The objective of the study was to compare landmark-based and ultrasound-guided techniques of central venous catheter insertion (CVC).
DesignRandomized controlled trial (2021–2023).
SettingZagazig University Hospitals (ZUH), a tertiary care center.
PatientsAdult patients in whom CVC insertion is indicated.
Main variables of interestDemographic and clinical peri-procedural data, the safety of the technique, time of performance, and cost-effectiveness were compared.
ResultsPatient ages ranged from 17 to 80 years with 56% being males. Urgent indications were found in around 22% without significant differences between groups. Regarding the time of performance, the ultrasound-guided method had slightly but significantly less time of performance (25.7 ± 4.3; range: 18−33) compared to the blind technique (26.9 ± 7.4; range: 15−45) (P-value < 0.001) with a higher but non-significant number of patients without complications (64% vs 52%; P-value = 0.2). Failure to insert the CVC into the IJV occurred in 12 patients (12%) with the blind technique and in eight patients (8%) with the ultrasound-guided technique (P-value = 0.04). Carotid artery puncture with neck hematoma occurred in only 8 (8%) patients with the blind technique (P-value = 0.04). Excess cost was consumed in only 36 patients (36%) in the blind technique group (P-value = 0.001).
ConclusionPoint-of-care ultrasonography bundle for CVC insertion is considered superior to, safer, and more cost-effective than the blind technique.
El objetivo del estudio fue comparar las técnicas de inserción de catéteres venosos centrales (CVC) guiadas por ecografía y basadas en puntos de referencia.
DiseñoEnsayo controlado aleatorizado.
ÁmbitoHospitales Universitarios de Zagazig (ZUH), un centro de atención terciaria.
PacientesPacientes adultos a los que se les indica la inserción de un CVC.
Principales variables de interésComparamos ambos grupos en cuanto a datos demográficos, datos clínicos periprocedimiento, seguridad de la técnica, tiempo de realización y relación coste-efectividad.
ResultadosLa edad de los pacientes osciló entre 17 y 80 años, y el 56,0 % eran varones. Se encontraron indicaciones urgentes en alrededor del 22 % sin diferencias significativas entre los grupos. En cuanto al tiempo de realización, el método guiado por ecografía tuvo un tiempo de realización ligeramente pero significativamente menor (25,7 ± 4,3; rango: 18−33) en comparación con la técnica a ciegas (26,9 ± 7,4; rango: 15−45) (valor P < 0,001) con un número mayor pero no significativo de pacientes sin complicaciones (64% vs 52%; valor P = 0,2). El fracaso en la inserción del CVC en la vena yugular interna ocurrió en 12 pacientes (12,0%) con técnica a ciegas y ocho pacientes (8,0%) con técnica guiada por ecografía (valor P = 0,04). La punción de la arteria carótida con hematoma en el cuello ocurrió en solo 8 (8,0%) pacientes con técnica a ciegas (valor P = 0,04). El sobrecosto se consumió en solo 36 pacientes (36,0%) con técnica a ciegas (valor P = 0,001).
ConclusiónEl paquete de ecografía en el punto de atención para la inserción de CVC se considera superior, más seguro y más rentable que la técnica a ciegas.
Placement of a central venous catheter (CVC) is considered a routine procedure in intensive care medicine and anesthesiology.1 Unfortunately, it can lead to mechanical, infectious, and thrombotic complications, with a total complication rate of around 5%.2 Iatrogenic pneumothorax, carotid artery puncture, and CVC malposition are the most common mechanical complications.3 As these complications are potentially of great detriment to the patient’s health, the current guidelines require that a chest X-ray be routinely performed after CVC placement. This is, however, cost-ineffective, time-consuming, and exposes the patient to additional ionizing radiation.4
Traditionally, CVC placement is performed using anatomical landmark techniques based on the knowledge of known anatomic structures and palpation of arteries next to the veins, but this method cannot be helpful in all cases.5 Another possible problem that may increase the difficulty of CVC insertion is venous thrombosis which should be considered especially in oncologic and long-standing critically ill patients making the whole process impossible or risky for the patient.6
Using ultrasound as a guidance tool during CVC placement has repeatedly been shown to improve procedural safety.1,7 In developing low-income countries, this intervention is frequently performed by physicians in training and without the availability of ultrasound guidance.7,8 While ultrasound (US) use for internal jugular CVC placement is standard of care in North America, most developing countries have not adopted this practice. Previous surveys of North American physicians have identified lack of training and equipment availability as the most important barriers to the use of the US.9 To prevent these possible life-threatening iatrogenic complications, an ultrasound-guided CVC insertion bundle has been employed in a stepwise manner. Ultrasound guidance is nowadays considered the best practice to prevent possible complications according to recent guidelines including the American Society of Anesthesiologists (ASA),10,11 and the Centers for Disease Control and Prevention (CDC).12,13 Although several studies attempted to evaluate the US-guided CVC insertion,11,13–17 the available evidence from our country and region is scarce. Therefore, this study aimed to compare the landmark technique and the ultrasound-guided technique regarding patient safety, time effectiveness, and cost. It is noteworthy that we implemented a comprehensive and stepwise approach. Furthermore, by distributing this study's methods and results, we aim to raise awareness among the community about the importance of using the US in guidance of CVC insertion provided the available equipment.
Patients and methodsStudy population and settingsA total of 200 patients who were admitted to the emergency hospital at the Zagazig University Hospital (ZUH) from October 2021 to April 2022 were included in a prospective comparative randomized clinical study. Patients aged between 18 and 70 years of both genders who were admitted to emergency hospital units including the emergency room (ER), operation theater (OT), or emergency intensive care unit (ICU) and indicated for both elective and emergent CVC insertion were enrolled in our study. Emergency indications were defined as emergency hemodialysis, transvenous pacing, difficult peripheral intravenous (IV) line, and hypovolemic shock. On the other hand, patient refusal, patients <18 years and >70 years, platelet count < 500000/cc, INR > 1.5, PT > 15 s, PTT > 40 s (not necessarily in emergencies), thrombosis of the internal jugular vein (IJV), recent surgery, trauma or skin infection at the target insertion site, and cervical spine collar were all absolute contraindications to CVC placement into IJV and hence were excluded from the study. Randomization was done using a computer-generated randomization table in which participants were randomized in a parallel design, to either Group A or Group B in a 1:1 ratio and allocated the patients into two equal groups.
