Evaluate the incidence of hypotension during the weaning phase of vasopressors.
DesignA single-center, open-label randomized clinical trial between May and December 2022.
Settinga tertiary care academic medical center.
Patients91 adult patients over 18 years of age with septic shock (according to Sepsis-3).
InterventionPatients were divided into two groups: initial reduction of norepinephrine or initial reduction of vasopressin.
Main variables of interestThe primary outcome was the incidence of hypotension within the first 24&#¿;h after reducing vasopressors. Additionally, the clinical impact of this hypotension was assessed through mortality, length of hospital stay, duration of vasopressor use, incidence of arrhythmias, and prevalence of hemodialysis.
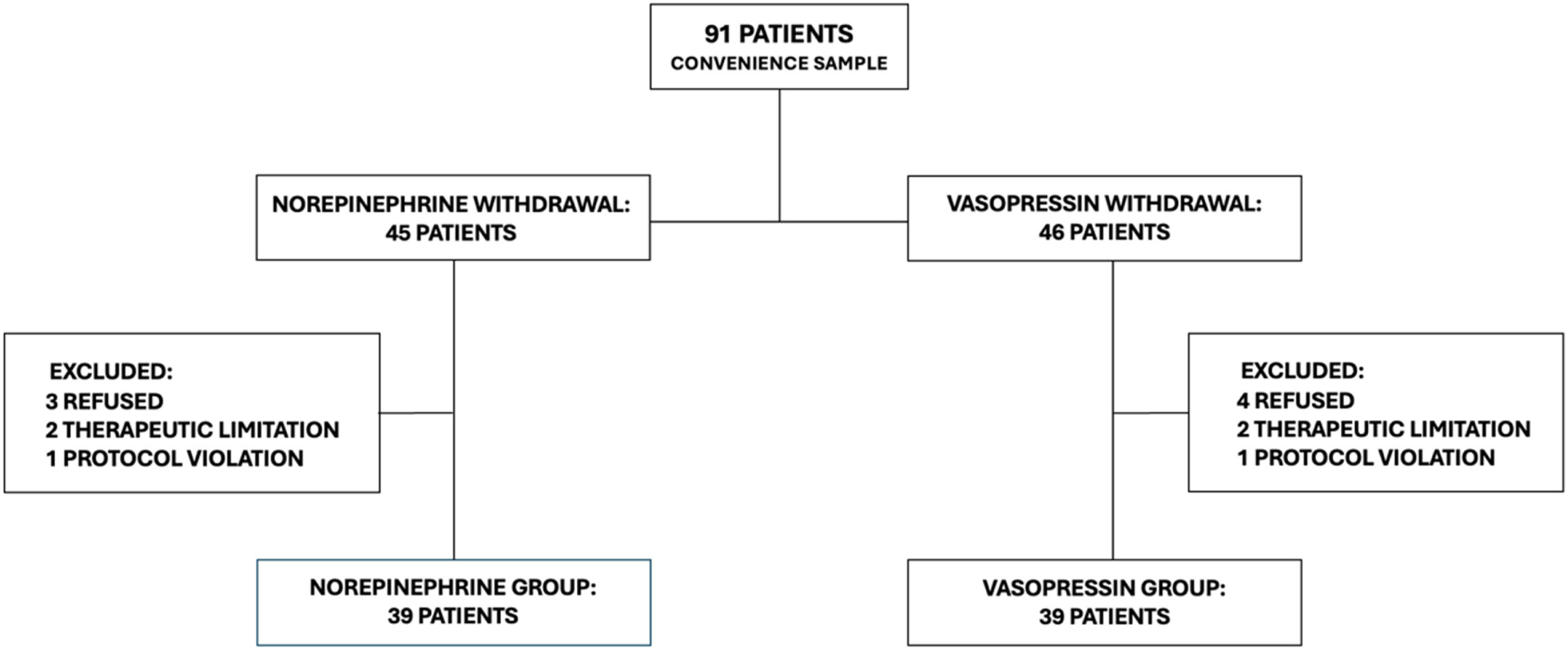
ResultsOut of a total of 91 patients, 78 were included in the analysis: 39 in the norepinephrine group and 39 in the vasopressin group. Despite a numerically significant difference in the incidence of hypotension between the groups (norepinephrine 43.6%, vasopressin 25.6%), there was no statistical difference (p&#¿;=&#¿; 0.153, relative risk = 1.7, 95% confidence interval: 0.9–3.2). In this sample, vasopressin withdrawal was predominantly titrated. There were no differences between the groups in terms of the evaluated clinical outcomes.
