Burned patients may need prolonged admissions in the Intensive Care Service, both for initial care and for the pre and postoperative treatment of the multiple surgeries they require. The initial resuscitation of critically burned patients requires adequate monitoring to calculate the fluid therapy necessary to replenish the losses and ensure tissue perfusion, but without excesses that increase interstitial edema. In addition, monitoring can evaluate the systemic inflammatory response that can lead to shock and organic dysfunctions. After this initial phase we will find a critical patient who requires multiple reinterventions in non-optimal situations, so he will need special care over a long period of time. In addition, the SMI offers specific postoperative care for reconstructive surgery and the transplantation of composite tissues (upper limb and face) in which its success depends on a rigorous control through adequate monitoring and treatment.
Los pacientes quemados pueden necesitar ingresos prolongados en el Servicio de Medicina Intensiva (SMI), tanto para la atención inicial como para el tratamiento pre y postoperatorio de las múltiples cirugías que precisan. La reanimación inicial del paciente quemado crítico requiere una adecuada monitorización para calcular la fluidoterapia necesaria para reponer las pérdidas y asegurar la perfusión tisular, pero sin excesos que aumenten el edema intersticial. Además, la monitorización puede evaluar la respuesta inflamatoria sistémica que puede llevar a shock y disfunciones orgánicas. Tras esta fase inicial nos encontraremos con un paciente crítico que requiere múltiples reintervenciones en situaciones no óptimas por lo que necesitará unos cuidados especiales durante un largo periodo. Además, el SMI ofrece la atención postoperatoria específica para la cirugía reconstructiva y el trasplante de tejidos compuestos (miembro superior y cara) en los que su éxito depende de un riguroso control mediante una monitorización y tratamiento adecuado.
Burns are a worldwide public health problem that according to the World Health Organization (WHO) cause almost 200,000 deaths each year, mostly in poor countries. There are no precise data on the incidence of burn injuries, though in our setting it has been estimated that there are about 300 burn cases per 100,000 inhabitants and year, of which 15 require hospital admission and only a small proportion are admitted to intensive care. The treatment of burns has evolved in recent years, with a decrease in mortality but an increase in the functional, aesthetic and quality of life consequences. This situation in turn implies very high direct and indirect costs as a result of patient loss of income, the prolonged care of deformities, and emotional trauma. The functional sequelae remain very important in this group of patients, with movement restrictions in 20% of the cases, even 5 years after injury. Aesthetic alterations in turn affect 43% of the patients, even in those with minor burns. The occupational impact is also very great, affecting up to 50% of all adult burn patients, with an incidence of occupational incapacitation of up to 5%.1
Patients with extensive burns are critical patients that require multiple surgeries during their prolonged hospital stays. The prognosis is conditioned to correct initial resuscitation, good surgical planning and adequate continued care between surgeries. Early excision and covering have improved the outcomes, but the timing of surgery and the surface area to be treated in each operation must be adjusted to the clinical situation of the patient. Likewise, in the subsequent surgeries and cures, the patient condition must be taken into account in order for such procedures to be carried out under the best conditions possible, with a view to obtaining the best results. This is also extendable to conditions such as toxic epidermal necrolysis, which requires a similar management approach.2
Other surgeries, such as the reconstruction of tissue loss using flaps or grafts, reimplantation and the transplantation of composite tissues, require adequate management and monitoring in order to be successful.