Study procedures and interventionsPatients were divided into two groups according to the technique of CVC insertion with 100 patients in each, Group A receiving the blind technique for CVC insertion, and Group B receiving the ultrasound-guided technique for CVC insertion. Equipment used included an Amecath triple lumen CVC kit size 7 French × 20 cm and the ultrasound machine GE Vivid E95.
Pre-procedural assessmentPatients were assessed regarding the site of planned insertion, explanation to the patient if conscious and cooperative, and checking platelet count and coagulation profile in elective cases. Routine Regarding anticoagulant and antiplatelet therapy; routine CVC insertion procedures were considered adequate in patients on monotherapy with acetylsalicylic acid, non-steroidal anti-inflammatory drugs, or prophylactic anticoagulants (e.g., low-dose heparin, low-molecular-weight heparin, pentasackarides, thrombin inhibitors). However, in case these drugs were combined, in particular clopidogrel and acetylsalicylic acid, in addition to therapeutic anticoagulant and emergency cases; CVC insertion was performed by experienced operators using the safest possible techniques.18 Before CVC insertion, we started by insertion of a peripheral intravenous (IV) line, if possible; for the potential need in special situations when procedural sedation was necessary in semiconscious or uncooperative patients, rapid fluid administration or medication during the procedure; like antiarrhythmic medications and medications for cardiopulmonary resuscitation. Applying a full monitor (pulse oximeter, non-invasive blood pressure, and ECG) was initiated, and then the patient was positioned in a supine flat position with lower limbs slightly elevated up to 30°, a wedge was placed behind the upper back in between shoulders and the head was turned gently to the opposite side of insertion.19,20 Xie et al. and Gok et al. concluded that leg elevation for a few minutes before insertion simulates the passive leg raising test, pooling blood from lower limbs to central circulation; thus, making neck veins more engorged, dilated, and more easily cannulated making first trial more successful.19,20 Once the site was chosen, a topical antiseptic such as chlorhexidine, in case of chlorhexidine allergy we used povidone-iodine instead, was applied circularly to the skin in ever-enlarging circles under complete aseptic conditions.
