The host and microbes play complex roles in balancing the pro- and anti-inflammatory pathways that cause sepsis. It is now increasingly recognized as a disorder of the mitochondrial system intrinsically or as a consequence of microcirculatory abnormalities leading to hypoperfusion/hypoxia ("microcirculatory and mitochondrial distress syndrome”). It is expected that improvements in endothelium or mitochondrial level therapy will lower sepsis-related morbidity and mortality. This article aimed to clarify the mitochondrial and microcirculation abnormalities in patients with sepsis and the futuristic research agenda for the management of sepsis.
El huésped y los microbios desempeñan funciones complejas en el equilibrio de las vías proinflamatorias y antiinflamatorias que causan la sepsis. Ahora se reconoce cada vez más como un trastorno del sistema mitocondrial intrínsecamente o como consecuencia de anomalías microcirculatorias que conducen a hipoperfusión/hipoxia ("síndrome de dificultad microcirculatoria y mitocondrial"). Se espera que las mejoras en la terapia a nivel del endotelio o mitocondrial reduzcan la sepsis. morbilidad y mortalidad relacionadas. Este artículo tuvo como objetivo aclarar las anomalías mitocondriales y de la microcirculación en pacientes con sepsis y la agenda de investigación futurista para el tratamiento de la sepsis.
Sepsis is a life‐threatening organ dysfunction caused by dysregulated inflammatory and immune host response to inflammation and infection. The clinical continuum includes localized infection, organ breakdown, septic shock, multiorgan failure, and death with varying prognosis.1 It affects all ages, with hospital deaths ranging from 14 to 45% in developed countries and higher in under-reported countries. 1,2 The initiating factors include a range of stimuli including a critical level of microorganisms. These converge to a common pathway leading to a mismatch between the oxygen supply and demand at tissue levels. The hypoxic tissues produce acute-phase molecules and these, in turn, stimulate and release a cascade of local and then systematic pro-inflammatory and pro-coagulant molecules. These further propagate an intense generalized neuro-humoral response. The complex interaction between the initiators and propagators is not yet fully understood.1,3,4
Management of sepsis is currently targeting the macro-circulatory dysfunction as seen by observable parameters such as low cardiac filling pressures, low systemic vascular resistance, and profound vasopressor-unresponsive hypotension. However, the optimal dosage of medications including vasopressors and fluids for the management are still not known. Few patients continue to show sudden deterioration of the clinical