The critical burn patientExtensive burns not only destroy tissue but also activate the cytokine-medicated inflammatory response. The first consequences are fluid loss, causing hypovolemia and even shock. However, there also may be a cardiogenic component due to low cardiac output, with a distributive component secondary to the inflammatory response.3 Pulmonary problems and upper airway alterations may also develop. Thus, severe burn patients require admission to a critical burn unit, since they need closely monitored fluid therapy and often also the use of vasoactive drugs, mechanical ventilation, analgesia-sedation, renal replacement techniques, etc.4
First careA number of formulas have been proposed for fluid replacement, the most widely used being that of the Hospital of Parckland.5 This formula estimates the needs as 4ml of crystalloids×kilogram body weight×percentage burn surface in 24h, of which one-half is infused in the first 8h and the rest in the following 16h. Ringer lactate has classically been used in order to avoid the hyperchloremic acidosis produced by saline solution when administered in large amounts. Other balanced crystalloids are being used lately, however.6 There is greater controversy regarding the use of colloids, though the most widely used practice is albumin from the first 8–12h in patients with a burn surface of over 30%.7 Nevertheless, these formulas are only a first orientation, since they do not take into account the depth of the injuries, the comorbidities, the presence or not of inhalation syndrome, etc.8
In the absence of major alterations in blood pressure and heart rate, and if hourly diuresis is maintained at 0.5–1ml/kg, we can assume that there is no great volume defect.9 However, in severe cases we require more complex monitoring, since these parameters may be influenced by many factors, and their correlation to cardiac output and preload is not sufficiently adequate.
Some years ago it was evidenced that in many cases much more volume was actually administered than that calculated by the formulas – a situation that caused increased fluid penetration into the interstitial space. This phenomenon, known as “fluid creep”, is particularly attributable to increased opiate use, a greater use of mechanical ventilation, or the definition of inadequate resuscitation objectives or targets.10
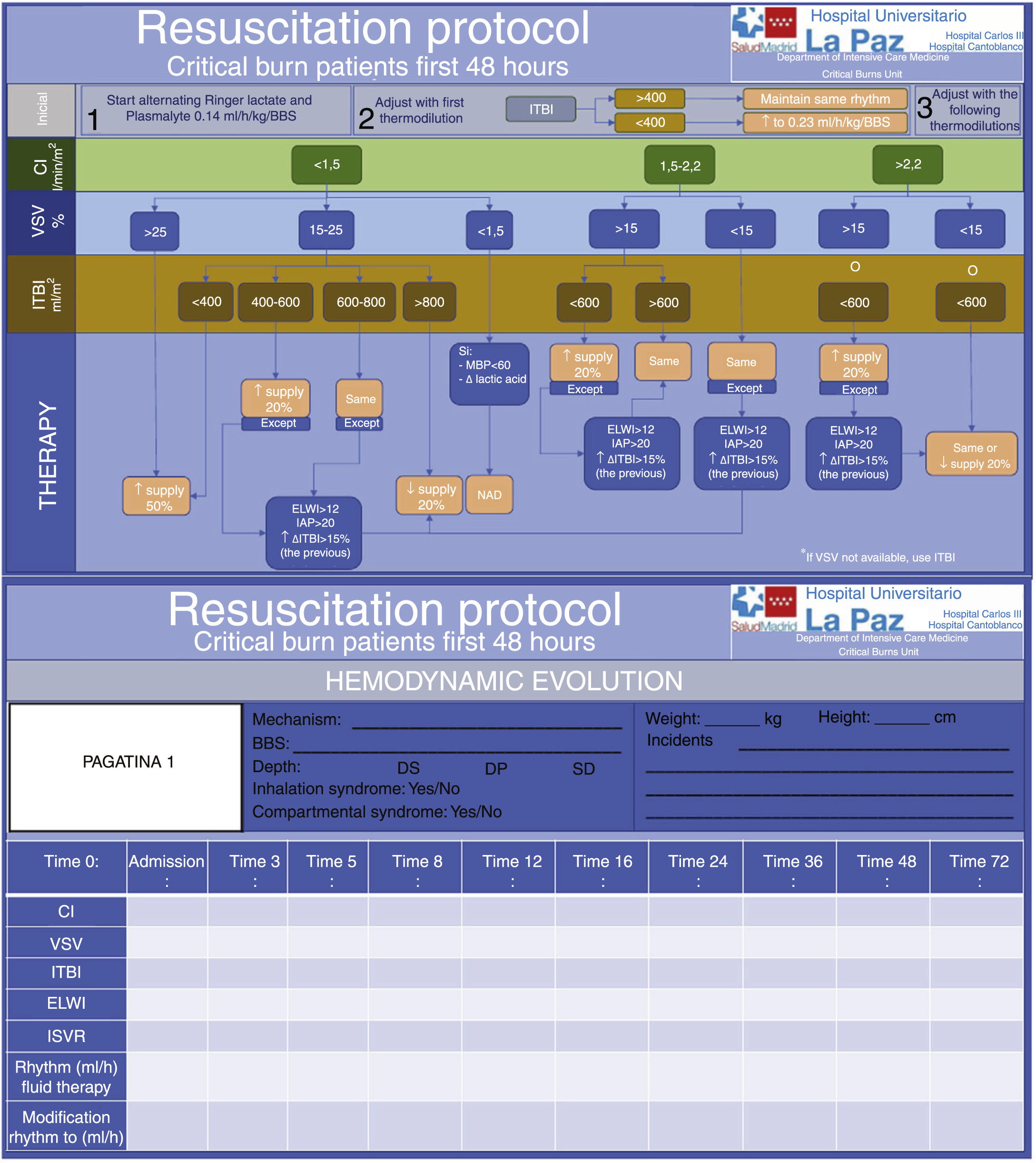
At present, the most widely used monitoring protocol is transpulmonary thermodilution, which calculates cardiac output, preload volumes and extravascular lung water (EVLW). In addition, it can offer continuous calculations of cardiac output and variation of systolic (stroke) volume. Patient resuscitation can be guided by the latter two parameters. The variation of stroke volume is a good predictor of fluid response, but in patients with spontaneous