CVC insertionAll the CVC placements were performed by specifically trained 3rd-year intensive care residents under the supervision of a specific attending physician in the same room with the same ultrasound device. In group A, CVC was inserted using the blind standard technique. In group B, the ultrasound machine was employed using the linear probe, the probe was covered by a sterile cover, the ultrasound probe was placed perpendicularly making an angle that should be close to 90° with the skin, and then localization of IJV was done. The IJV was assessed for collapsibility along its whole length to ensure patency, to choose the site of the widest diameter, shallowest depth, and farthest site from the carotid artery (better not overlying) that appears non-collapsible and pulsatile, then adjusted to be the center of the ultrasound monitor view. Afterward, a local anesthetic was injected into the subcutaneous tissue at the site of planned insertion, and then the needle was carefully introduced through the skin and carefully advanced into the IJV with real-time ultrasound guidance, The needle was introduced in continuous aspiration at an angle of approximately 60° in relation to the skin in out-of-plane technique. The angle between the needle and the skin should be 30−45º in the in-plane technique.
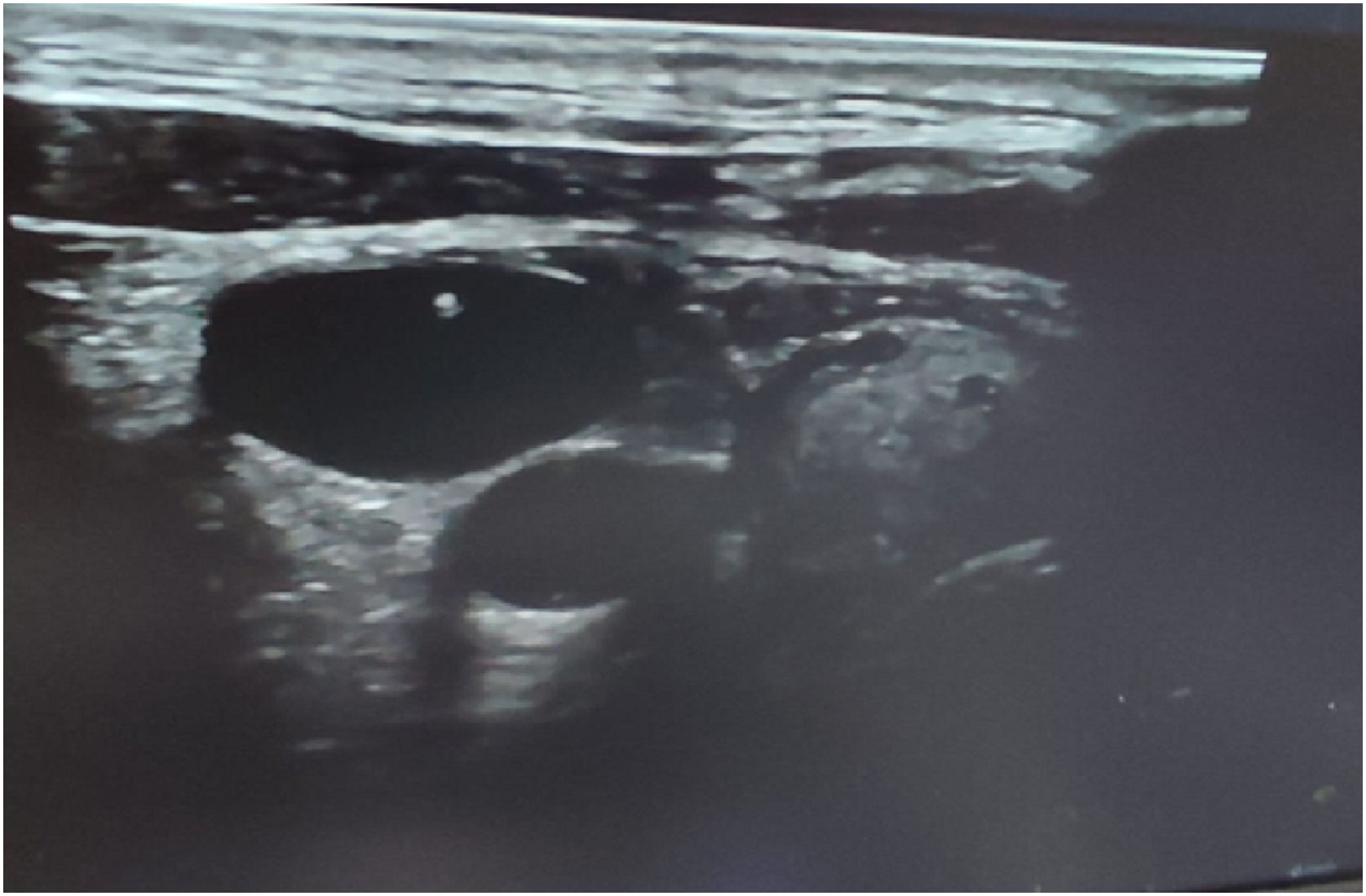
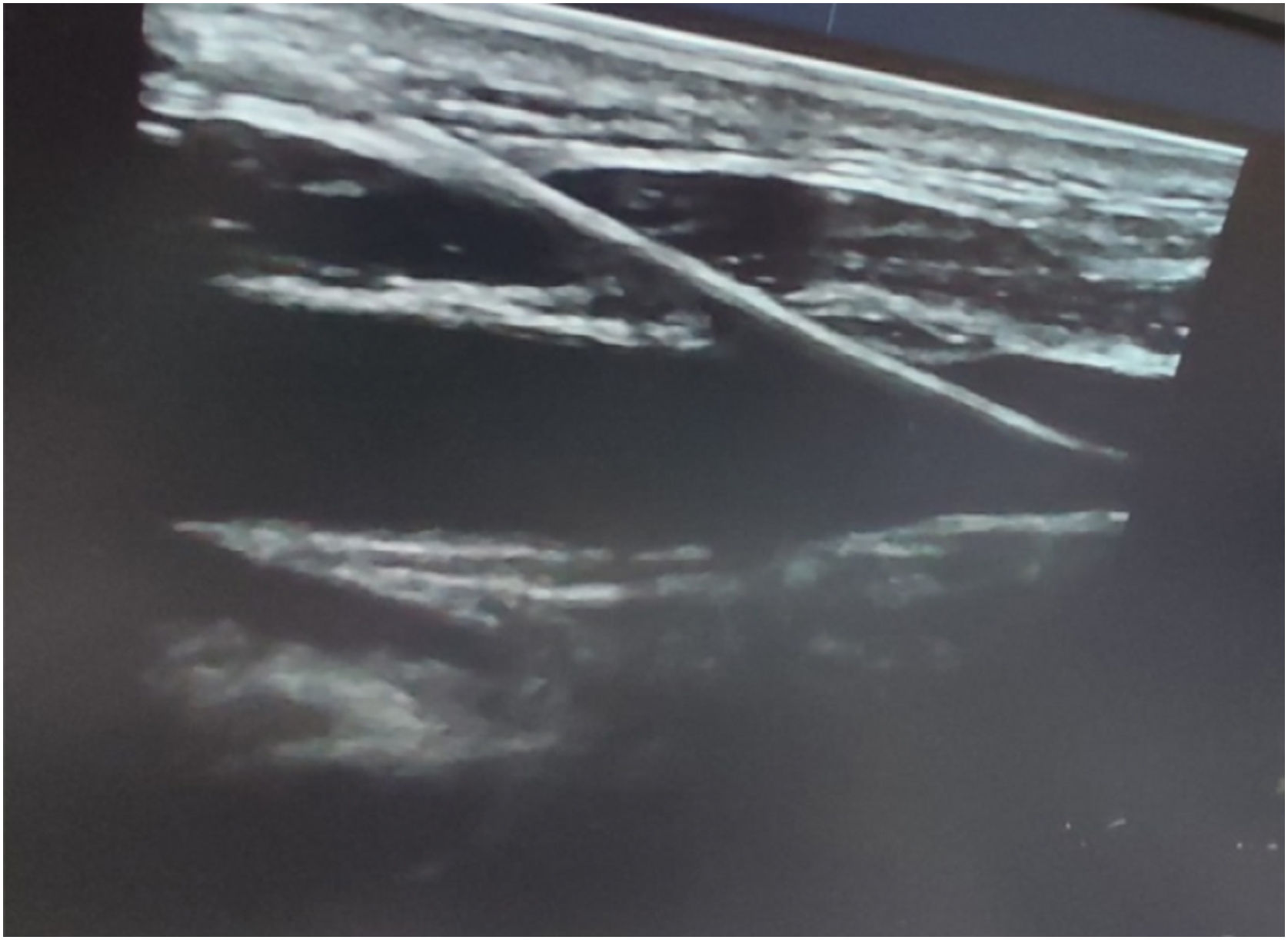
Different US approaches were used; short-axis probe orientation and an out-of-plane view of the needle and a long-axis probe orientation and an in-plane view of the needle. In the case of short-axis/out-of-plane view, the needle appeared on the screen as a point (Fig. 1) while it appeared as an echogenic line in the case of long-axis/in-plane view. When the needle tip was seen inside the IJV, then aspiration of dark venous blood was done to confirm the needle tip position, and the syringe was removed. A guide wire was inserted through the needle into the vein, to a depth of at least 15 cm and it was visualized after introducing inside the IJV as an echogenic dot if the probe was placed out of plane or as an echogenic thread if the probe was placed in plane (Fig. 2), and finally the catheter was visualized inside the IJV.15
Finally, it is important to point out that we planned in case of failed insertion of CVC into IJV (maximum three attempts in case of causes other than hypovolemic shock where only one attempt was allowed) due to very high collapsibility of IJV making its cannulation hard and difficult guidewire advancement, immediate shift to ipsilateral subclavian or femoral vein was done without further trials in IJV to avoid negative consequences of multiple trials like neck hematoma, carotid artery puncture, and pneumothorax.