status despite initial normalization of these parameters. This led to a focus on the role of micro-circulation in sepsis. With the introduction of hand-held vital microscopes for bedside monitoring at tissues levels, it was demonstrated that unregulated release of vasoactive mediators persisted in the microcirculation even after improvement of the clinical parameters. This observed imbalance between macro- and micro-vascular circulation produces heterogeneity in inter- and intra-organ blood flow and local tissue oxygen availability and has been postulated to be responsible for the initiation of the entire cascade of the response to inflammation and infection. The molecules that may be involved in sepsis including the signaling pathways, and the crosstalk between pro-inflammatory and pro-coagulant molecules, signaling pathways and vital organs are potential therapeutic targets. Search is on to target the source and to prevent the production and release of which are responsible for the mortality and morbidly in sepsis.1,2,4
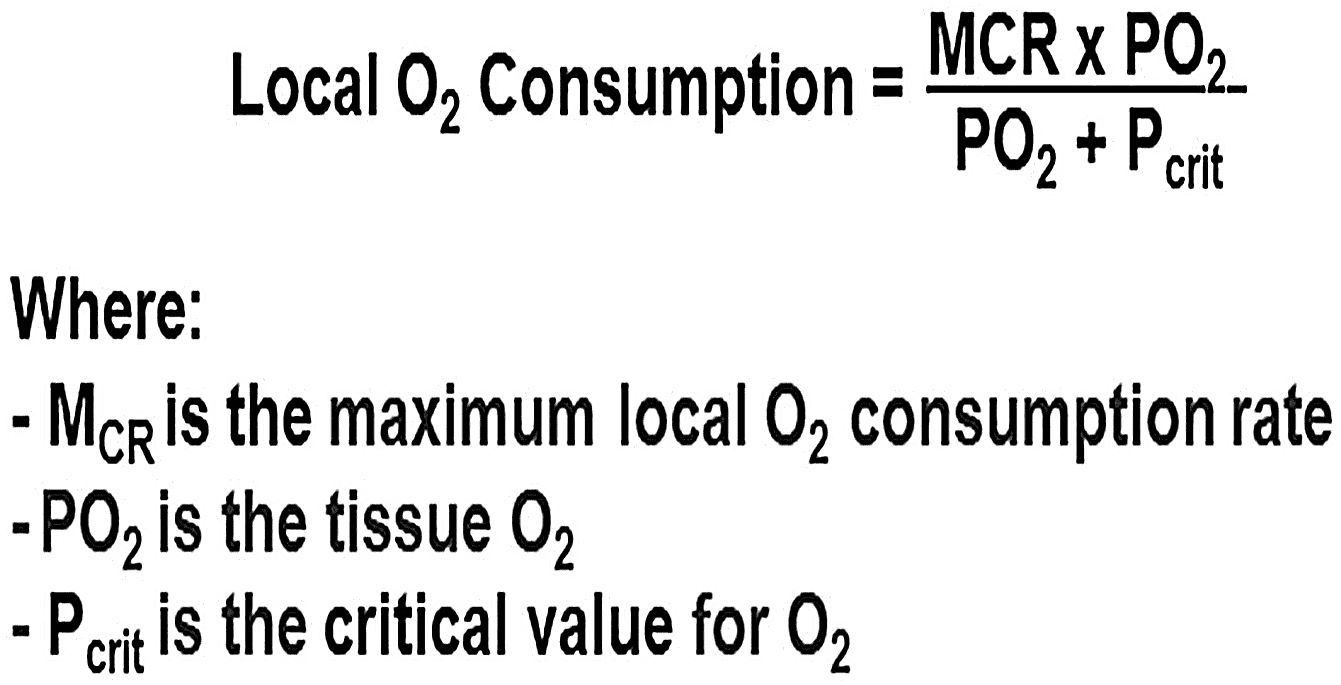
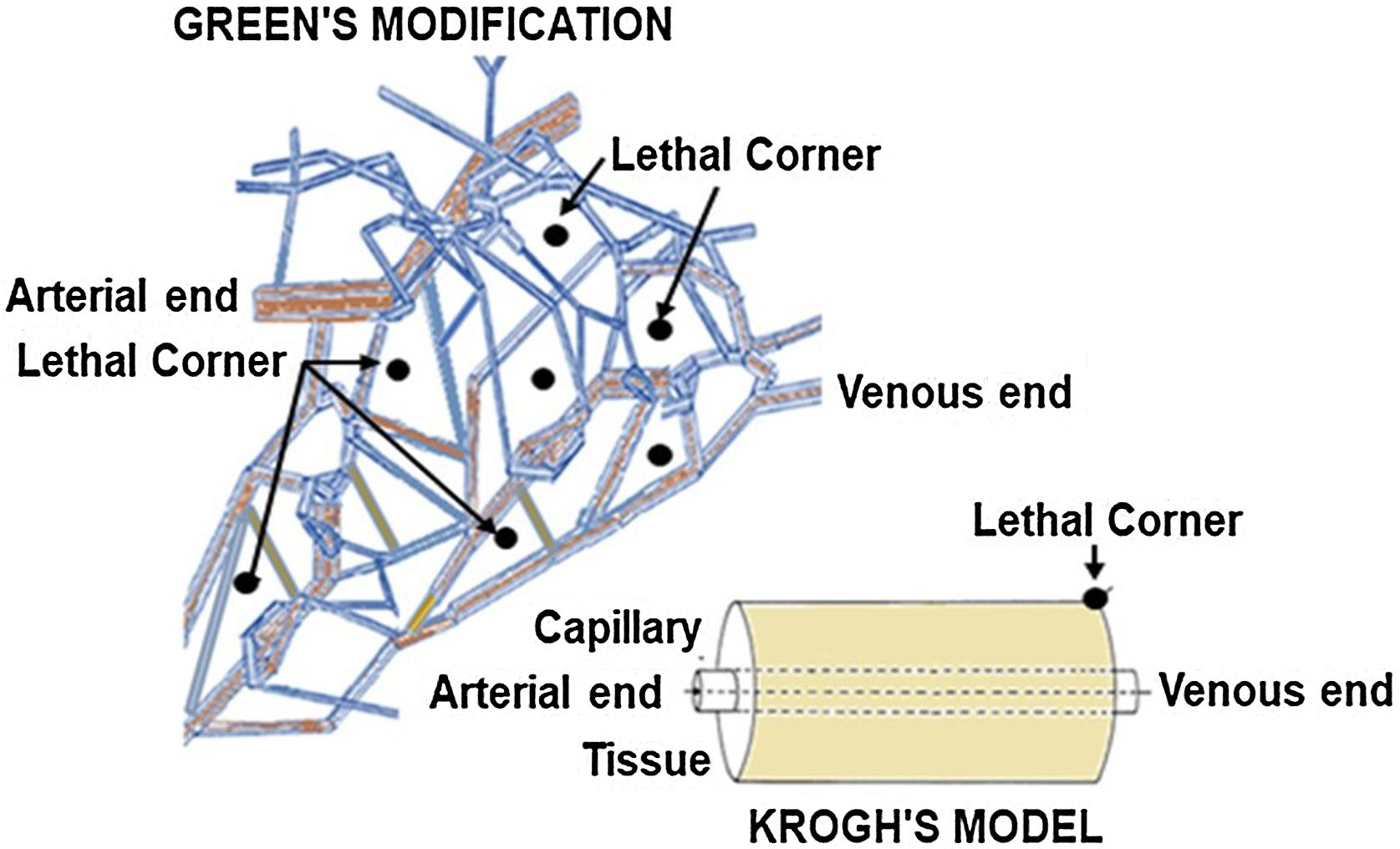
Role of microcirculation in sepsisMicrocirculation is defined as capillaries <20 μm in diameter. Krogh and Erlang defined a microvascular bed as a portion of tissue supplied by a single capillary with oxygen flux occurring in an axisymmetric (tissue cylinder) geometry around it. Tissue oxygen levels are low at the periphery of this cylinder, being greater near the arterial end, and lowest at the venous end of the microvascular bed. The authors called these ‘Lethal Corners”. Under normal blood flow and tissue compliance conditions, different "cylinders of perfusion" overlap with no lethal corners. Application of a Michaelis‒Menten mathematical model based on first-order kinetics to the original Krogh–Erlang solution, further postulated that at low oxygen flux levels, smaller cylinder radii as seen in vasoconstriction, or increased tissue geometry due to fluid accumulation, these corners become at highest risk for under-perfusion(Fig. 1). Hypoxia causes a switch to anaerobic metabolism and the production of localized acidosis through the release of inflammatory molecules. This was implicated as the origin and perpetuator of multiorgan failure. The Oxygen Pressure Field Theory, with the concept of "lethal corners" as the source of inflammation and sepsis, won the Krogh Nobel Prize in 1920.5,6
Green et al. later proposed the three-dimensional concept of a branching network of microvessels inside tissues.7 The "lethal corners" were proposed to be within the tissues instead of at the periphery (Fig. 2). The authors also highlighted a substantial role of tissue myoglobin in conditions of low tissue oxygen levels. The P50 of tissue myoglobin is relatively small (∼2–5 mmHg), and the slope of the myoglobin–oxygen dissociation curve before this value is steeper than the corresponding haem–oxygen dissociation curve. This is an adaptive mechanism to prevent tissue hypoxia. 7,8
