Wernicke's encephalopathy (WE) is a neurological entity due to thiamine deficiency that is classically associated with alcoholism although it may be present in non-alcoholics too.1,2 Its estimated prevalence is 0.4–2.8 per cent, but the coexistence of conditions of the same profile and the inaccessibility to diagnostic tests make the real incidence of this condition unknown.1 Due to the severity and innocuousness of the treatment, the immediate administration of thiamine is recommended and it stops the progression of the disease and favours recovery.1,3 In order to prevent the occurrence of WE, we should know all those therapeutic situations and interventions where there is an increased metabolic demand that may lead to its deficiency.3 In view of this situation, we hereby present the case of one female patient with thiamine deficient-encephalopathy of rapid progression.
It is the case of a fifty-seven year old female with a history of duodenal bulbitis only. She had been experiencing compartmental alterations, vertigo, vomit, abdominal pain, and food intolerance for two (2) months that finally lead to acute renal failure, and severe metabolic acidosis that ended up with the patient being admitted to her reference hospital. At admission, symptomatic treatment and fluid therapy was initiated and renal function was recovered, but the persistence of the patient's digestive clinical manifestations recommended the initiation of parenteral nutrition (PN). One abdominal computed tomography (CT) scan was performed together with one digestive endoscopy, and several other serological and microbiological tests–all of them with anodyne results. In a progressive way, the behavioural changes became more and more significant and deteriorated the patient's level of consciousness until she scored 9–10 points in the Glasgow Coma Scale (GCS). One cranial CT scan and one lumbar puncture were performed and both were not pathological. Given the diagnostic uncertainty, the patient was transferred to our unit specialised in the management of neurocritical conditions.
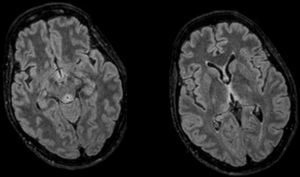
Upon arrival the patient scored 8–12 points in the GCS. Her only physical focality was the inexistence of oculocephalic reflexes. Given the patient's clinical manifestations, chronopathology, and negative tests, the initial suspicion included the possibility of WE and the patient was administered IV thiamine; 24–48h later the patient improved significantly and was bradypsychic, yet aware and collaborative. One cranial MRI was performed that confirmed the presence of thalamic, periaqueductal hyperintense, symmetrical lesions, mammillary bodies and quadrigeminal tubercles–all typical findings of WE and the patient was discharged early. Yet despite the patient's initial recovery, in the posterior follow-ups she showed sequelae noticeable in her gait and memory.
There are multiple publications on WE. The classical clinical triad includes the alteration of the mental state, oculomotor signs and cerebellous alterations.1–3 The coexistence of these symptoms is variable, but the series coincide in that this entity is more common in alcoholic patients than in non-alcoholic patients, in whom the course of the disease is faster and more fatal, making it detection harder.1,3 The diagnostic sensitivity of the triad is limited (20 per cent), but it improves when nutritional deficit is included: if 2 out of these 4 symptoms are present, we may detect up to 85 per cent,4 coexisting all in our case. However, occasionally it is done by exclusion.3
The thiamine reserves are limited and we need a regular supply in our food.5 It is hydrolysed in the stomach and its local absorption is saturable, which is why its oral administration is not effective if we want to increase its levels rapidly,4,5 being the parenteral administration essential.1,3,5 Its function is being the cofactor of enzymes of the metabolism of carbohydrates that are essential in the production of cerebral energy, and its deficit3,5 causes neurotoxicity, and neuronal death.
If there is insufficient intake or incremental losses, its deposits are depleted in 3–4 weeks.5 Intake after prolonged fasting and/or the administration of glucose solutions increases the needs, which is why it is recommended to administer it before or together with glucose solutions.3
Its leading cause is alcoholism although it may be due to malnutrition, losses (vomits, dialysis), increased requirements (severe sepsis, burned) and genetic metabolic disorders,1,2 being the coexistence of various of these factors the ideal scenario for its rapid progression. In our case, prolonged hyperemesis was initially responsible, although the introduction of PN increased the metabolic demand in one patient in whom the deficit was unnoticed, which worsened the progression. Multivitamin supplements usually include between 3 and 3.5mg of thiamine. Based on the actual guidelines, patients at risk who initiate PN should receive between 100 and 300mg/day during the first three (3) days,1,3,6 especially patients with malnutrition.7 However, compliance is poor. There is no general consensus on dose in WE.
Diagnosis is clinical even though there are tests for assessment and support. The cerebral nuclear magnetic resonance is the modality of choice1,3 thanks to its specificity (93 per cent) and positive predictive value (89 per cent) being the CT scan discarded due to its low sensitivity. The typical lesions are symmetrical and they affect the medial thalamus, the mammillary bodies, the tectal plate, and the periaqueductal region8 (Fig. 1). The thiamine deficiency may be analysed using high resolution liquid chromatography or from the activity displayed by erythrocyte transketolase1,3,7 where it acts as a cofactor. In our patient we were unable to obtain any levels due to an extraction mistake that made our sample useless. The fast recovery after the administration is the best diagnosis as it occurred with our patient.
Vitamins and other coexisting deficits should be supplemented. Magnesium is an important cofactor of the thiamine pathways. One recent study confirmed a significant increase of the activity of erythrocyte transketolase in the group treated with magnesium and thiamine compared to the group that received thiamine only, which means that the administration of magnesium plus thiamine may speed up the recovery of metabolic pathways.9,10
The prognosis of WE is closely associated with the precocity of diagnosis and treatment.1,3,5 Its low cost and innocuousness support its early administration. The residual deficits are common as it occurred with the case presented here.
We would like to finish with a thought. The diagnosis of WE is clinical and the response to treatment with thiamine is the best test there is. We should always take it into consideration with non-related neurological manifestations in patients with risk factors (increased metabolic demand, or deficits). When in clinical suspicion, we should supplement with IV thiamine, since it has a good profile of safety and innocuousness that may prevent and even stop the devastating effects of WE.
FundingThe authors declare that they have received no funding while conducting this study.
Please cite this article as: Freire-Aragón MD, Fernández Delgado E, Carbajal-Guerrero J, Ribera-Rubiales G. Encefalopatía progresiva de origen carencial: identificar a los pacientes en riesgo de déficit de tiamina. Med Intensiva. 2017;41:439–441.