Post-procedural assessmentImmediately after CVC placement, a saline flush consisting of 5 mL of normal saline was injected into the distal hub of the CVC by the interventionist while focused ultrasonography on the right atrium using the echo probe was conducted by a second resident by visualization of the heart using subcostal 4 chamber view. The appearance of opacification of the right atrium known as Rapid Atrial Swirl Sign (RASS) or Bubble Sign was judged as “immediate” (less than two seconds after injection), “delayed” (appearing more than two seconds after injection), or “absent”. Ultrasonography was performed by the resident placing the catheter and the result was recorded by another investigator. Overall, a positive RASS (negative screening test for misplacement) translated into a correctly positioned catheter, whereas a delayed or absent flush (negative RASS or positive screening test) implied a potentially misplaced catheter requiring further evaluation by chest x-ray.21
After finishing CVC insertion, chest ultrasonography was done by the intensivist to exclude the presence of pneumothorax through the presence of a lung sliding sign at the lung apex or hemothorax through assessment of the basal lung lobe in adherence to the diaphragm. This step is performed with the patient in the supine position and with a curvilinear probe. The probe was placed in a sagittal position on the anterior surface of the thorax, close to the second intercostal space, in the midclavicular line. Sonographic references include the presence of two ribs with their respective posterior acoustic shadow and the pleural line between them.16
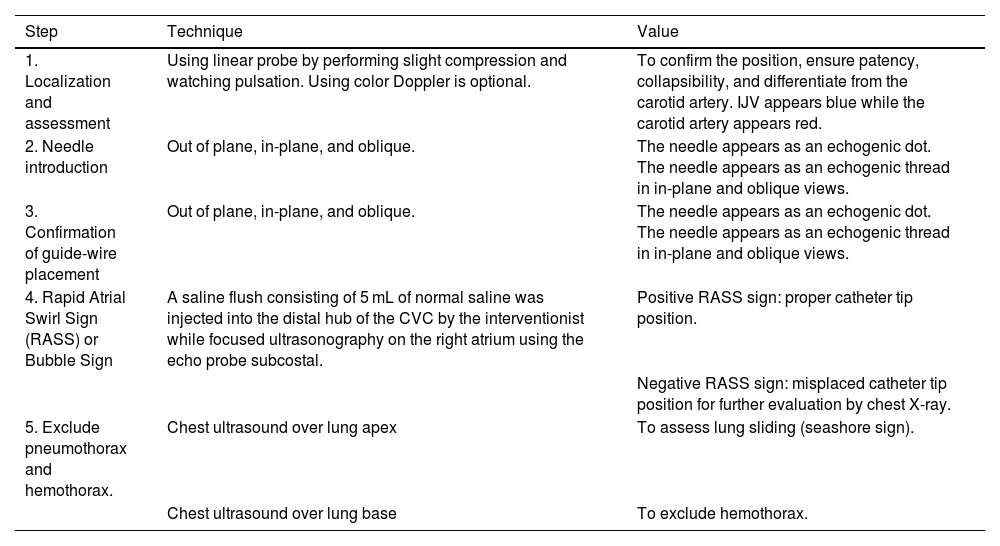
All of the previous steps that were carried out on patients in group B in the previously mentioned sequence could be considered as the point-of-care ultrasound (POCUS) guided CVC insertion bundle; that included CVC insertion with real-time ultrasound guidance followed by confirmation of placement by application of RASS screening test and finally screening for early postprocedural complications like pneumothorax by chest ultrasound (Table 1).
Ultrasound-guided central venous catheter insertion bundle.
| Step | Technique | Value |
|---|---|---|
| 1. Localization and assessment | Using linear probe by performing slight compression and watching pulsation. Using color Doppler is optional. | To confirm the position, ensure patency, collapsibility, and differentiate from the carotid artery. IJV appears blue while the carotid artery appears red. |
| 2. Needle introduction | Out of plane, in-plane, and oblique. | The needle appears as an echogenic dot. The needle appears as an echogenic thread in in-plane and oblique views. |
| 3. Confirmation of guide-wire placement | Out of plane, in-plane, and oblique. | The needle appears as an echogenic dot. The needle appears as an echogenic thread in in-plane and oblique views. |
| 4. Rapid Atrial Swirl Sign (RASS) or Bubble Sign | A saline flush consisting of 5 mL of normal saline was injected into the distal hub of the CVC by the interventionist while focused ultrasonography on the right atrium using the echo probe subcostal. | Positive RASS sign: proper catheter tip position. |
| Negative RASS sign: misplaced catheter tip position for further evaluation by chest X-ray. | ||
| 5. Exclude pneumothorax and hemothorax. | Chest ultrasound over lung apex | To assess lung sliding (seashore sign). |
| Chest ultrasound over lung base | To exclude hemothorax. |
The two studied groups were compared regarding patient characteristics (age, sex, and BMI), causes of emergency hospital admission, and indications for CVC insertion. Relevant clinical and laboratory data including the use of anticoagulant therapy, INR values, and platelets counts were collected in addition to systolic, and diastolic blood pressure and heart rate during insertion. Time of performance was estimated for each patient which was defined as time from skin draping to the end of fixing CVC by sutures. Post-insertion, the proper position of CVC was assessed using a chest x-ray. The incidence of complications was estimated among the two studied groups. Manifestations suggesting infection were evaluated for including fever and chills most commonly. Still, they may be masked if the patient is immunocompromised, where atypical presentations of sepsis occur including altered mental status, hypotension, lethargy, and fatigue. The exit site examination was also done to search for signs of inflammation with inspection and palpation of the subcutaneous track. Patients may report pain, swelling, or discharge from the exit site and redness surrounding or along the subcutaneous track when exit site or tunnel infections are present. If previous manifestations developed within less than seven days of insertion, it suggested early extraluminal CVC infection while it was considered late intraluminal infection if occurring after more than seven days.22 The cost of the entire procedure was calculated. The excess cost was defined as the need for more than one CVC set (price about 11 dollars), in addition to the cost of managing associated complications like intercostal chest tube insertion in case of pneumothorax, carotid artery doppler in case of accidental carotid artery puncture or antibiotics needed to treat CVC infection (average > 20 dollars is considered excess cost)21 and finally we considered the cost of post-insertion chest x-ray that was performed for all cases (price 2.8 dollars).