ventilation or with arrhythmias it is not so useful, and we need to employ static preload parameters (intrathoracic blood volume or global end-diastolic volume). However, fluid administration until achieving normalization of the parameters is not indicated in all fluid-responding patients, since the alteration of permeability can cause fluid to not be retained within the intravascular compartment, and the objective of achieving normalization would only cause an increase in edema. Consequently, other parameters such as EVLW or intraabdominal pressure and the trend in preload parameters may alert us to an excess of supply. With these and other data, such as mean blood pressure and lactate levels, we can establish an interventional algorithm or decision tree seeking to correct the hemodynamic alterations and ensure tissue perfusion11,12 (Fig. 1).
Fluid therapy protocol in patients with burns affecting over 20% of total body surface.
DP: deep dermal burn; DS: superficial dermal burn; ELWI: Extravascular Lung Water Index; IC: cardiac index; ITBI: Intrathoracic Blood Index; MBP: mean blood pressure; IAP: intraabdominal pressure; ISVR: indexed systemic vascular resistances; BBS: burnt body surface; SD: subdermal burn; VSV: variation of stroke volume.
Another issue is the respiratory repercussions secondary to altered pulmonary vascular permeability, which associated to hypoproteinemia and diminished oncotic pressure can increase EVLW. Consequently, mechanical ventilation proves necessary in many patients, and although there is not enough supporting evidence, the same protective ventilation measures used in other disease conditions are advised.13 In addition, the decrease in thoracic compliance in chest burn patients, and intraabdominal hypertension, contribute to further alter respiratory function.
In the event of coexisting smoke inhalation, airway and lung parenchyma problems may be present from the start. Smoke inhalation syndrome can be suspected when the accident has occurred in a closed space, in the presence of facial burns, burnt nostril hairs, carbon-tainted sputum, stridor and elevated carboxyhemoglobin levels. Early intubation is recommended due to the possibility of airway obstruction caused by edema, but positive pressure ventilation may increase the fluid requirements and worsen the prognosis, and thus should only be used when necessary. In the rest of cases it is better to monitor and, if the patient has been intubated, assess the possibility of early extubation.14,15 The routine use of corticosteroids or antibiotic prophylaxis is not advised, and nebulized anticoagulants have not demonstrated sufficient effectiveness.16,17 Furthermore, high-dose cyanocobalamin is indicated in the event of suspected cyanide intoxication, and 100% oxygen in the event of suspected carbon monoxide exposure.