Assessment of microcirculationThe microcirculatory oxygenation level is currently evaluated by serum lactate concentration. However, confounding factors in critically ill patients including use of Ringer’s lactate solution as the resuscitative fluid have the potential for misinterpretation and resultant under- or over-resuscitation. The Capillary Refill Time or Mottling Score has limited prognostic value. Microcirculatory dysfunction has been variously assessed by erythrocyte deformability, peripheral to central temperature gradient, and perfusion indices of superficial tissues. Sublingual microcirculation flow index (MFI) is a good indicator for the indirect assessment of splanchnic microcirculation. The MFI significantly correlated with red corpuscle velocity and the fraction of perfused small vessels. An abnormally low MFI (defined as <2.6), especially on the first day of Intensive care unit admission, is associated with adverse outcomes.9 CritiView (CRV, CritiSense Ltd., Tel Aviv, Israel), a new fiberoptic-based optical device, is used to assess the urethral perfusion index (uPI). This measures four different physiological parameters from the urethral wall of animals in real-time: the mitochondrial NADH redox state, microcirculatory blood flow, blood volume, and hemoglobin saturation. An indwelling urinary catheter equipped with a photoplethysmography sensor in contact with the urethral mucosa allows continuous evaluation of the uPI.10 The MFI and uPI have demonstrated mucosal perfusion alterations associated with hypotension or excessive doses of vasopressors. However, further studies are needed to evaluate these devices in a clinical context.4,9,10
Direct viewing of lingual microcirculation with orthogonal polarization spectral imaging, side stream dark field imaging or incident dark field illumination, and partial sublingual carbon dioxide pressure are now available. Others include laser Doppler flowmetry and near-infrared spectroscopy.11
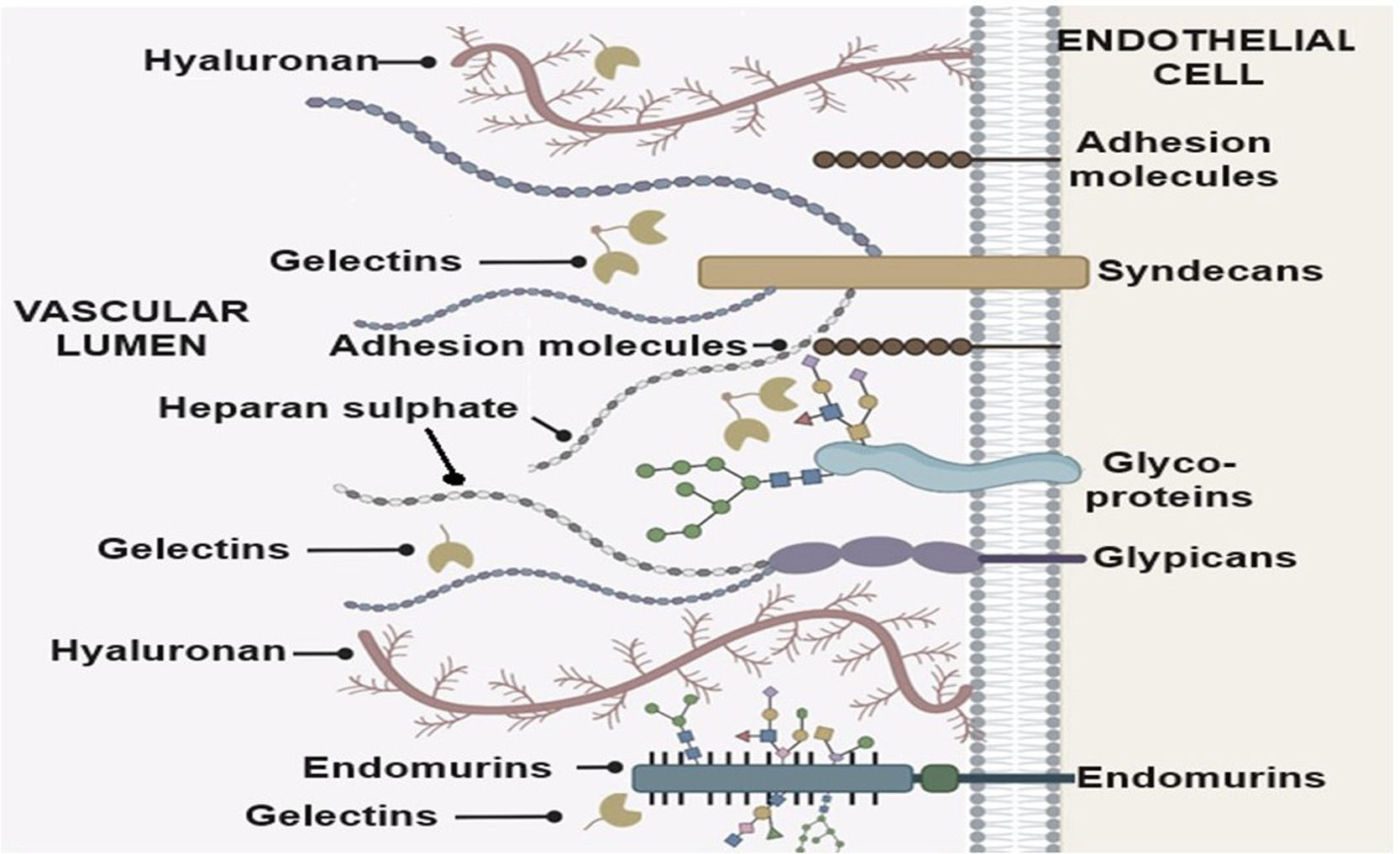
Role of endothelial cells including the glycocalyx in release of inflammatory moleculesEndothelial cells are covered by a multicomponent layer, the vascular endothelial glycocalyx (EGC). It is a network of endothelium- and plasma-derived soluble molecules that contain abundant proteoglycans (PGs) and glycosaminoglycans (GAGs), such as hyaluronic acid, chondroitin sulphate, dermatan sulphate, keratan sulphate, and heparan sulphate (Fig. 3).