Ethical considerationsWritten informed consent was obtained from all patients or their first-degree relatives and the study was approved by the research ethical committee of the Faculty of Medicine, Zagazig University (IRB#6827/30-3-2021) and registered on clinicaltrials.gov (NCT05338138). The work has been performed uniformly with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans.
Statistical analysisAll data were entered in Excel and then imported and analyzed using the Statistical Package for Social Sciences (SPSS), version 27 (IBM, Chicago, Illinois, USA). Categorical data were presented as frequencies and percentages. The chi-square test or Fisher’s exact test was used to compare the proportion between the groups. The Kolmogorov-Smirnov test was used initially to determine the normality of continuous data. Continuous data with normal distribution were described as mean ± standard deviation (SD) and compared with the independent t-test. A P-value of <0.05 indicates significant results.
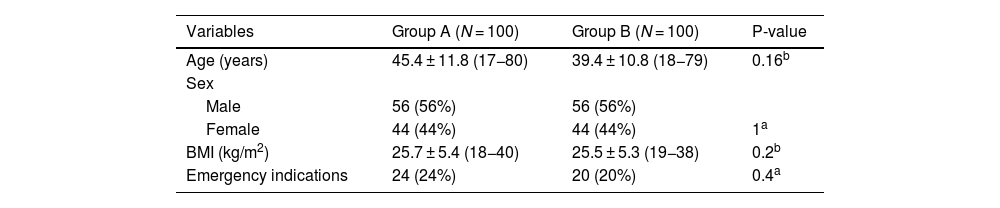
ResultsPatients’ characteristics among the two studied groupsThis controlled clinical trial included 200 patients, whose ages ranged from 17 to 80 years with a BMI ranging from 18 to 40 kg/m2, and 56% were males. Urgent indications were found in around 22% (24% in group A and 20% in group B). No statistically significant difference was observed between the two studied groups regarding age, sex, BMI, and emergency indications (Table 2). There was no statistically significant difference between the two studied groups concerning causes of emergency hospital admission. Obstetric causes (16%) followed by respiratory failure secondary to pneumonia (12%) and traumatic brain injury (12%) were the most common causes of emergency hospital admission among group A, while traumatic brain injury (20%) followed by obstetric causes (20%) then respiratory failure secondary to pneumonia (18%) were the most common causes of emergency hospital admission among group B as shown in Fig. S1 (Supplementary material).
Patients’ characteristics and emergency indications of CVC insertion among the two studied groups.
| Variables | Group A (N = 100) | Group B (N = 100) | P-value |
|---|---|---|---|
| Age (years) | 45.4 ± 11.8 (17−80) | 39.4 ± 10.8 (18−79) | 0.16b |
| Sex | |||
| Male | 56 (56%) | 56 (56%) | |
| Female | 44 (44%) | 44 (44%) | 1a |
| BMI (kg/m2) | 25.7 ± 5.4 (18−40) | 25.5 ± 5.3 (19−38) | 0.2b |
| Emergency indications | 24 (24%) | 20 (20%) | 0.4a |
Data were expressed as Mean ± SD (range) or number (%).
Group A: the standard blind technique for CVC insertion into the internal jugular vein.
Group B: ultrasound-guided technique for CVC insertion into the internal jugular vein.