ConclusionNo differences were detected in the incidence of hypotension when weaning was initiated with norepinephrine or vasopressin, although it was non significantly higher in norepinephrine group. In our sample, vasopressin withdrawal was titrated, which differs from North American practice. Brazilian Clinical Trials Registry (REBEC: RBR-10smbw65). ClinicalTrials.gov platform (NCT 05506319).
Evaluar la incidencia de hipotensión en la fase de retirada de vasopresores: noradrenalina y vasopresina.
DiseñoEnsayo clínico unicéntrico, abierto y aleatorizado.
Ámbitoun Hospital Universitario de tercer nível.
Pacientes91 pacientes mayores de 18 años con shock séptico (según Sepsis-3).
IntervenciónLos pacientes se dividieron en dos grupos: reducción inicial de noradrenalina o reducción inicial de vasopresina.
Principales variables de interésLa incidencia de hipotensión en las primeras 24 horas posteriores a la reducción de vasopresores. El impacto clínico de esta hipotensión a través de la mortalidad, la duración de la estancia hospitalaria, el tiempo con vasopresor, la incidencia de arritmias y la prevalencia de hemodiálisis.
ResultadosDe 91 pacientes, 78 fueron incluidos en el análisis: 39 en el grupo noradrenalina y 39 en el grupo vasopresina. La incidencia de hipotensión fue mayor en el grupo que inició la retirada de noradrenalina (43,6% vs 25,6%), aunque no hubo diferencias estadísticamente significativas (p&#¿;=&#¿;0,153, RR = 1,7, IC 95%: 0,9−3,2). En esta muestra, la retirada de vasopresina se tituló en la mayoría de los casos. No hubo diferencias entre los grupos en cuanto a los resultados clínicos evaluados.
ConclusiónNo se detectaron diferencias en la incidencia de hipotensión cuando el destete se inició con norepinefrina o vasopresina, aunque fue no significativamente mayor en el grupo de norepinefrina. En nuestra muestra, la vasopresina se retiró de forma titulada, lo que refleja la realidad nacional, lo que difiere de la práctica norteamericana.
Registro Brasileño de Ensayos Clínicos (REBEC: RBR-10smbw65).
Plataforma ClinicalTrials.gov (NCT 05506319).
In a syndromic analysis, among the causes of shock, the vasoplegic profile predominates, and within this subgroup, septic etiology is the most common. Sepsis is the leading cause of death among critically ill patients1 and is the main indication for the use of vasopressors. Regarding the pathophysiology, there is hyporesponsiveness of vascular smooth muscle to norepinephrine, as well as a relative deficiency of vasopressin.2 Norepinephrine is the first choice vasopressor in septic shock.3 However, high doses of catecholamines are associated with side effects such as tachyarrhythmias.4 Vasopressin has a norepinephrine-sparing effect.5 Though there is no conclusive evidence, it is speculated that there is also nephroprotective action.6,7 Currently, vasopressin is the second option in septic shock, used in conjunction with norepinephrine.3
In 2012, the phased approach to shock management was developed – resuscitation, optimization, stabilization, and evacuation – along with a growing interest in deresuscitation.8–10 In 2010, Bauer et al.11 published the first study focused on vasopressor weaning. This observational and retrospective study observed a higher incidence of hypotension among those who initiated withdrawal with vasopressin. However, the study found no difference in other outcomes, such as length of hospitalization or mortality. Later, Kyeongman et al.12 published the first clinical trial with 78 patients in this area. Contrary to findings thus far, there was a higher incidence of hypotension among those who started weaning with norepinephrine.
Although there are guidelines on initiating vasopressors during the resuscitation phase, little is known about how to proceed during the weaning phase.13 This is a routine decision in daily ICU practice but lacks robust scientific foundation. This clinical trial assesses the incidence of hypotension during vasopressor weaning and its associated clinical impact, comparing primary weaning with norepinephrine versus vasopressin.