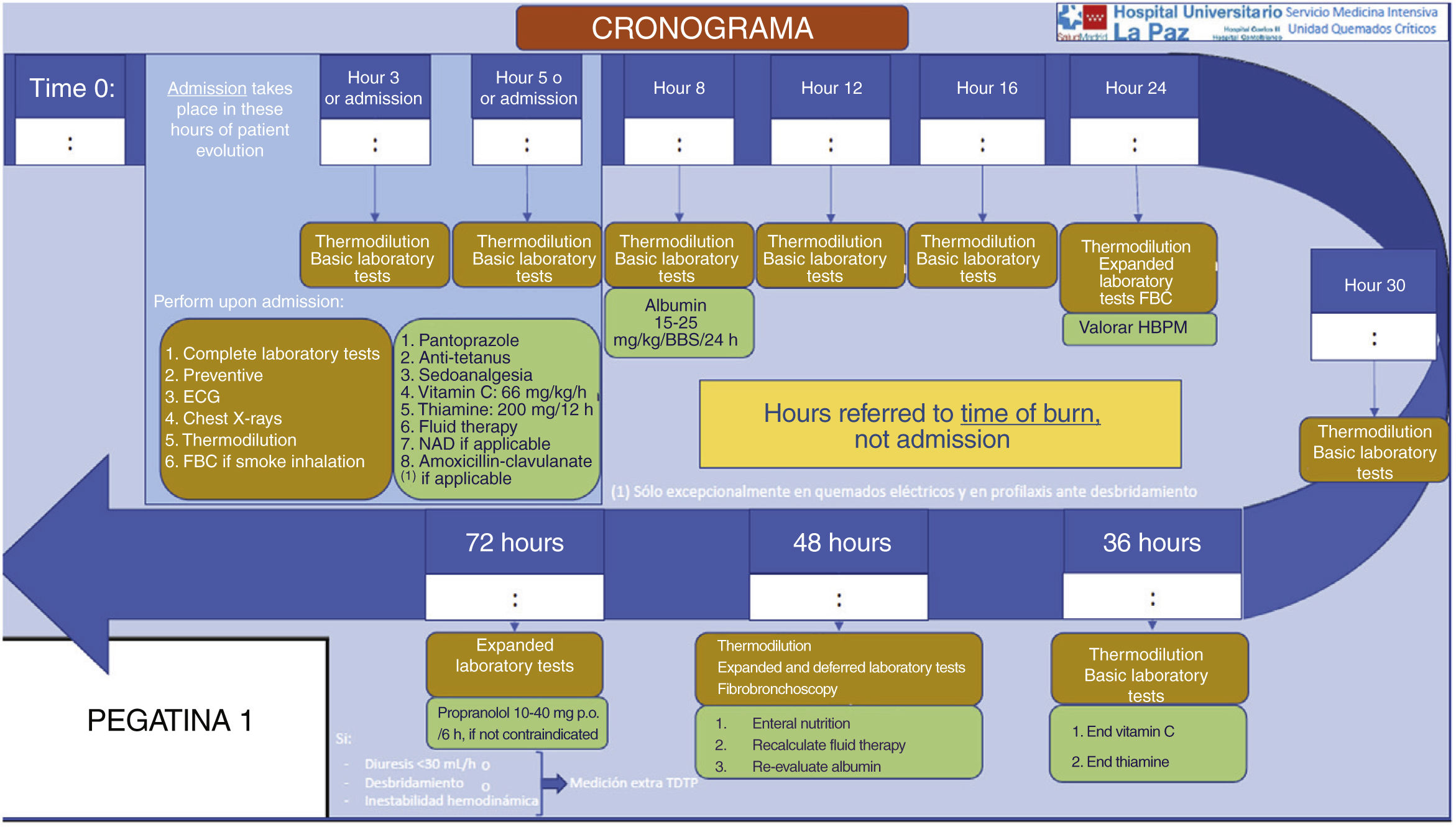
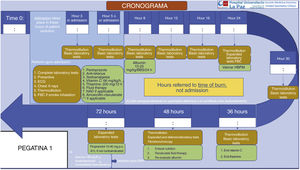
Other necessary measures are the treatment of pain and the prevention and correction of hypothermia. High-dose vitamin C has also been suggested in order to reduce the inflammatory response18 (Fig. 2).