The EGC constitutes a first-line barrier to regulate the cellular and molecular traffic and is a powerful antioxidant, and transducer of shear stress to the endothelium.
The EGC is damaged in microcirculatory hypoxia and sepsis which exposes the endothelium. The denuded endothelial cells experienced morphological changes, including degranulation, rupture, and even apoptosis. The content of the intracellular vesicles, the Weibel-Palade bodies (tissue factor; TF, P-selectin, vWF, angiopoietin-2) are released into the vasculature thereby promoting a systemic proinflammatory and procoagulant phenotype. The leukocytic accumulation at the site may enhance tissue damage by further generating inflammatory cytokines, reactive oxygen, and proteases. Leukocyte adherence to the endothelium may also aggravate microvascular blood flow alterations. Pathways, like the angiopoietin-2/tyrosine-protein kinase receptor (Ang2/Tie) axis are critical for maintaining vascular integrity, and on disruption, leads to increased microvascular permeability. The Krogh–Erlang model is affected and microcirculatory hypoxia releases reactive species which further cause cellular damage contributing to organ failure and death. Cytokines also reduce the innate microcirculatory vasomotor response despite an increase in the circulating catecholamine concentrations.
In addition, small vesicles derived from activated or apoptotic endothelial cells with various surface antigens called endothelial microparticles (EMPs) are released into the circulation. Some surface antigens amplify the coagulation and inflammatory response, dispersing it away from the initial site of infection. Heparan sulphate embedded EMPs penetrate hippocampal tissues with remarkable specificity. These oligosaccharides do not affect other systemic and neuronal vascular beds.12 By blocking brain-derived neurotrophic factor (BDNF), a neurotrophin crucial for memory and learning, these have been linked to post-sepsis cognitive impairment.13
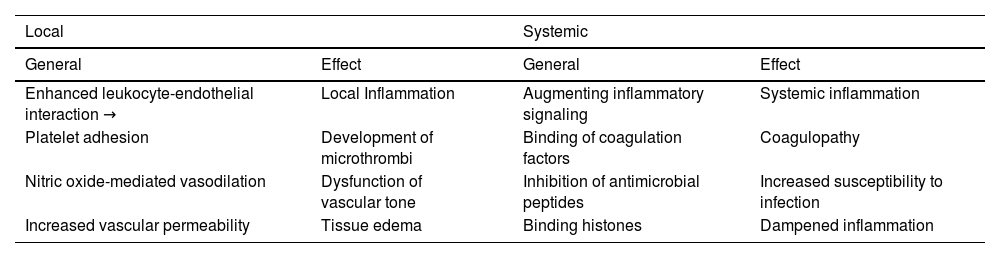
The local and systemic effects of EGC damage are listed in Table 1.
Consequences of septic endothelial glycocalyx degradation.
| Local | Systemic | ||
|---|---|---|---|
| General | Effect | General | Effect |
| Enhanced leukocyte-endothelial interaction → | Local Inflammation | Augmenting inflammatory signaling | Systemic inflammation |
| Platelet adhesion | Development of microthrombi | Binding of coagulation factors | Coagulopathy |
| Nitric oxide-mediated vasodilation | Dysfunction of vascular tone | Inhibition of antimicrobial peptides | Increased susceptibility to infection |
| Increased vascular permeability | Tissue edema | Binding histones | Dampened inflammation |
EGC breakdown in sepsis is influenced by acute illnesses, chronic renal disease, age, and comorbidities, but the role of other factors is less understood. Restoration of the EGC starts five to seven days after an acute injury, and complete restoration takes longer. This may explain the lag in correcting the microcirculatory environment in sepsis patients, even after macro-circulatory hemodynamic correction.14,15
The evaluation of the extent of vascular endothelium and EGC damage in sepsis is important not only for prognostication of endothelial damage and repair but also research for future target drugs.
