteria for this review, including 222 adults with ARDS (Fig. 1). The basic information and methodological quality of the included studies are reported in Table 1. Table 2 summarizes baseline patient characteristics and the varied PEEP protocols used between studies.
Methodological quality of included studies.
| Author | Zhao | Eronia, N | Weber | Kastern | Heines | Becher | Jacopo | Scaramuzzo |
|---|---|---|---|---|---|---|---|---|
| Year | 2019 | 2017 | 2020 | 2018 | 2018 | 2016 | 2019 | 2020 |
| Country | China | Italy | Germany | Germany | The Netherlands | Germany | USA | Italy |
| Study design | RCT | NRCT | RCT | NRCT | NRCT | NRCT | NRCT | NRCT |
| Scale | Jadad 6 | MINORS 18 | Jadad 6 | MINORS 18 | MINORS 18 | MINORS 18 | MINORS 18 | MINORS 18 |
Baseline patient characteristics and the varied PEEP protocols.
| Study | Zhao | Eronia, N | Weber | Kastern | Heines | Becher | Jacopo | Scaramuzzo |
|---|---|---|---|---|---|---|---|---|
| Sample (C/T) | 31/24 | 14/14 | 23/25 | 15/15 | 39/39 | 15/15 | 14/14 | 20/20 |
| Gender (M/F) | 37/18 | 14/2 | 30/18 | 9/6 | 25/14 | / | 6/8 | 13/7 |
| Interpretation: | ||||||||
| Control group | Pressure–volume loop | PEEP/FiO2 table | PEEP 5 cmH2O | Best Compliance approach | PEEP 4 cmH2O | The ARDSnet protocol | PEEP/FiO2 table | End expiratory PL/FiO2 sliding table |
| EIT group | ODCL | EELI | CRS | ODCL | ODCL | EELI | ODCL | SStot |
| Outcomes | ①② | ①② | ① | ① | ①② | ①② | ①② | ② |
ODCL, percentage of over distension/collapse; PL, transpulmonary pressure; SStot, total Silent Spaces; ①, lung compliance; ②, PaO2/FiO2-ratio.