Electrical burns constitute a special case, since they can be accompanied by arrhythmias and rhabdomyolysis. In these patients we need to supply more fluids in order to secure greater diuresis, and we can also add sodium bicarbonate, mannitol or furosemide.19
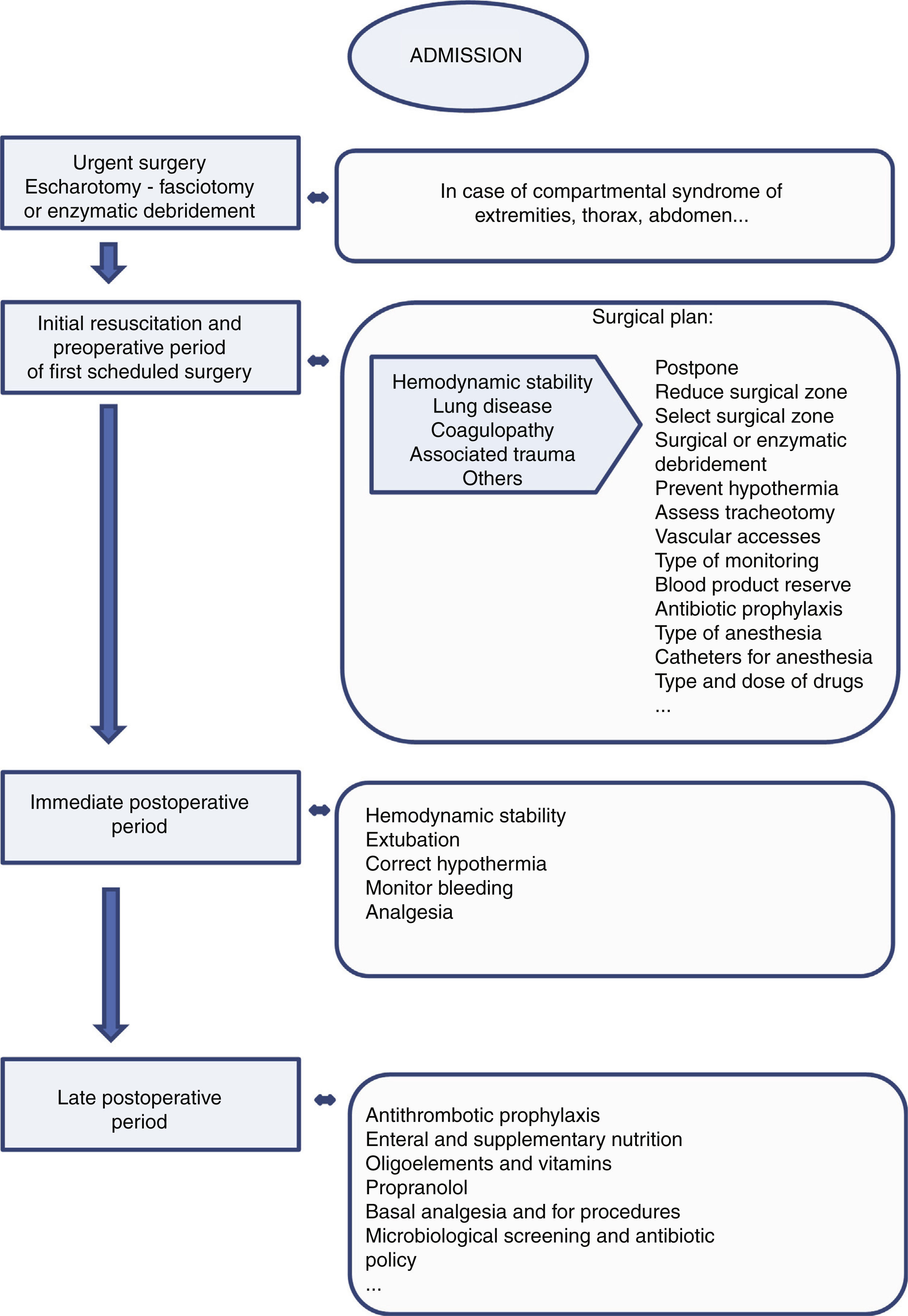
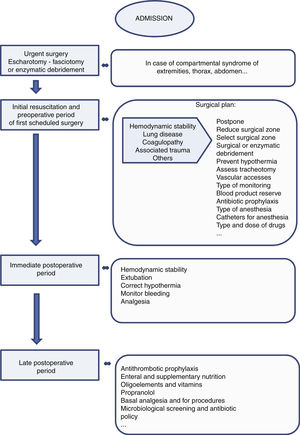
A necessity from the first moment is adequate coordination with plastic surgery in order to avoid the development of compartmental syndromes through the adoption of conservative measures such as postural adjustments and the monitoring and limitation of fluid administration. If compartment syndrome appears, urgent surgical treatment (escharotomy - fasciotomy) or enzymatic debridement of the affected location is indicated (Fig. 3).