Evaluation of the EGC in sepsisIn sepsis, there are increased circulating levels of GAG oligosaccharides and PG extracellular domains produced by the degradation and core PG cleavage by two interconnected "sheddase" mechanisms. Upstream regulators of “sheddases” include the activation of the GAG sheddase heparanase (HPSE1) by proinflammatory cytokines, particularly endothelial-derived TNF-α. The Ang2/Tie pathway has antagonistic effects on EGC sheddases. During sepsis, increased levels of Ang2 lead to elevated circulating markers of EGC breakdown, increased permeability, thrombosis, and vascular inflammation. In mouse models of sepsis, suppression of Ang2 has been shown to decrease EGC shedding. Numerous other molecules, such as phorbol esters, tissue inhibitors of matrix metalloproteinases (TIMPs), and macrophage migratory inhibitor factor (MIF1), are implicated in the degradation of EGC on non-endothelial surfaces and in other diseases, indicating their possible relevance to the degradation of EGC in septic endothelial cells.14–17
Evaluation of the vascular endothelium in sepsisTwo particles are of special interest: EMPs and endothelial progenitor cells (EPCs). Survival in patients with sepsis and elevated EMPs has been positively correlated in a few studies. In contrast, in cardiovascular disorders, elevated EMPs indicate endothelial dysfunction and have shown an unfavorable prognosis. However, other researchers discovered no increase in EMPs in sepsis patients, with an inverse correlation with the Sequential Organ Failure Assessment score.18,19
EPCs stem from the bone marrow and differentiate into mature endothelial cells after release of pro-inflammatory molecules released following tissue ischemia. EPCs form cell-to-cell connections and, by tunneling nanotubes (TNTs), transfer mitochondria and other organelles to endothelial cells, thereby aiding in endothelial repair and capillary network creation. This mitochondrial transfer of TNTs can rescue senescent endothelial cells and change cell fate. A positive correlation was observed between the quantity of circulating EPCs in sepsis patients and patient survival. EPCs are crucial therapeutic targets in sepsis, as they promote endothelium restoration and are impacted by various treatment approaches targeting endothelial dysfunction.3,14,19,20
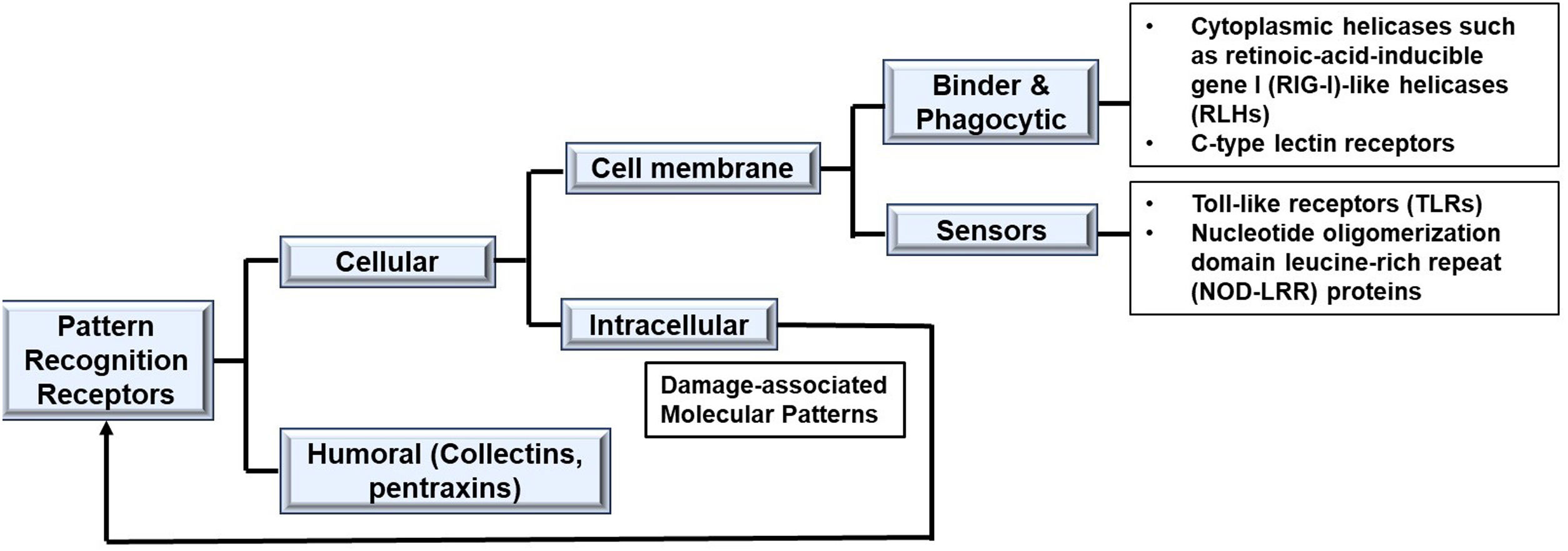
Evaluation of bacterial induced sepsis moleculesLipopolysaccharide (LPS) on the wall of gram-negative bacteria is able to induce an inflammatory response through both intracellular and extracellular pathways. It is a specific microbial molecular signature known as pathogen-associated or microbial-associated molecular pattern (PAMP or MAMP). LPS is composed of lipid A (endotoxin), core oligosaccharide and O-antigen. The specific PAMP of LPS is Lipid A. Pattern recognition receptors (PRRs) are present in innate immune cells. At least four families of PRRs are recognized (Fig. 4). The PRR which recognizes Lipid A is the receptor complex containing at least three essential cell surface components: CD14, Toll-like receptor 4 and myeloid differentiation factor 2 (TLR4/MD-2) complex. Binding with the complex stimulates an upregulation of proinflammatory cytokines including nuclear factor-κB (NF-κB), activator protein-1 (AP-1), members of the cytosine-cytosine-adenosine-adenosine-thymidine (CCAAT)-enhancer-binding protein (C/EBP) family, early growth response protein 1 (EGR-1), p53 and signal transducer and activator of transcription 1 (STAT1). Additionally, following secondary tissue damage, intracellular moieties (e.g., DNA, proteins, or pieces of inorganic crystals), collectively called Damage-Associated Molecular Patterns (DAMPs) or alarmins, are produced. DAMPs, like PAMPs, also bind to PRRs. This process increases the production of chemokines, growth factors, adhesion molecules, gaseous compounds, vasoactive peptides, and cell stress markers exponentially. Clinical sepsis-associated cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-1α, IL-1β, and IL-6, are also produced. Together, these inflammatory cells participate in a positive feedback loop that perpetuates tissue inflammation.3,16,17 While trace amounts of Lipid-A can be beneficial in human hosts, leading to immune protection against severe infection, high levels of LPS induce an overwhelming inflammatory response causing fevers, hypotension, and septic shock.
Role of mitochondria in critical illnessThe disruption of the electron transport chain (ETC) within the mitochondria has been implicated in many diseases. It is the final common pathway for cellular metabolism. Symptoms such as exhaustion, weakness, various skeletal muscle dysfunctions, acute lung and kidney injuries, severe immune dysfunctions, developmental and cognitive disabilities and metabolic encephalopathy are observed in the clinically diverse category of ailments called mitochondrial diseases. Similar symptoms characterize sepsis. Alterations in the ETC leads to decreased adenosine triphosphate (ATP) synthesis, reduced biogenesis, and increased reactive species generation. Damaged mitochondria produce mitochondria-derived DAMPs (mtDAMPs), which include oxidized cardiolipin (oxCL), cytochrome c, and mitochondrial DNA (mtDNA). Blocking mtDNA and inhibiting endothelial cell proliferation in animal models of inflammatory lung injury enhanced the regeneration of endothelial cells. On the other hand, OxCL promotes inflammation by drawing more monocytes to the blood vessel intima layer.