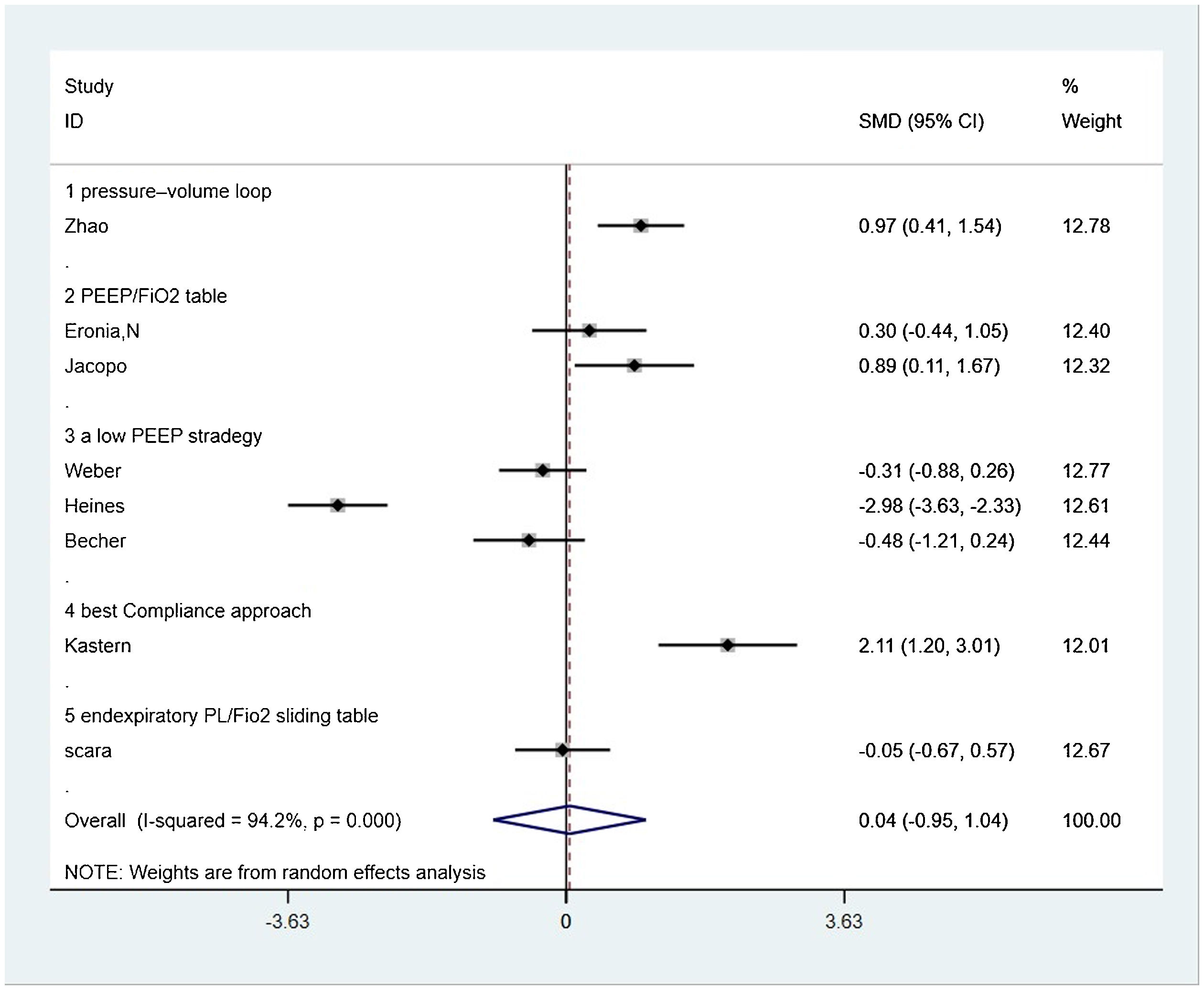
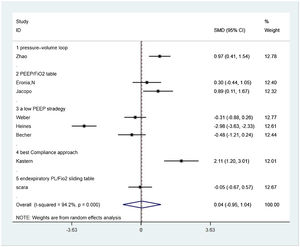
Seven from eight included studies reported respiratory system compliance and were included in the merger. After the data were merged, the heterogeneity of the studies was large (I2 = 95.1%), so the random-effects model was used and the subgroup analysis was carried out according to the different control PEEP strategies. Two trials compared the effects of PEEP strategy of PEEP/FiO2 table and EIT-drived PEEP titration on respiratory system compliance, of which the final meta-analysis result showed a significant difference between two groups that respiratory system compliance increased in EIT group [SMD = 0.582, 95% CI (0.044, 1.121), p < 0.05] (n = 30); Three trials compared the effects of the strategy of a low PEEP and EIT-drived PEEP titration on respiratory system compliance and the meta analysis either showed no significant difference [SMD = −1.216, 95% CI (−1.585, 0.847), p > 0.05] (n = 102); One trial comparing the effects of PEEP strategy of pressure–volume loop and EIT-drived PEEP titration on respiratory system compliance revealed a significant rise in respiratory system compliance with the EIT-drived PEEP titration [SMD = 0.974, 95% CI (0.410, 1.538), p = 0.001] (n = 55); One trial which compared the effects of PEEP strategy of pressure–volume loop and EIT-drived PEEP titration on respiratory system compliance uncovered a significant difference that the respiratory system compliance with EIT-drived PEEP titration increased [SMD = 2.106, 95%CI (1.203, 3.010), p = 0.000] (n = 15) (Fig. 2).