Preoperative period and surgeryFollowing the initial alterations, the main cause of morbidity-mortality in these patients is sepsis. In this regard, excision and early covering of the burn reduce the risk of colonization and sepsis, and thus improve the prognosis.20 However, such surgery must be performed under adequate clinical conditions, and in many cases this is not done, since the patient might not have recovered hemodynamic stability and may have respiratory problems.21 In these scenarios, close communication is required between the surgical team and the intensivists in order to adjust the timing, type and magnitude of surgery to the situation of the patient.
In recent years, and in addition to the usual surgical techniques, enzymatic debridement with bromelain cream has been introduced. This method is capable of eliminating the eschar in a more selective manner, and can be applied in the Intensive Care Unit (ICU) without having to move the patient to the operating room. While this technique appears to have advantages, it may trigger an added inflammatory response.22,23
The surgical plan needs to estimate the blood losses in order to procure the required blood product reserves. The need for transfusion has decreased with the use of topical thrombin, tourniquets and topical vasoconstrictors,24,25 and also with reduction of the target minimum hemoglobin concentration to 7g/dl.26 The utilization of enzymatic debridement instead of classical surgical excision appears to have reduced the need for transfusion.27 Autologous blood hemodilution transfusion has been used in certain patients.28
Programming is also required of the vascular accesses, the necessary monitoring procedures, and the measures for avoiding hypothermia. Likewise, the need or not for tracheotomy must be assessed (due to neck and face injuries, or because prolonged intubation is anticipated).
Sedoanalgesia is fundamental from the start.29 It must be taken into account that intravascular protein loss results in hypoalbuminemia, which in turn modifies the volume of distribution of many drugs. On the other hand, the decrease in cardiac output, and in renal and liver flow, can reduce the elimination of certain drugs. In this regard, and as an example, increased opiate and propofol doses may prove necessary. In subsequent surgeries, or if first surgery is delayed more than 48h, succinylcholine should be avoided, since there is an increased presence of acetylcholine receptors that release potassium upon depolarizing - and this may result in rapid hyperpotassemia.30 Regional anesthesia can facilitate the surgical procedure, lessen postoperative pain and contribute to rehabilitation, though it should not be used on an isolated basis when the burns or the graft donor zones are located in different areas. Epidural or spinal anesthesia should not be performed in extensive debridement procedures, due to the risk of bleeding and hypovolemia.