ETC is composed of five multisubunit enzyme complexes: complex I (ubiquinone oxidoreductase), complex II (succinate dehydrogenase), complex III (cytochrome c reductase), and IV (cytochrome c oxidase), and Complex V.20,21 The movement of electrons to the terminal electron acceptor of the chain, i.e., oxygen, is an energy-yielding reaction (52.5 kcal/mol for every pair of electrons transported). Up to 4% of oxygen undergoes incomplete reduction during imperfect electron flow through the ETC. As a result, superoxide anion, the "primary" reactive oxygen species (ROS), is produced. Superoxide further generates “secondary” ROS (hydroxyl radical, singlet oxygen, hypochlorous acid, hydroperoxyl radical, lipid hydroperoxide, peroxyl radical, and alkoxyl radical) and reactive nitrogen species (RNS) (nitric oxide (NO), peroxynitrite, nitric dioxide, oxinitrite, and S-nitrosothiols).14,18,21
Inflammatory mediators, hormones, gaseous mediators (such as hydrogen sulphide and NO), and reactive species (ROS/reactive nitrogen species [RNS]) may play a role in mitochondrial dysfunction. The peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α) is particularly activated. These proteins interact with thyroid glucocorticoid and estrogen-related receptors (ERR α and γ), nuclear respiratory factors (NRF 1 and 2), mitochondrial transcription factor A (Tfam), uncoupling proteins (UCP2), and other transcription factors to control the process of mitochondrial biogenesis. Mitochondrial biogenesis is controlled by two important enzymes thought to function as metabolic sensors, AMP-activated protein kinase (AMPK) and silent information regulator 2 (SIRT-2). An increase in both during energy depletion enhances PGC-1α-dependent transcription. There is also an increase in the mitochondrial gene expression of factors such as hypoxia-inducible factor 1 (HIF-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) and in the inhibition of complex IV. Under normal circumstances, antioxidants (carcinoids and flavonoids) and enzymes (superoxide dismutase 2, coenzyme Q10, glutathione reductase, and oxidase) are present in the mitochondria as defenses to counter these species. The levels of these counteroxidants are lower in patients with sepsis.22
This diminished mitochondrial function, known as “mitochondrial hibernation", may be an adaptive mechanism to tissue hypoxia and hypoperfusion by reducing global oxygen and ATP consumption and downregulating critical cellular processes.3,14,21,22
Assessment of mitochondria in clinical settingsMitochondrial genome encodes 13 proteins, all of which are components of the ETC. mtDNA replication is carried out independent of the cell cycle by the nuclear DNA (nucDNA) encoded polymerase γ, the only DNA polymerase found in the mitochondria. mtDNA can be measured from either frozen tissue or fresh isolates. Mitochondrial extraction from frozen tissues is done using commercial kits (E.g., QIAamp DNA mini kit, QIAGEN, Chatworth, CA). The analysis includes mRNA expression of nuclear-encoded mitochondrial proteins, protein expression of both nuclear and mitochondrial-encoded proteins, and electron microscopy-based mitochondrial determinations. However mtDNA levels were observed to be significant only between patients with infection and those with septic shock with no difference between infection and sepsis levels and sepsis and septic shock levels.23 In the fresh tissue isolates, two marker genes Nicotinamide Adenine Dinucleotide + Hydrogen (NADH) dehydrogenase subunits 1 and 4 are measured using a polymerase chain reaction system (E.g., PE Biosystems, Foster City, CA). Mitochondrial protein concentration can also be measured using colorometric assays (E.g., Bio-Rad DC Protein Assay) using a microplate spectrophotometer.
Implications of current resuscitative guidelines on the microcirculation and sepsis moleculesVarious current international guidelines on the management of sepsis focus only on macro-circulatory stabilization. The implications of these guidelines on microcirculatory perfusion, EGC and endothelial cells and mitochondrial resuscitation need to be addressed, although no effective clinical strategies are available to guide practice.
Resuscitation fluids may exacerbate or ameliorate coagulation dysfunction, endothelial activation, and EGC shedding. However, inconsistently, crystalloids and gelatins may contribute to the disintegration of EGCs. Endothelial cells and EGCs are protected by plasma and albumin. Treatment of sepsis may be improved by therapeutic plasma exchange and fresh frozen plasma replacement, which inhibits the circulation of proinflammatory signals such as heparan sulfate, which acts through DAMP signaling pathways.24 Fresh frozen plasma (FFP) can replace healthy plasma elements in EGCs, restoring balance and promoting hemodynamic changes, enhancing cytokine profiles, and improving endothelial barrier function. FFP therapy alone during sepsis may also preserve and regenerate EGCs.3,18 Recommendations to increase the priority of hyperoncotic albumin solutions and FFP as resuscitative fluids requires extensive clinical studies for firm recommendations.3,11,14,25 The usage of hydroxyethyl starch (HES) is severely limited due to adverse effects such as coagulation malfunction and damage to renal tubules, even though solutions with a higher mean molecular weight (MW), molar substitution (MS), and C2/C6ratio have been shown to preserve and restore EGC. Further research is needed for HES 200/0.4.
A significant correlation was detected between the volume of resuscitation fluids and the degree of EGC damage in two patient cohorts with sepsis. Prospective clinical research has shown increased vasopressor dependence and duration of mechanical ventilation in patients receiving liberal fluid resuscitation (30 mL/kg/hr) vs. conservative fluid resuscitation (10 mL/kg/hr), with syndecan-1 levels increasing, indicating EGC breakdown and worsening mortality. These findings imply that EGC degradation may mediate the adverse clinical outcomes associated with intensive fluid resuscitation during sepsis.11,14,15 The optimum dose of fluids in sepsis needs to be studied further.