Patients and methodsThis is a single-center, open-label, randomized controlled trial (RCT). Patients from the ICU of Hospital Nossa Senhora da Conceição (HNSC), a public hospital with 784 beds, located in the south of Brazil (Porto Alegre, Rio Grande do Sul) were included. The ICU consists of 59 beds, serving both medical and surgical patients. The medical team comprises 67 intensivists. The nursing staff provides care at a ratio of one nurse for every five beds. According to the National Health Surveillance Agency (ANVISA), there must be at least 1 nurse for every 8 beds. In our institution we also have the licensed practical nurse: 1 for every 2 beds. Adults over 18 years of age with septic shock (according to The Third International Consensus Definitions for Sepsis and Septic Shock: Sepsis-314), admitted to the ICU and concurrently using norepinephrine and vasopressin, were included. Interventions prior to randomization, such as the time of initiation of vasopressin, were performed by the assistant team, without intervention from the research team. Those for whom the reduction of norepinephrine or vasopressin occurred due to the attending team's decision not to add therapies, prioritizing palliative care, were excluded, as were cases where, in addition to the reduction of norepinephrine or vasopressin, a third drug with a predominantly vasopressor effect was added prior to randomization. The association of inodilator drugs—dobutamine or milrinone—did not result in exclusion from the study. Patient selection was done by convenience sampling between May and December 2022. Randomization was performed using sealed opaque envelopes, in a 1:1 ratio, identified by numbers and selected randomly. The study was approved by the Scientific Advisory Committee of the Grupo Hospitalar Conceição (GHC), as well as by the institutional Research Ethics Committee (Ethical Appreciation Presentation Certificate Platform: 57213022.0.0000.5530; Opinion Number: 5.415.614). For study inclusion, informed consent was obtained from the patient or their direct representative. Patients were divided into two groups: initial reduction of norepinephrine, defined as the norepinephrine group, and initial reduction of vasopressin, defined as the vasopressin group. The study is registered in the Brazilian Clinical Trials Registry (REBEC: RBR-10smbw65) and also on the ClinicalTrials.gov platform (NCT 05506319). Data reporting follows the CONSORT15 guidelines.
Patients’ chart number, age, and gender, as well as the use of other vasoactive drugs besides norepinephrine and vasopressin, and the prescription of hydrocortisone, were recorded. The dose of norepinephrine (in mcg/kg/min) and vasopressin (in U/min) at the time of the first vasopressor reduction was documented. The norepinephrine formulation used in the institution is hemitartrate 2&#¿;mg/mL, in a 4&#¿;ml ampoule (equivalent to 4&#¿;mg of norepinephrine base), manufactured by Hypofarma, Brazil. The available vasopressin formulation is synthetic vasopressin (8-arginine vasopressin), 20 U/mL, in a 1&#¿;ml ampoule, marketed under the brand name Encrise by Biolab Sanus Farmacêutica, Brazil. These companies did not participate in any stage of the study. The presence of comorbidities was retrieved from medical records. The use of mechanical ventilation and hemodialysis was documented. The Sequential Organ Failure Assessment (SOFA) score was calculated for all patients at the time of study inclusion. Pre-existing left ventricular dysfunction, defined as ejection fraction ≤ 40% and identified through an echocardiogram performed within the last 6 months, was recorded.
The primary outcome was defined as the incidence of hypotension within the first 24&#¿;h after initiating the reduction of either vasopressor (norepinephrine or vasopressin), defined as a decrease in mean arterial pressure below 65&#¿;mmHg, leading to one or more of the following interventions: administration of crystalloid or colloid fluids, increased dosage of the remaining vasopressor drug, or reinstatement of the reduced vasopressor drug. The design of the primary outcome was based on the original study by Bauer et al.11 This outcome was assessed through patient follow-up, review of medical records and prescriptions. The titration of vasoactive drugs was at the discretion of the attending team, without a weaning protocol or interference from the research team.
Among those randomized for initial reduction of vasopressin, the withdrawal method was assessed: abrupt versus titrated, without influence from the research team, through patient follow-up and bedside record review.
The following secondary outcomes were evaluated:
- &#¿;
length of stay in the intensive care unit,
- &#¿;
28-day mortality;
- &#¿;
number of days on vasoactive drug use after the reduction of the first drug (norepinephrine or vasopressin), during a follow-up period of up to 7 days;
- &#¿;
incidence of arrhythmias with hemodynamic consequences within the first 24&#¿;h after the reduction of the first drug (norepinephrine or vasopressin), defined as hemodynamic deterioration requiring electrical or chemical cardioversion;
- &#¿;
prevalence of hemodialysis within the first 72&#¿;h after the reduction of the first drug (norepinephrine or vasopressin), regardless of dialysis modality, excluding patients already on dialysis prior to the current hospital admission.