Systematic antibiotherapy should be avoided in burn patients, though such treatment is indicated in the perioperative period. In these patients we should adjust the dose to the alterations in the volume of distribution.31,32
Immediate postoperative periodIn this period the most frequent complications are bleeding and the hemodynamic alterations derived from new aggression in the form of surgery. The hemodynamic alterations are particularly relevant, since adequate peripheral perfusion is decisive for the success of wound covering with autologous grafts and for determining whether the depth of the burn and donor zones will increase or not. Another important decision is the timing of weaning from mechanical ventilation.4
On the other hand, analgesia should be adjusted taking into account the basal needs and the extra requirements in cases of changes in patient body posture, cures or other procedures. In this sense, protocols are needed in which other drugs such as ketamine, dexmedetomidine or clonidine are added to the administered opiates, together with the use of catheters for locoregional analgesia.3
Late postoperative or between-surgeries periodIn this period the patient is in critical condition and continuity of care is very important in order to facilitate recovery, avoid complications and prepare the patient for the next surgery. In this sense, we should reintroduce antithrombotic prophylaxis and nutrition as soon as possible. Enteral nutrition should be supplemented with vitamins and oligoelements, and in some cases with parenteral nutrition because of the frequent perisurgical and peri-procedure under sedation fasting periods. In addition to attempting to minimize the peri-procedure fasting periods, we also must seek to control the hypercatabolic response with propranolol.33
This period is characterized by great tolerance to opiates, and neuropathic pain may moreover develop, as well as pruritus and even hyperalgesia. Acetaminophen, nonsteroidal antiinflammatory drugs (NSAIDs), clonidine, gabapentin and tricyclic antidepressants can reduce nociceptive and neuropathic pain.34,35
Cures and therapeutic bathing cause hypothermia, and represent new aggression, with the release of cytokines. Such procedures therefore require sedoanalgesia, in which ketamine is particularly important, for in addition to its excellent analgesic effect, it preserves hemodynamic stability and does not depress spontaneous respiratory function. It therefore may avoid orotracheal intubation, though premedication is required with benzodiazepines or propofol at low does in order to avoid possible dysphoria. Other alternatives are dexmedetomidine, which also affords sedation and analgesia with scant respiratory depression, and inhaled anesthesia. Lastly, there are non-pharmacological coadjuvant treatments that reduce patient anxiety and pain, including particularly music therapy.36
Prophylactic antibiotherapy is not recommended, though burn patients are more vulnerable to infection due to absence of the skin barrier, the vascular accesses, the increased probability of bacterial translocation, and the associated immune depression. Frequent microbiological screening is therefore needed to know the colonizing flora and – in the case of infection – to adjust antibiotherapy.37
It is very common for these patients to require multiple reoperations, either because the large extent of the burnt surface does not make complete excision and covering in a single step advisable, or because the percentage of successfully grafted zone is insufficient.
Perioperative period in reconstructive surgeryFree flaps are often used for the reconstruction of tissue or substance loss in plastic surgery. Flaps are differentiated from grafts in that they are portions of tissue preserve a vascular axis. They involve great complexity, and this sometimes requires careful postoperative monitoring and clinical management, particularly in operations lasting more than 6h. Patients with surgical complications or certain risk factors must be monitored and treated in the ICU, while the rest of patients at least require intermediate care areas with specialized nursing staff.38 The risk factors to be taken into account are advanced age,39 ischemic heart disease, alcohol dependency, diabetes mellitus,40 smoking41 and obesity.42
Intraoperative and postoperative periodThe most important factor is the maintenance of adequate blood flow through the vascular pedicle. Hypovolemia, hypothermia and pain are the main complications to be avoided.43 Adequate volume supply is therefore required: neither too little (as this may negatively affect flow) nor too much (since edema and thrombosis of the flap may result). Vasoactive drugs are frequently needed in order to ensure adequate perfusion pressure.44 We also must avoid hematocrit readings above 40%, due to the elevated viscosity this implies, and transfusion is only recommended with hematocrit values of under 25%.45
A special case is head and neck microsurgery, since in these cases the patient may need a prolongation of mechanical ventilation to ensure that there is no upper airway obstruction, and sedation may also have to be prolonged in order to avoid complications due to poor body posture or other reasons.46