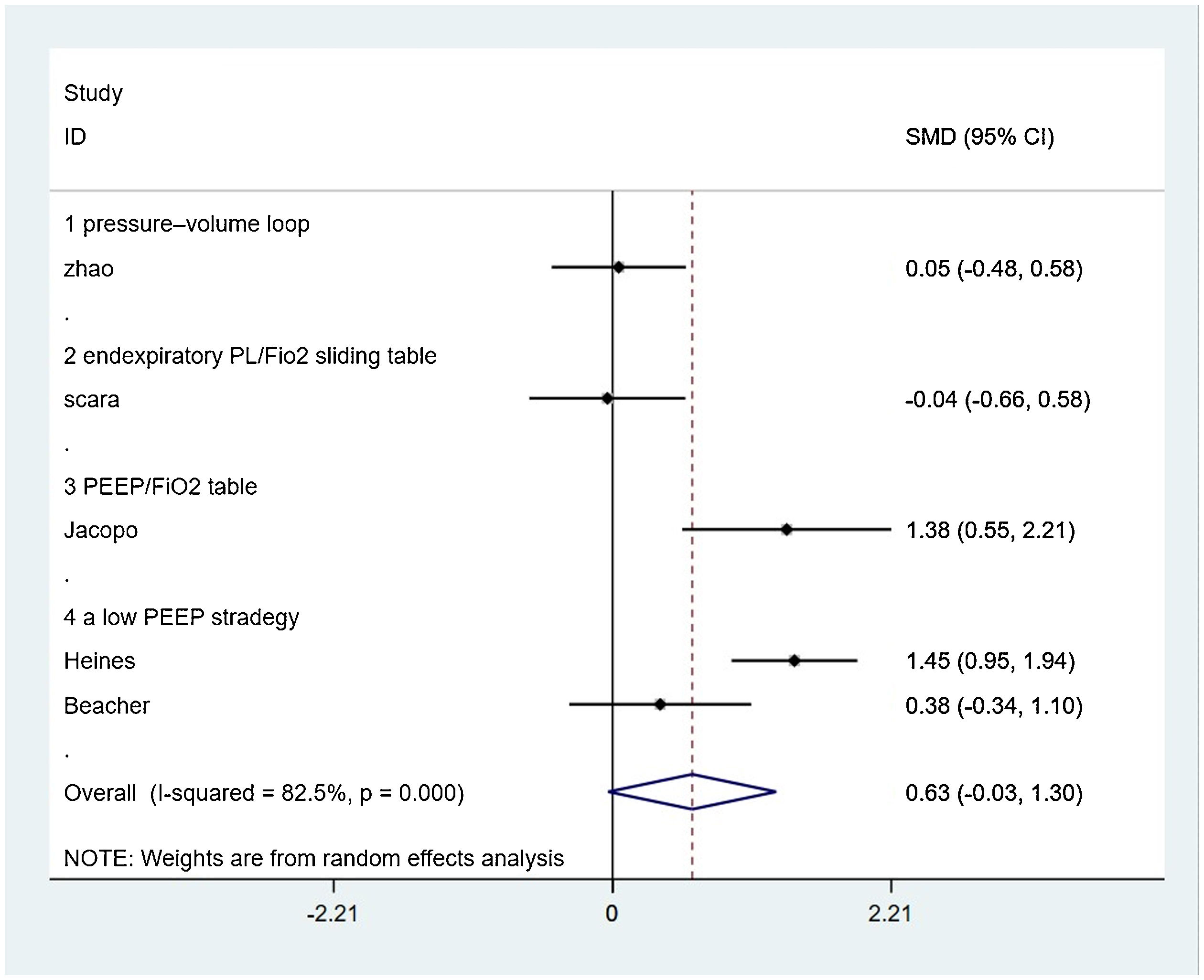
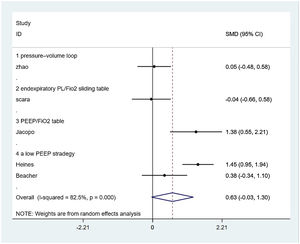
PaO2/FiO2-ratioFive from eight included studies reported the PaO2/FiO2-ratio and were included in the merger. After the data were merged, the heterogeneity of the studies was large (I2 = 82.5%), so the random-effects model was used and the subgroup analysis was carried out according to the different control PEEP strategies. One trial compared the effects of PEEP strategy of PEEP/ FiO2 table and EIT-drived PEEP titration on PaO2/FiO2-ratio, of which the final meta-analysis result showed a significant difference between two groups that PaO2/FiO2-ratio increased in EIT group [SMD = 1.383, 95% CI (0.533, 2.214), p < 0.05] (n = 14); Two trials compared the effects of the strategy of a low PEEP and EIT-drived PEEP titration on PaO2/FiO2-ratio and the meta-analysis either showed no significant difference [SMD = 0.945, 95% CI (−0.097, 1.988), p > 0.05] (n = 54); one trial comparing the effects of PEEP strategy of the pressure-volume loop and EIT-drived PEEP titration on PaO2/FiO2-ratio revealed no significant difference between the two groups [SMD = 0.050, 95% CI (−0.483, 0.583), p > 0.05] (n = 55). One trial which compared the effects of PEEP strategy of end-expiratory transpulmonary pressure (PL)/FiO2 sliding table and EIT-drived PEEP titration uncovered no significant difference t on PaO2/FiO2-ratio [SMD = −0.041, 95% CI (−0.661, 0.579), p > 0.05] (n = 20) (Fig. 3).