For the assessment of these outcomes, patient follow-up and review of medical records and prescriptions were conducted.
The sample size calculation was based on the only randomized clinical trial available in the literature on this subject so far. In this study, the incidence of hypotension was higher in the group that withdrew norepinephrine first (68.4% versus 22.5%, p&#¿;<&#¿; 0.001).12 To detect the difference in the incidence of hypotension between the groups, the online version of the PSS Health tool was used.16 Considering a power of 80%, significance level of 5%, hypotension incidence of 68%,12 and estimated relative risk of 0.5, a total sample size of 78 patients was determined, divided into two groups of 39.
The statistical analysis was conducted using the SPSS software (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA), with normality of variables assessed using the Shapiro-Wilk test, using a significance level of p&#¿;<&#¿; 0.05 to indicate statistical significance.
Quantitative variables with normal distribution were presented using measures of central tendency, mean, and standard deviation. Those with non-normal distribution were described using the median and interquartile range.
The Student's t-test was used to compare the means of independent samples with normal distribution for the variables "age", "weight" and "SOFA" with the categorical variables "norepinephrine" and "vasopressin". It was also used for the variables "norepinephrine dose", "vasopressin dose" and "PaO2/FiO2 ratio", with the same categories.
To assess the association between the categorical variables "norepinephrine", "vasopressin" and "hypotension", as well as for the vasopressin subgroup (abrupt suspension versus gradual), Fisher's Exact Test was used. Results were presented with relative risk and a 95% confidence interval. Per-protocol analysis was conducted.
The Chi-square test was applied to determine the presence of statistically significant association between the categorical variables "norepinephrine" and "vasopressin" with the variables "mortality", "arrhythmias" and "hemodialysis".
The non-parametric variables "norepinephrine" and "vasopressin" were related to the continuous variables "length of stay in the ICU" and "days free of vasoactive drugs" using the Mann-Whitney U test to compare these independent samples.
ResultsNinety-one patients were screened. In seven cases, a legal representative refused to include the patient in the study. In four cases, the withdrawal of the vasopressor was linked to therapeutic limitations. There were protocol breaches in 2 cases: in the first, the randomization group was not respected; in the second, there was a parallel reduction of norepinephrine and vasopressin. In the per-protocol analysis, 78 patients were included: 39 patients in the norepinephrine group and 39 patients in the vasopressin group (Fig. 1).
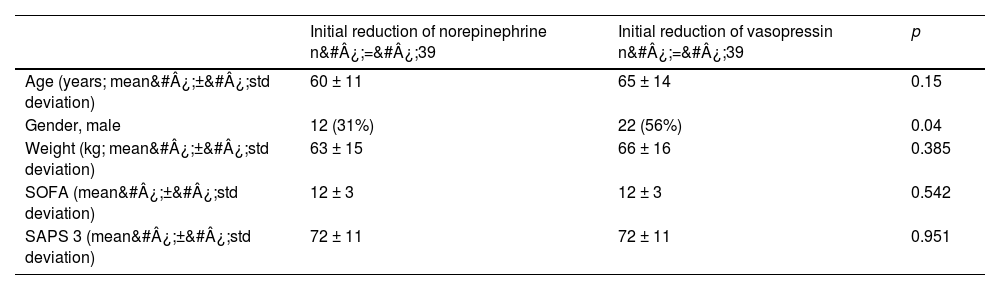
The most frequent focus of infection was respiratory. The average dose at inclusion was 0.5 mcg/kg/min of norepinephrine and 0.03 U/min of vasopressin. In the norepinephrine group, five patients used dobutamine, none used milrinone. In the vasopressin group, four used dobutamine and only one used milrinone. There was a homogeneous distribution of patients, as inferred by the SOFA score of 12 in both groups (SOFA: mean 12, standard deviation ± 3, in both groups). The cohort profile can be found in Table 1.