Monitoring of the flap requires trained staff that frequently and for about three days must check capillary filling, color, edema, temperature and bleeding. Doppler ultrasound, oxygen saturation, laser doppler flowmetry and fluorescein angiography allow more objective monitoring, and implantable doppler and microdialysis are particularly useful in buried flaps.47
The current recommendation is to apply antibiotic prophylaxis protocols in clean-contaminated surgery during 7 days, with special attention to patients with risk factors, prolonged surgical times, the use of bone flaps and malnutrition.48
Postoperative antithrombotic treatment is very controversial, though a recent meta-analysis has concluded that antithrombotic agents do not reduce the risk of thrombosis or of flap loss, but do increase the risk of hematoma.49
Postural measures can avoid venous overload and edema, particularly of the lower extremities; the limb subjected to microsurgery is therefore raised.50
Lastly, the most frequent causes of flap loss are thrombosis (venous/arterial proportion 2:1) and pedicle obstruction (due to local causes or infection).51
Reimplants and transplantationReimplantation of the upper extremity has experienced great advances in the last two decades, particularly due to better patient selection, improvement of the microsurgical technique, better equipment, and expansion of the indication of venous grafts – reaching success rates of 80–90 %. However, the purpose of surgery should not be limited to graft survival but should also contemplate the functional outcome of reimplantation referred to sensitivity, joint mobility range, strength, cold intolerance and a return of the patient to work.52
In these patients the surgeries are lengthy, and the complications may be similar to those described for flaps. The postoperative management and monitoring requirements are therefore analogous to those of free flaps.53 Monitoring of the extremity with pulsioximetry is simple and affords continuous information; it therefore may allow early identification of different problems that complicate perfusion of the extremity. Doppler ultrasound is a simple monitoring procedure that can be used periodically and in situations where pulsioxymetry shows alterations. Furthermore, we need to use the monitoring procedures commonly used in critical patients in order to ensure an adequate hemodynamic status. Despite all the advances in monitoring, the most important factor for the success or failure of reimplantation remains the microsurgical technique used. However, the quality of the skin, tendon and bone reconstruction, as well as sensory recovery are the elements that define the functional outcome and therefore the ultimate success or failure of reimplantation.
Cost-benefit studies comparing reimplantation versus direct stump closure show that those centers with a larger number of patients and which perform reimplantation on a regular basis obtain higher success rates, with greater patient satisfaction and fewer complications.54 Different studies show that in functional terms, reimplantation is superior to stump closure and to the fitting of a prosthesis when the damage affects the forearm.55,56
Treatment comprises antibiotic prophylaxis and the rest of usual measures. Despite the improvements in surgical technique, there is still great controversy regarding the risk-benefit ratio of antiplatelet medication or anticoagulation. At present there is no uniform criterion referred to the use of antiplatelet medication or anticoagulation,52 though the recommendation is to avoid anticoagulants for reimplantation procedures above the elbow, due to the risk of bleeding.57,58
In addition to reimplantation of the patient’s own extremity, transplantation of an upper extremity from a donor is possible. Such operations must be made in highly selected patients, and the surgical times are usually very long. As a result, in the immediate postoperative period we can encounter the complications commonly seen in reimplantation procedures. Hypothermia and systemic inflammatory responses are common, and in some cases can even result in multiorgan failure (MOF). In fact, attempted quadruple transplants of extremities have failure for this reason.59 Given the risks, at present inferior extremity transplants are not recommended on a general basis. The monitoring requirements would be the same as in the case of reimplantation. Furthermore, in the late postoperative period and also in later stages, we must monitor the immunosuppressor drug levels in order to avoid rejections and the side effects of these drugs. Attention also needs to be paid to possible infectious complications in the immunosuppressed patient.
ConclusionsBurn patients are critical patients that require multiple surgeries and procedures. This makes integral, coordinated and dynamic management essential, adjusted to the clinical situation in each moment. Furthermore, more or less complex but constant monitoring is required. Likewise, the perioperative period of reconstructive surgery needs an integral approach in order to avoid systemic complications and ensure success of the flap, reimplantation or transplant.
FundingNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to Eva Herrero de Lucas, Eva Flores-Cabeza, Lucia Cachafeiro-Fuciños, and to all the medical and nursing staff of the Critical Burns Unit of Hospital Universitario La Paz.
Please cite this article as: Sánchez-Sánchez M, Martínez JR, Civantos B, Millán P. Perioperatorio de cirugía plástica reconstructiva y quemados en Medicina Intensiva. Med Intensiva. 2020;44:113–121.