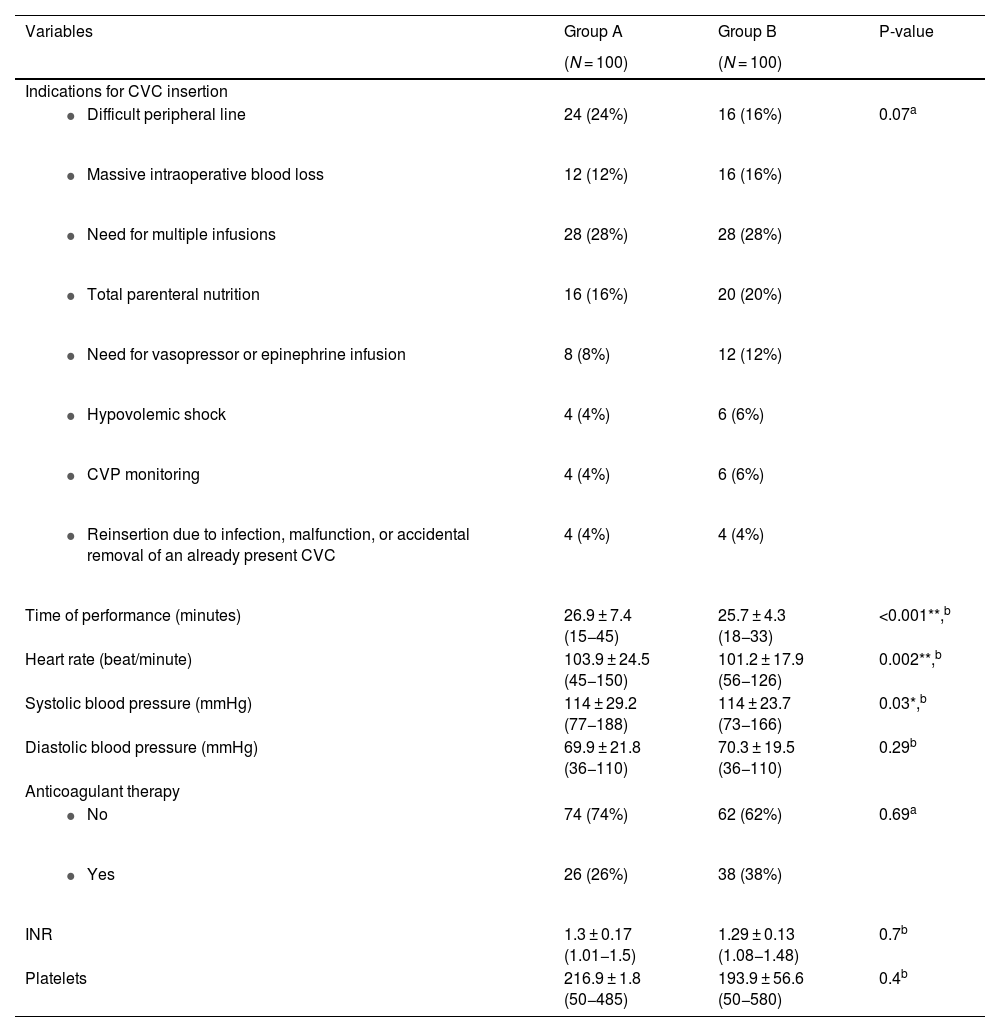
Regarding indications for CVC insertion, there was no statistically significant difference among the two studied groups (p-value = 0.07). In group A, the need for multiple infusions (28%) followed by a difficult peripheral intravenous line (24%), then the need for total parenteral nutrition (TPN) (16%) were the common indications while in group B, they included the need for multiple infusions (28%) and total parenteral nutrition (20%) then difficult peripheral intravenous line (16%). There were no significant differences in the number of patients receiving anticoagulant therapy, INR values, or platelet counts (P-value = 0.69, 0.7, and 0.4 respectively). Regarding the time of performance, group B had slightly but significantly less time of performance (25.7 ± 4.3; range: 18−33) compared to group A (26.9 ± 7.4; range: 15−45) (P-value < 0.001). Similarly, the mean heart rate was significantly lower in group B (101.2 ± 17.9; range: 56−126) than in group A (103.9 ± 24.5; range: 45−150) (P-value = 0.002). The details of clinical and peri-procedural data are shown in Table 3.
Clinical and peri-procedural data among the two studied groups.
| Variables | Group A | Group B | P-value |
|---|---|---|---|
| (N = 100) | (N = 100) | ||
| Indications for CVC insertion | |||
| 24 (24%) | 16 (16%) | 0.07a |
| 12 (12%) | 16 (16%) | |
| 28 (28%) | 28 (28%) | |
| 16 (16%) | 20 (20%) | |
| 8 (8%) | 12 (12%) | |
| 4 (4%) | 6 (6%) | |
| 4 (4%) | 6 (6%) | |
| 4 (4%) | 4 (4%) | |
| Time of performance (minutes) | 26.9 ± 7.4 (15−45) | 25.7 ± 4.3 (18−33) | <0.001**,b |
| Heart rate (beat/minute) | 103.9 ± 24.5 (45−150) | 101.2 ± 17.9 (56−126) | 0.002**,b |
| Systolic blood pressure (mmHg) | 114 ± 29.2 (77−188) | 114 ± 23.7 (73−166) | 0.03*,b |
| Diastolic blood pressure (mmHg) | 69.9 ± 21.8 (36−110) | 70.3 ± 19.5 (36−110) | 0.29b |
| Anticoagulant therapy | |||
| 74 (74%) | 62 (62%) | 0.69a |
| 26 (26%) | 38 (38%) | |
| INR | 1.3 ± 0.17 (1.01−1.5) | 1.29 ± 0.13 (1.08−1.48) | 0.7b |
| Platelets | 216.9 ± 1.8 (50−485) | 193.9 ± 56.6 (50−580) | 0.4b |
Data were expressed as Mean ± SD (range) or number (%).