Population characteristics.
| Initial reduction of norepinephrine n&#¿;=&#¿;39 | Initial reduction of vasopressin n&#¿;=&#¿;39 | p | |
|---|---|---|---|
| Age (years; mean&#¿;±&#¿;std deviation) | 60 ± 11 | 65 ± 14 | 0.15 |
| Gender, male | 12 (31%) | 22 (56%) | 0.04 |
| Weight (kg; mean&#¿;±&#¿;std deviation) | 63 ± 15 | 66 ± 16 | 0.385 |
| SOFA (mean&#¿;±&#¿;std deviation) | 12 ± 3 | 12 ± 3 | 0.542 |
| SAPS 3 (mean&#¿;±&#¿;std deviation) | 72 ± 11 | 72 ± 11 | 0.951 |
| Comorbidities | |||
|---|---|---|---|
| Hypertension | 20 (51%) | 21 (53%) | > 0.999 |
| Heart failure | 3 (7%) | 3 (7%) | > 0.999 |
| Ischemic heart disease | 4 (10%) | 8 (20%) | 0.346 |
| Diabetes | 9 (23%) | 13 (33%) | 0.45 |
| AIDS | 4 (10%) | 3 (7%) | > 0.999 |
| Neoplasm | 14 (35%) | 16 (41%) | 0.641 |
| COPD | 10 (25%) | 3 (7%) | 0.068 |
| Chronic kidney disease | 3 (7%) | 3 (7%) | > 0.999 |
| Chronic dialysis patient | 0 (0%) | 2 (5%) | 0.494 |
| Ejection fraction ≤ 40% | 4 (10%) | 6 (15%) | 0.735 |
| Vasoactive drugs | |||
|---|---|---|---|
| Norepinephrine dose (mcg/kg/min; mean&#¿;±&#¿;std deviation) | 0,56 ± 0,33 | 0,52 ± 0,34 | 0.553 |
| Vasopressin dose (U/min; mean&#¿;±&#¿;std deviation) | 0.03 ± 0.009 | 0.03 ± 0.01 | 0.813 |
| Maximum dose of norepinephrine (mcg/kg/min; median, p25 – p75) | 0,7 (0,6 – 0,8) | 0,7 (0,6 – 0,8) | 0.818 |
| Maximum dose of vasopressin (U/min; median, p25 – p75) | 0.04 (0.03 – 0.04) | 0.04 (0.03 – 0.04) | 0.65 |
| Dobutamine | 5 (12%) | 4 (10%) | > 0.999 |
| Milrinone | 0 (0%) | 1 (2%) | > 0.999 |
| Other therapies | |||
|---|---|---|---|
| Mechanical ventilation | 39 (100%) | 35 (89%) | 0.115 |
| PaO2/FiO2 ratio (mean&#¿;±&#¿;std deviation) | 210 ± 94 | 245 ± 95 | 0.108 |
| Hydrocortisone | 33 (84%) | 34 (87%) | > 0.999 |
| Onset of hemodialysis | 15 (38%) | 19 (48%) | 0.493 |
| Infectious focus | |||
|---|---|---|---|
| Respiratory | 23 (59%) | 20 (51%) | 0.649 |
| Catheter | 4 (10%) | 4 (10%) | > 0.999 |
| Abdominal | 12 (30%) | 12 (30%) | > 0.999 |
| Genitourinary | 0 (0%) | 3 (7%) | 0.24 |
| Cutaneous | 2 (5%) | 4 (10%) | 0.675 |
| Central nervous system | 0 (0%) | 1 (2%) | > 0.999 |
| Undefined | 1 (2%) | 2 (5%) | > 0.999 |
SOFA: sequential organ failure assessment score; AIDS: acquired immunodeficiency syndrome; COPD: chronic obstructive pulmonar disease; PaO2/FiO2 ratio: arterial oxygen partial pressure/fraction of inspired oxygen ratio.