Steroids are advised for some patients with septic shock despite their conflicting clinical utility. Glucocorticoids regulate different aspects of endothelial physiology including expression of adhesion molecules, production of pro-inflammatory cytokines and maintenance of endothelial barrier integrity.3,18,21,26 Levels of syndecan-1 and heparan sulphate in the serum, markers of EGC stability were found decreased in isolated guinea pig hearts following exhibition of steroids.19 Administering hydrocortisone via injection before TNF-α infusion reduced vascular permeability and EGC breakdown treatment.25 However, the regulation of EGC sensitivity remains still incompletely understood.
Dobutamine infusion (5 μg/kg/min) has been shown to improve microcirculatory perfusion and increase tissue oxygen supply in septic shock patients. Increasing the dobutamine dosage (over 5 μg/kg/min) did not improve microcirculation. Further studies are needed to determine the usefulness of dobutamine in microcirculation in low doses.27 Dexmedetomidine, an alpha-agonist, has demonstrated significant inhibition of the host response to infection by boosting the pressure sensitivity of norepinephrine to a lower dosage, eventually leading to the normalization of haemodynamics.18,28 The role in sepsis needs to be further explored.
Various studies have targeted mitochondrial dysfunction. Indomethacin, a cyclooxygenase inhibitor, impacts mitochondrial activity in rats, potentially contributing to aging, ischemic reperfusion injury, and mitophagy, but further in vivo studies are needed to fully understand its effects. Antioxidants such as vitamin C and hydrocortisone have been shown to slow sepsis complications, reduce mortality, and reduce organ failure, while melatonin regulates gastric microcirculatory oxygenation by reducing mitochondrial damage.1,7,20,29–31
Potential research targets in the management of sepsisPatients with vasopressor-dependent septic shock have demonstrated increased capillary density and enhanced microcirculatory perfusion following nitroglycerin-induced vasodilatation. Nitroglycerin is a potent stimulant of inducible nitric oxide synthetase (iNOS). NO is a potent endothelium-derived relaxing factor and a ubiquitous signaling molecule that acts through different signaling molecules at different concentrations. Processes mediated by cyclic guanosine monophosphate (cGMP) are activated by concentrations less than 30 nM; between 30 and 100 nM, the protein Akt is phosphorylated; between 100 and 300 nM, hypoxia-inducible factor-1α is stabilized; and above 400 nM, p53 phosphorylation is triggered.10,12,14,32 Few inconclusive studies have linked NO and its derivatives to re-injury by inhibiting complex I and complex IV of the ETC, thereby limiting mitochondrial respiration. However, inhaled NO has not demonstrated improvement in microcirculatory perfusion after macro-circulatory optimization in septic patients. Tetrahydrobiopterin, an endogenous nucleic acid derivative cofactor of iNOS, has been shown to be effective in restoring sublingual microcirculation in animal tests.14,20,29,33,34 More studies are needed to include NO donors in sepsis-guided treatment strategies.
A novel group of compounds called glycomimetics, in particular thiosugars (salaprinol, ponkoranol and kotalanol), has demonstrated restoration in NO production; binding and modulation of different glycan-binding proteins, galectins, and β-galactoside-binding lectins; and antioxidant activity in an in-vitro model of lipid-induced endothelial dysfunction. These compounds also cause glycosylation and inactivation of interalpha-trypsin inhibitor heavy chains (ITIHs) and fibronectin, both of which are associated with sepsis. These compounds show promise for improving the colony formation, proliferation, migration, differentiation, apoptosis, and angiogenesis of EPCs and promoting vascular repair.35,36