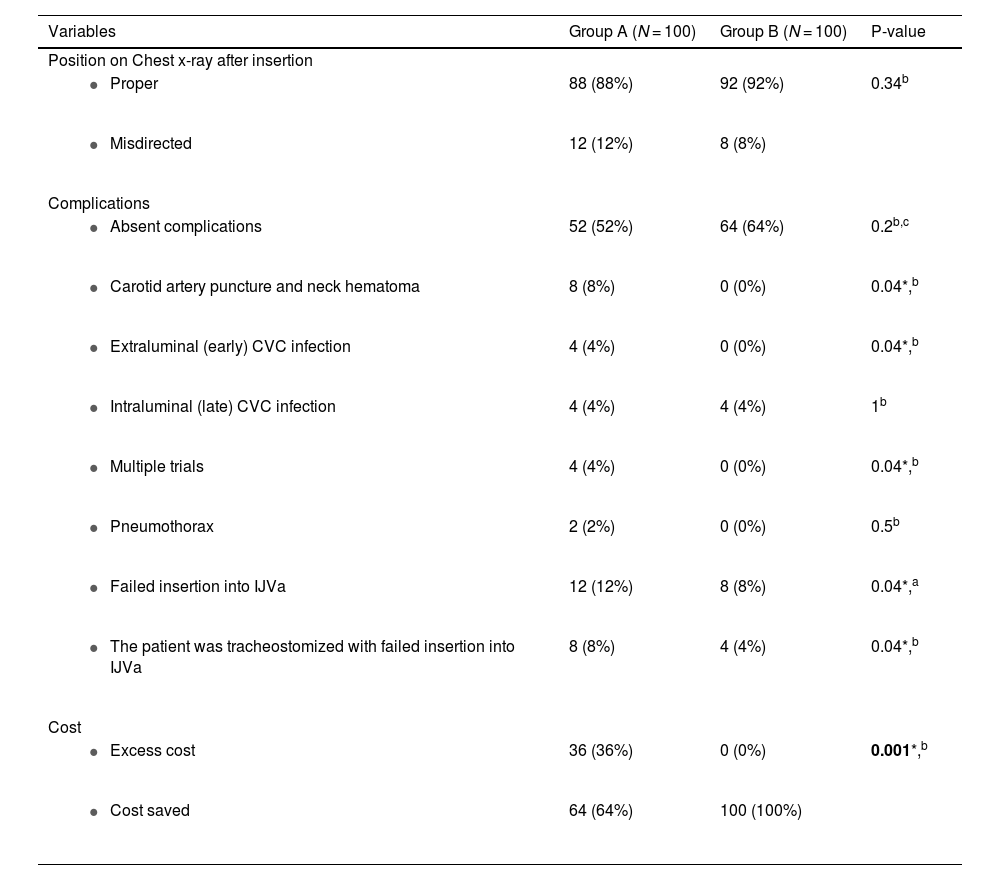
The proper position was confirmed using chest X-rays in both groups without significant differences (88% vs 92%; P-value = 0.34). Group B had a higher but non-significant number of patients without any complications (64% vs 52%; P-value = 0.2). However, concerning complications, carotid artery puncture with neck hematoma occurred in 8 (8%) patients in group A compared to none in group B (P-value = 0.04). While both groups had the same rate of late CVC infection, only four patients (4%) in group A experienced early CVC infection compared to none in group B (P-value = 0.04). The most common complication was failure to insert the CVC into IJV which occurred in 12 patients (12%) in group A and eight patients (8%) in group B (P-value = 0.04). Notably, in such cases, conversion into an ipsilateral subclavian vein was performed for access as a backup plan. Regarding tracheostomized patients with failed insertion into IJV. Conversion into the ipsilateral subclavian vein was also done in eight patients (8%) in group A and four patients (8%) in group B. Regarding cost, the excess cost was consumed in 36 patients (36%) in group A while all group B had usual costs (P-value = 0.001) (Table 4).
Post-procedural assessment data among the two studied groups.
| Variables | Group A (N = 100) | Group B (N = 100) | P-value |
|---|---|---|---|
| Position on Chest x-ray after insertion | |||
| 88 (88%) | 92 (92%) | 0.34b |
| 12 (12%) | 8 (8%) | |
| Complications | |||
| 52 (52%) | 64 (64%) | 0.2b,c |
| 8 (8%) | 0 (0%) | 0.04*,b |
| 4 (4%) | 0 (0%) | 0.04*,b |
| 4 (4%) | 4 (4%) | 1b |
| 4 (4%) | 0 (0%) | 0.04*,b |
| 2 (2%) | 0 (0%) | 0.5b |
| 12 (12%) | 8 (8%) | 0.04*,a |
| 8 (8%) | 4 (4%) | 0.04*,b |
| Cost | |||
| 36 (36%) | 0 (0%) | 0.001*,b |
| 64 (64%) | 100 (100%) |
Ultrasound-guided technique for CVC insertion into the IJV is considered superior to the standard technique. Nowadays, all over the world, the ultrasound-guided technique has been increasingly standardized,11 but in most low-income countries still the majority of central lines inserted by the standard technique that carry a lot of potential hazards that may constitute major health issues either immediately or in the future even with the best hands.8,9 Herein, we found that the application of our bundle has reduced the incidence of complications, improved outcomes, and raised awareness about the importance and benefits of the employment of ultrasound-guided technique for CVC insertion.
Several studies have been done to ensure the benefits of ultrasound-guided technique for CVC insertion over the standard technique2,3,7,11,13–15,17; but what is considered novel in our study is the comprehensive bundle that we applied in a stepwise approach that can prevent and detect any abnormality or hazard before (like IJV thrombosis), during (like carotid artery puncture) and even after the whole procedure (like pneumothorax). Notably, patients’ characteristics, emergent indications, and causes of emergency hospital admission did not differ significantly between the two studied groups. The heart rate and blood pressure values were higher in the blind-technique group which may be attributed to the higher number of attempts and longer time of performance that may represent extra stress on patients.
The US can easily predict the collapsibility of IJV better than blind maneuver, making the first puncture success higher in the US-guided method and avoiding multiple trials and the time consumed and consequences of such. Notably, the maximal number of attempts of IJV cannulation was restricted in this study to one attempt in case of hypovolemic shock and three attempts in other causes to reduce the likelihood of accidental injuries or complications such as neck hematoma and pneumothorax. Previous studies showed results favoring the use of the US.1,14,17,23 One of these similar studies that was performed by Sazdovet et al found that real-time ultrasound guidance improves success, decreases the number of attempts, decreases average time to the return of blood, and reduces mechanical complications rate.20 Similarly, Lennon et al found that ultrasound may reduce the incidence of procedural-related complications.18 In the present study, although there is no difference in overall complication-free patients, US guidance reduces the rate of carotid puncture, multiple trials, and failure of insertion into IJV. Only two patients had pneumothorax in this study, in the conventional method group without significant difference from the US-guided group. Namely, accidental carotid artery puncture and pneumothorax did not occur with the US-guided method and possibly indicated improved first puncture success.