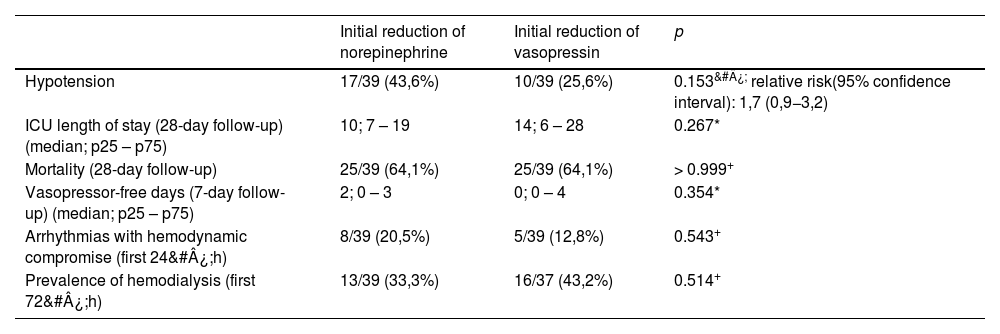
In a per-protocol analysis of 78 patients, the incidence of hypotension in the first 24&#¿;h tended to be higher in the group that began weaning with norepinephrine (norepinephrine: 43.6%, vasopressin: 25.6%), but with no statistically significant difference (p&#¿;=&#¿; 0.153, relative risk = 1.7, 95% confidence interval: 0.9–3.2) (Table 2). The intention-to-treat analysis of 84 patients does not alter the interpretation of the results (norepinephrine: 40.4%, vasopressin: 23.8%; p&#¿;=&#¿;0.16, relative risk = 1.7, 95% confidence interval: 0.8–3.2). In the norepinephrine group, among those who experienced hypotension, in 71% of cases, the instituted management was an increased dosage of the remaining vasopressor drug. In 29% of cases, the administration of crystalloid or colloid fluids was chosen. In the vasopressin group, in 90% of cases, the management was an increased dosage of the remaining vasopressor drug. In neither group was the reinstatement of the reduced vasopressor drug chosen in any hypotensive events.
Outcomes.
| Initial reduction of norepinephrine | Initial reduction of vasopressin | p | |
|---|---|---|---|
| Hypotension | 17/39 (43,6%) | 10/39 (25,6%) | 0.153&#¿; relative risk(95% confidence interval): 1,7 (0,9−3,2) |
| ICU length of stay (28-day follow-up) (median; p25 – p75) | 10; 7 – 19 | 14; 6 – 28 | 0.267* |
| Mortality (28-day follow-up) | 25/39 (64,1%) | 25/39 (64,1%) | > 0.999+ |
| Vasopressor-free days (7-day follow-up) (median; p25 – p75) | 2; 0 – 3 | 0; 0 – 4 | 0.354* |
| Arrhythmias with hemodynamic compromise (first 24&#¿;h) | 8/39 (20,5%) | 5/39 (12,8%) | 0.543+ |
| Prevalence of hemodialysis (first 72&#¿;h) | 13/39 (33,3%) | 16/37 (43,2%) | 0.514+ |
| Abrupt cessation of vasopressin | Gradual reduction of vasopressin | p | |
|---|---|---|---|
| Hypotension | 2/3 (66,7%) | 8/36 (22,2%) | 0.156&#¿; relative risk (95% confidence interval): 3 (1,1−8,2) |
The median length of stay in the Intensive Care Unit was 14 days in the vasopressin group (interquartile range: 6–28) and 10 days in the norepinephrine group (interquartile range: 7–19), with no statistical difference (p&#¿;=&#¿; 0.267). There was no difference in mortality between the groups. There was no statistically significant difference in the time free of vasoactive drugs. The incidence of arrhythmias with hemodynamic repercussions was 20.5% in the norepinephrine group and 12.8% in the vasopressin group (p&#¿;=&#¿; 0.543). In the norepinephrine group, there were 6 episodes of atrial fibrillation, one of flutter, and one of paroxysmal supraventricular tachycardia. In the vasopressin group, there were 5 episodes of atrial fibrillation. The prevalence of hemodialysis was 33.3% in the norepinephrine group and 43.2% in the vasopressin group (p&#¿;=&#¿;0.514) (Table 2).
In a post-hoc analysis, the method of vasopressin withdrawal was evaluated. Among the 39 patients who began the reduction with vasopressin, only three underwent abrupt weaning, stopping the infusion without dose titration. In this group, a 66.7% incidence of hypotension was observed among those who underwent abrupt vasopressin withdrawal, compared with 22.2% among those who underwent gradual reduction (p&#¿;=&#¿; 0.156, relative risk: 3, 95% confidence interval: 1.1–8.2) (Table 2). No adverse events (acute coronary syndrome, cardiorespiratory arrest, mesenteric ischemia, or digital ischemia) were recorded during the 28-day follow-up.