Publication biasConsidering not enough studies enrolled, it is not available for the funnel plot method to publication bias. There is a potential risk of publication bias.
DiscussionOur study indicates that ARDS patients who have optimized PEEP with Electrical Impedance Tomography have a significantly higher PaO2/FiO2-ratio than those titrated with the conventional type. However, the respiratory system compliance was similar in the two groups. PEEP was not significantly decreased in the EIT group compared with the conventional group indicating that EIT-drived PEEP has similar efficacy to conventional strategies.
Raised compliance compared to subgroups and raised PaO2/FiO2-ratio in the EIT group was maintained across important subgroups associated with patient demographics, the severity of hypoxemia, patient position, imaging feature, tidal volume, other clinician therapies, and use of prophylactic measures. This observation suggests that our findings reflect a true effect of Electrical Impedance Tomography, rather than an artifact of statistical heterogeneity or specific patient or procedural characteristics. Notably, noninvasive and visible are the specific feature of EIT. Despite this difference, the conventional strategies are maintained, suggesting that the effect of dynamic imaging design does not take place of that of conventional strategies in treatment guidance.
In the recent decade, Electrical Impedance Tomography (EIT) is becoming emerging as an easily accessible imaging radiation-free techniques that allow continuous and functional respiratory monitoring at the bedside, based on the repeated measurement of the surface voltages resulting from a rotating injection of high frequency and low-intensity alternating current that circulates between the electrodes located around the chest. The electrodes collect the information on impedance by forming a relative image concerning a reference. The tissues allow the passage of current with little resistance, however, with gas the opposite occurs. In this way, it is possible to perform plethysmography of the volume entering upon each inspiration and in each region of interest.13 Previous studies mainly focus on randomized controlled trials of ventilation strategies and monitoring, animal models on cardio-pulmonary perfusion, and reviews of clinical practice,8,9,13–21 but without a meta-analysis of EIT-drived PEEP titration for ARDS patients yet. No meta-analysis of EIT presents up to December 2020, probably because of EIT coming out not long and being not widely used. Thus we performed this meta-analysis investigating the use of EIT-drived PEEP titration for the management of patients with ARDS and establish evidence. The previous meta-analysis reported a lot of conventional PEEP settings (including Gas exchange, compliance, pressure-volume curve, stress index, esophageal manometry, lung volume, Imaging) for patients with ARDS, of which mostly compared the effect of high PEEP level and low PEEP level on outcomes.22–31 Our findings stand in clear distinction from those of past studies that did not reach a consensus on this topic. A previous meta-analysis22–26 had limitations and focused on the conventional and indisputable PEEP setting, whereas our study is broad in its scope with robust analyses done by a multidisciplinary team.
However, our study is not without limitations. First, only eight studies were included, all of which are small-sample trials, resulting in poor test efficiency. At the same time, all trials did not report the allocation concealment scheme, which may have selection bias. Only two of the studies were RCT. Six studies did not report their blinded implementation schemes, and the measurement biases might exist. These defects will affect the internal and external authenticity of the results.
In conclusion, we found that EIT-guided PEEP titration was associated with a raise respiratory system compliance and significant raise in PaO2/FiO2-ratio, and had similar efficacy to their conventional counterparts. Our findings suggest that the EIT group retains a favorable balance between safety and efficacy when compared with the conventional type. These results provide clinicians and health-care policymakers with a comprehensive assessment and high-quality evidence on the safety and efficacy of atraumatic needles as a superior option for patients with ARDS.
Disclosure statementThis research received grant from health special talents program of Suzhou high tech Zone. The authors report no conflicts of interest.
CRediT authorship contribution statementMengnan Yu: Conceptualization, Formal analysis, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Yanjun Deng: Conceptualization, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. Jun Cha: Data curation. Lingyan Jiang: Data curation, Formal analysis, Validation, Visualization. Mingdeng Wang: Data curation, Supervision. Shigang Qiao: Data curation, Formal analysis, Investigation, Supervision. Chen Wang: Conceptualization, Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
The authors would like to thank Ms Yan Wang and Ms Li Huang for their assistance in the screening of abstracts and data extraction.