Consequently, early CVC infection was higher with the standard method which is possibly related to higher risk catheter tip malposition and repeated trials 24. Concerning other complications like late intraluminal infection there was no statistically significant difference between the two methods. This result was in concordance with the result of Imataki et al. who studied the Effect of ultrasound-guided central venous catheter insertion on the incidence of catheter-related bloodstream infections (CRBSI) and mechanical complications and found that US-guided CVC insertion did not decrease the incidence of CRBSI.22
In the present study, the conventional method had a longer time of performance and excess cost than the ultrasound-guided technique. This may be attributed to extra time consumed in IJV localization by anatomical landmark technique, and in some cases, IJV cannulation failed or arterial puncture in the carotid artery occurred requiring compression for a few minutes before the next attempt, where the time needed for IJV localization and cannulation was less in patients undergoing ultrasound-guided insertion where carotid artery puncture never occurred. This is in agreement with other studies10,18 which detected that some healthcare providers prefer the use of POCUS to guide vascular access in emergency and internal medicine as it is easy to use and provides real-time guidance for central line insertion, its clinical effectiveness, safety, and cost-effectiveness compared with other non-POCUS guidance techniques such as the traditional landmark methods remain to be elucidated. Choi et al. concluded that also in the case of peripherally inserted central catheter (PICC) procedure, US guidance was associated with shorter procedure time.11
The dependence on traditional landmarks in the blind maneuver introduces higher risks of injury, complications, and failure of cannulation and hence not only longer time consumption but also excess cost and resource use. While none of the US-guided patients caused excess cost, around a third of the conventional group had excess cost of the procedures. This excess cost can be due to the cost of antibiotics needed to treat early CRBSI caused by early CVC infection, in addition to extra CVC set and surgical drapes that were required in some cases after changing the site of the targeted central vein from IJV to subclavian vein in case of failed IJV cannulation.25 We also considered the cost of a carotid artery Doppler that was performed in patients complicated by carotid artery puncture. This result agreed with the result of Smit et al who studied the ultrasound-guided placement of CVC in a systematic review and found that the price of ultrasound machines is steadily decreasing; recently introduced and more affordable handheld scanners are available and have already been shown to be a feasible tool in most hospitals with cost saving benefit when compared to blind technique.3 Chest radiography was performed in our institution as part of the standard protocol at the time of the study, primarily to confirm catheter tip position, especially given the learning curve associated with POCUS at that time. This added extra cost over the non-performance of chest X-ray.
Lastly, we can think of POCUS’s advantages as being its quickness, lack of ionizing radiation, affordability, and ability to be used at the patient’s bedside without requiring them to go to the operating room or radiology department. The primary drawbacks are patient and operator dependence (i.e., insufficient ultrasound views and insufficient expertise).26
Strengthens and limitationsOur study is the first to evaluate the use of POCUS in CVC insertion in Egyptian hospitals. Furthermore, our study distinguishes in the comprehensive bundle that we applied in a stepwise approach that can prevent and detect any abnormality or hazard before (like IJV thrombosis), during (like carotid artery puncture), and even after the whole procedure (like pneumothorax). We also evaluated measures that were missing in other similar studies as heart rate and blood pressure monitoring during insertion, in addition to the cost-benefit of the study.
Our study faced certain limitations though. The resources utilized as a result of complications like prolonged ICU stay and for management of other complications, unfortunately, could not be assessed and were not included in the statistical analysis. In our study, we found that when CVC insertion was indicated due to hypovolemic shock; the IJV was highly collapsible even with very gentle light pressure with the ultrasound probe making the IJV cannulation and guidewire advancement a difficult and time-consuming process, so in these cases, we had to insert the CVC into the subclavian vein by blind landmark technique as it is known that subclavian vein less likely to collapse in response to hypovolemia compared to the IJV due to its proximity to the heart compared to IJV. Although this has the advantage of reducing the number of erroneous trials, this issue should be considered in real practice. This result was consistent with Killu et al who studied the internal jugular vein collapsibility index associated with hypovolemia in ICU patients.27 Regarding RASS, it is of great importance to mention that it may be delayed or absent in patients with long filling times like shock and heart failure, so alternative methods for confirming catheter placement should be considered in patients with compromised cardiac function.
Furthermore, another limitation of our study was that the pediatric patient population was excluded from our study as they needed a separate study to assess due to different body weights, neck circumference, the smaller size of veins, and the need for procedural sedation. The last limitation on clinical implication was that we neglected preprocedural cessation of antiplatelet therapy due to its long half-life, so we didn't have the luxury of time to stop for a suitable time before insertion and to assess results.
ConclusionThe present study showed that utilization of a comprehensive stepwise bundle of POCUS for placement of CVC during the whole insertion process is considered superior to and safer than the standard landmark technique as regards time and cost and should be considered a routine practice even during emergency scenarios.
Clinical trial registrationNCT05338138. Point of Care Ultrasonography Versus Standard Blind Technique for Central Venous Catheter Insertion in Emergency Hospital (https://clinicaltrials.gov/).
FundingNone.
Declaration of Generative AI and AI-assisted technologies in the writing processThe authors declare that no form of AI was used in this project or manuscript.
CRediT authorship contribution statementAhmed Beniamen: Conceptualization, Writing - original draft, Resources, Project administration, Methodology, Investigation. Ahmed Mosallem: Conceptualization, Writing - original draft, Resources, Project administration, Methodology, Investigation. Hossam Tharwat Ali: Writing - original draft, Writing - review & editing, Project administration, Conceptualization. Hanaa A. Nofal: Data curation, Formal analysis, Writing - review & editing. Essamedin M. Negm: Methodology, Supervision, Project administration.
The authors are grateful to all the medical and nursing staff in the ICU and emergency hospitals at Zagazig University Hospitals.