DiscussionIn our sample of septic patients, the option for primary weaning of norepinephrine or vasopressin was not associated with a higher incidence of hypotension. In this clinical trial, 43.6% of patients who began weaning from vasopressors with norepinephrine experienced hypotension, compared to 25.6% of patients whose weaning began with vasopressin. The lower incidence of hypotension in our study compared to the study that underpinned our sample size calculation resulted in the absence of a statistically significant difference in the primary outcome despite an 18 percentage point difference.12
Our result aligns with the only clinical trial published so far. The study by Kyeongman et al.,12 known as DOVSS, established a protocol for titration of vasopressors during the withdrawal phase. We opted for a pragmatic approach where titration of drugs during weaning was left to the discretion of the attending team without interference of the research team, better mimicking bedside practice. The institution has no protocol for titration of vasoactive drugs. Vasopressors are usually tapered off gradually keeping MAP&#¿;>&#¿;65&#¿;mmHg. Vasopressin is usually not withdrawn abruptly.
The results of these clinical trials diverge from observational studies, which indicate a higher incidence of hypotension with primary withdrawal of vasopressin.
The population of our study consisted of patients with severe shock, requiring high doses of vasopressors (average dose at inclusion: norepinephrine 0.5 mcg/kg/min; vasopressin 0.03 U/min). This data differs from other observational studies, where patients required lower doses of vasopressors.17–19 Song et al.19 retrospectively analyzed 961 septic patients, in the largest cohort available to date. In this study, the maximum dose of norepinephrine ranged from 0.24 mcg/kg/min to 0.28 mcg/kg/min.
In our study, nearly all patients underwent gradual withdrawal of vasopressin, in contrast to observational studies where vasopressin withdrawal occurred abruptly17–20; while in other studies, the method of vasopressin withdrawal is not mentioned.11,21,22 Hammond et al.23 published a study based on a questionnaire conducted in North American hospitals. In an assessment of vasopressin use in patients with septic shock, it was observed that approximately 70% of physicians wean off vasopressin rather abruptly, without dose titration. Nearly all observational studies on vasopressor weaning have been conducted in North America. The practice of abruptly withdrawing vasopressin, as opposed to gradual withdrawal, may explain the higher incidence of hypotension associated with vasopressin withdrawal in observational studies, a contrasting result to ours and the DOVSS study.
Another open question is the impact of hypotension on clinically relevant outcomes. Although we observed a higher prevalence of hemodialysis among those who initiated weaning from vasopressin, which could raise speculations about the potential nephroprotective effect, there was no statistically significant difference. Despite observing a significant difference in the incidence of hypotension, the DOVSS study did not find differences in the duration of vasopressor use, length of stay, or mortality. Similarly, the vast majority of observational studies did not identify differences in the clinical outcomes analyzed. Our evaluation is consistent with these findings, as we did not find statistically significant differences in the evaluated clinical outcomes. Although we observed a high mortality rate (64%), this data is consistent with what has been observed in Brazil and other developing countries. The SPREAD study (Sepsis Prevalence Assessment Database), a cohort conducted by the Latin American Institute of Sepsis (ILAS) in 227 Brazilian ICUs, demonstrated a mortality rate of 58.6%.24
As a strength of our study, we highlight that it is a randomized clinical trial conducted in a clinical-surgical ICU with critically ill patients, a population that is prevalent worldwide, particularly in low-income countries.
However, our study has limitations; for example, the sample size calculation was based on the incidence of hypotension observed in the DOVSS trial, where the hypotension incidence in the norepinephrine group was 68.4%, while in our study, it was 46.3% in this group. This difference compromised the strength of our study. The fact that it is a single-center study is also a limitation. Similarly, convenience sampling and lack of blinding open the possibility of potential biases.
ConclusionWe observed no difference between the norepinephrine and vasopressin groups regarding the hypotension outcome, nor in the clinical outcomes evaluated. The idea conveyed by available observational studies so far, that early reduction of vasopressin leads to a higher risk of hypotension, has been widely accepted. Jeon Kyeongman, through the DOVSS clinical trial in 2018, challenged the impression of previous studies. Our work is consistent with the DOVSS study by showing a trend towards higher incidence of hypotension in the norepinephrine group. Future trials with larger samples, as well as a better understanding of the clinical significance of hypotension during vasopressor weaning, will likely provide further insight into this issue.
CRediT authorship contribution statementCássio Mallmann: Study design, data collection, literature search, manuscript preparation.
Thizá Maria Bianchi Galiotto: data collection, analysis, manuscript preparation. Michele Salibe de Oliveira: data collection, manuscript preparation.
Rafael Barberena Moraes: Study design, manuscript review.
Declaration of competing interestThere is no conflict of interest.