Mitoquinone (ubiquinol conjugated to the lipophilic cation triphenylphosphonium) is a mitochondrion-targeted, potent free radical scavenger with anti-inflammatory and antioxidative properties. It improves vascular endothelial function by reducing mitoROS levels and has been used extensively in chronic inflammatory conditions by increasing oxidative stress biomarkers. Administration of mitoquinone at 3.5 mg/kg/day has been demonstrated to prevent endotoxin-induced cardiac mitochondrial damage, caspase-3 and caspase-9 activation, and endotoxin-induced reductions in cardiac pressure-generating capacity. It also ameliorated an increase in the protein carbonyl level, an index of tissue free radical generation. However, its role in sepsis needs elaboration.30
Gram-negative bacteria are especially difficult to treat due to the impermeable cell envelope and a large number of efflux pumps. The LPS molecules present on the outer leaflet of the bacterial cell membrane are directly inhibited by antibiotics such as polymyxin B and colistin. However, this class of drugs is not the first line of antibiotics in sepsis as the risk of multidrug resistant strains is higher. Besides, LPS-null strains of Neisseria meningitidis, Moraxella catarrhalis and A. baumannii have all been shown to survive in vivo. LPS is an attractive target for developing target-focused approaches. Biosynthesis of lipid A is via the Raetz pathway, which has nine catalytic enzymes in the following order: LpxA, LpxC, LpxD, LpxH, LpxB, LpxK, KdtA, LpxL and LpxM. Inhibition of these increases cell permeability and potentiate the activities of azithromycin, vancomycin, and rifampin. Various pharmaceutical companies are targeting these enzymes. LpxA inhibitors (Novartis, X-Biotix) and LpxC inhibitors (Merck) need further improvements in activity and pharmacokinetics as clinical testing of these new drugs has had limited success so far.37
DAMPs are effectively removed by either autophagy i.e., destruction of whole cell, or mitophagy, i.e., destruction of damaged mitochondria. When autophagy is harmful, mitophagy can be targeted. Beclin-1, a well-established regulator of autophagy by the kinase mammalian target of rapamycin (mTOR) is a key regulatory point suppressing Beclin-1-dependent autophagic activity, stimulates mitophagy by selectively increasing the Pink1/Parkinthe pathway in response to LPS-challenged cardiomyocytes. The damaged mitochondria are then attached to the E3 ubiquitin ligase in autophagosomes for degradation. Cyclosporine A and Calcein-AM has been shown to inhibit mitochondrial permeability pore (mPTP) permeability in animal models of traumatic brain injury and sepsis by attenuating mPTP opening, stimulating mitochondrial biogenesis, and reducing mitoROS production. Nevertheless, the possibility of significant adverse effects, including kidney damage and hyperlacticaemia, prevents their widespread use. 14,19,22,30
Mitochondria transplantation, derived from the endosymbiotic origin of mitochondria, has shown efficacy in treating dysfunctional mitochondrial disorders, restoring organ function, and increasing cellular energy and contractility in ischemic cardiac tissue.22,38
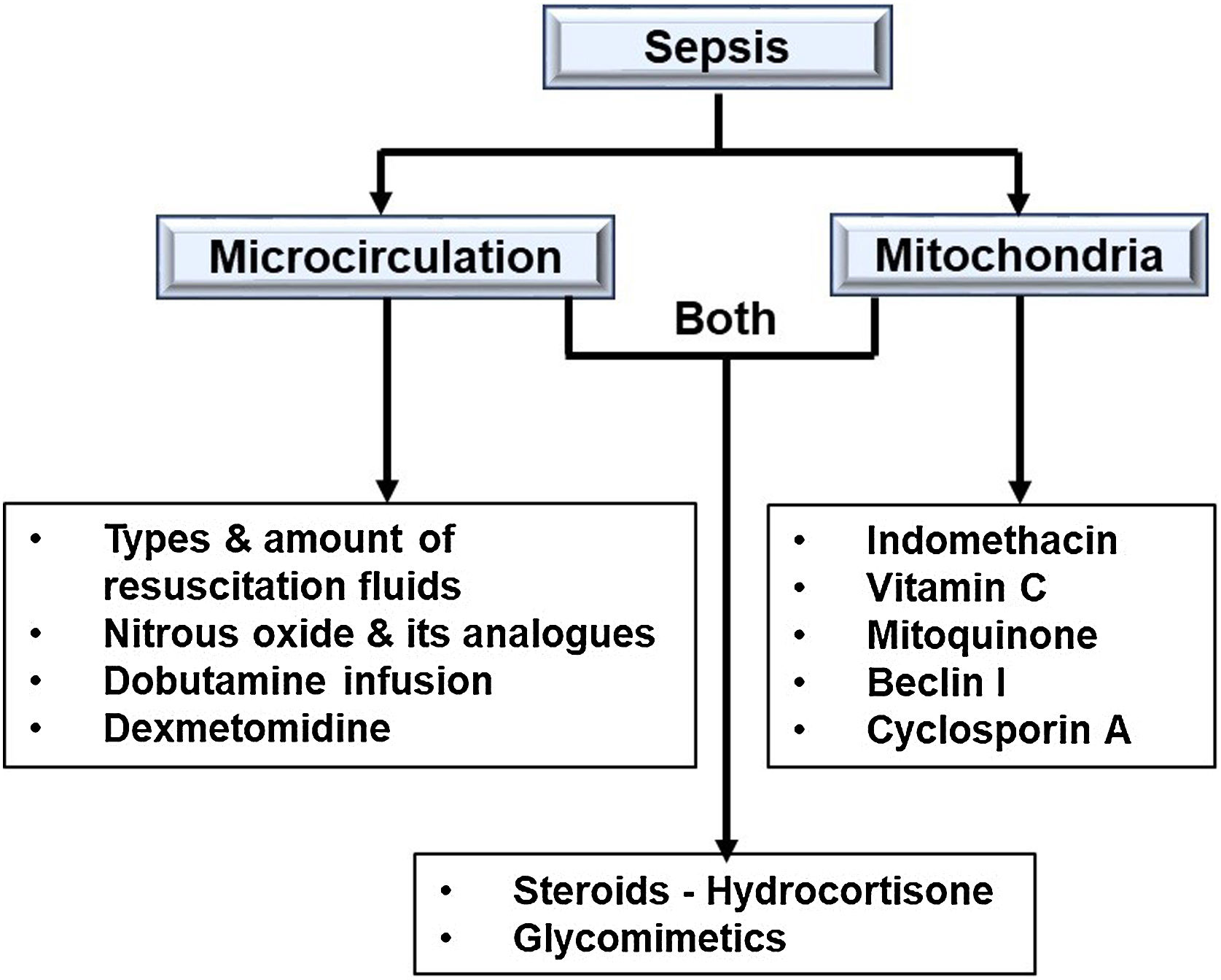
ConclusionsSepsis is now considered a microcirculatory and mitochondrial disease caused by hypoperfusion and hypoxia at the microvascular level. Emphasis is now focused on improving the status of the microcirculation and mitochondria in sepsis (Fig. 5). Sepsis-related mortality and morbidity may decrease in the future with coming advancements in endothelial- or mitochondrial-level therapy. To improve patient outcomes and provide more clarity, more research is necessary.
CRediT authorship contribution statementConcepts: Rashmi Datta; Design: Rashmi Datta; Definition of intellectual content: Rashmi Datta; Literature search: Shalendra Singh; Clinical studies:Rashmi Datta; Data analysis: Rashmi Datta; Manuscript preparation: Shalendra Singh; Manuscript editing: Shalendra Singh; Manuscript review: Shalendra Singh; Guarantor: Rashmi Datta.
Source(s) of support: NIL.
CTRI registration and IEC clearance: Not applicable for review article.
Presentation at a meeting: NIL.
Images in the article: All images and tables included in this work are owned by the author, who retains the rights to publish them, and no generative AI has been used in the creation of the text or images.
NIL.