To evaluate the Speckle Tracking values in the descending thoracic aorta (DTA) of a healthy population.
DesingThis is an observational study.
SettingHospital Universitario de Jaén.
ParticipantsOur study included 242 healthy people (102 women) with a mean age of 42.35±16.37 years.
InterventionsEvaluation of Speckle Tracking in DTA.
Main variables of interestStrain, velocity and displacement values in the DTA were evaluated.
ResultsThe DAT rotational velocity was 55.91±0.22º/s, the DTA radial velocity was 0.98±0.28cm/s, the circumferential strain of the DTA was −8.98±0.87%, while the circumferential strain rate was −0.96±0.15/s. The rotational displacement of the DTA was determined at 8.87±0.36º, while radial displacement was 0.82±0.11mm. Logistic regression found an association between the rotational values of the aorta and the longitudinal strain of both ventricles. In thirty people, strain, rotational velocity and displacement were also evaluated in the apical 4-chamber views and in the parasternal long-axis view. Both projections showed similar values.
ConclusionSpeckle Tracking could be used to study the descending thoracic aorta, and its values could suggest or rule out the presence of cardiovascular pathology.
Evaluar los valores de speckle tracking en la aorta torácica descendente (ATD) en población sana.
DiseñoEstudio observacional.
ÁmbitoHospital Universitario de Jaén.
Participantes242 personas sanas (102 mujeres) con una edad media de 42,35±16,37 años.
IntervencionesEvaluación del Speckle Tracking en la ATD.
Variables de interésprincipalesSe ha evaluado el valor de la deformación, sus velocidades y su desplazamiento en la ATD.
ResultadosLa velocidad rotacional del ATD fue de 55,91±0,22º/s, la velocidad radial del ATD fue de 0,98±0,28cm/s, la deformación circunferencial de la ATD fue de −8,98±0,87%, mientras que la velocidad de deformación circunferencial fue de −0,96±0,15/s. El desplazamiento rotacional del ATD se determinó en 8,87±0, 36º, mientras que el desplazamiento radial fue de 0,82±0,11mm. La regresión logística encontró asociación entre los valores rotacionales de la aorta con el strain longitudinal de ambos ventrículos. En treinta personas, también se evaluaron la tensión, la velocidad de rotación y el desplazamiento en las proyecciones apicales de 4 cámaras y en la proyección paraesternal de eje largo. Ambas proyecciones mostraron valores similares.
ConclusionesEl Speckle Tracking podría utilizarse para estudiar la aorta torácica descendente, y sus valores podrían sugerir o descartar la presencia de patología cardiovascular.
Classically, cardiovascular function has been based on LVEF as well as (later) diastolic dysfunction.1 These two functions are now considered the essential basis of heart failure. However, very little is known about the atrial functions that are currently being studied extensively2–4 and even less is known about the pathophysiology of the aorta, which is still considered only a simple conducting vehicle, although recent reports may hint at further functions.3 The aorta is a fundamental organ in cardiovascular physiology, its elasticity, structure, anatomy or age change its elasticity and can modify cardiovascular physiology and may influence condition the clinical situation. Patients with atheromatous plaques, aortic artery diseases or advanced age, naturally have lower aortic distensibility and this could translate into a worsening of the symptoms.7–9 The aorta is distributed in three layers, i.e., the Intima, Media and Adventitia layers. The middle layer also contains muscle cells and therefore could potentially be an indicator of muscle activity.5 Despite the muscular tissue of the aorta being well studied, its pathophysiology is currently unknown. However, its behavior could modify the prognosis for inducing heart failure or the prognosis of certain shocks.6,7 Our current knowledge of the aorta is based on the study of pressures, in addition to anatomical models, while the use of artificial intelligenceis now also beginning to provide new novel tools and theories. The modes of evaluation of the aorta are more limited. Although progress has been made in its knowledge, further progress is needed, since it could have more functions than being a reservoir or with only modifications of the pulse wave.8,9 However, at present there is no method available to reliably evaluate the aorta, and to provide comparisons between healthy or diseased patients, except when evaluating its complications, dilatation, or the existence of atheromatous plaques.10
Diagnostic imaging techniques of the aortic artery such as tissue Doppler, time velocity integral, MRI or Speckle Tracking could provide physiological information that could be reflected in diagnostic and therapeutic applicability. Speckle Tracking is an avant-garde and highly efficient echocardiographic tool that has revolutionized all cardiological knowledge.3,11–14 It is much more sensitive than 2D, tissue doppler imaging and its applicability is fundamentally defined by the ability to perform "strain or strain rate" measurements. However, there are also offline software tools available -e.g., vector velocity analysis12 -which can perform measurements of longitudinal, radial or circumferential velocities and explore longitudinal, radial or rotational displacements. Despite its unparalleled potential and variability, this technology has not yet been used in the aorta, notably to evaluate the velocities, displacement, strain or strain rate of the aorta. In Intensive Care Medicine, distributive shock often occurs, affecting the entire vascular system, but we do not have tools that can assess aortic function. The possibility that Speckle Tracking in the thoracic aorta could be performed and normal patterns detected would provide another tool, in which the next step would be to correlate it with various pathologies or different hemodynamic situations and even help in patient resuscitation. Our objective was to evaluate the possibility of finding normal patterns in DTA, evaluated in a healthy population, the radial and rotational velocities, circumferential strain, strain rate, and radial or rotational displacements by vector velocity analysis echocardiography.
MethodsStudy designThis is a transversal, cross sectional study aimed at creating a reference library of Speckle Tracking values and variables in the descending thoracic aorta in the healthy population. Consequently, no intervention or patient follow-up was required nor foreseen. Only anthropometric measurements were performed to assess body surface area, heart rate, weight, height, and age.
This work was approved by the local ethics committee and funded by the Department of Health of the regional government of Andalusia, Spain. The data were stored in a de-identified database to ensure the blinding of the study.
Inclusion criteriaThe study was performed between January 2018 and February 2020 and from March 2022 to May 2024, with a suspension during the Covid pandemic (2020–2022). Healthy adult volunteers, who requested the echocardiography unit of the cardiological intensive care section (CCU) to perform an echocardiography, and whose echocardiography was without abnormality, were included. In addition, the study cohort also included additional volunteers who were invited to participate in the study. Participants were between 18 and 45 years old.
Exclusion criteriaPeople who met any of the following conditions were excluded from the study. (1) Previous cardiovascular, neurological, or renal disease. (2) Any cardiac pathology detected during echocardiography. (3) Inadequate echocardiographic study, such as poor sonographic window, or if the descending thoracic aorta was not well visible in the parasternal long-axis projection. (4) Lack of informed consent. (5) People who have presented an episode of arrhythmia or arterial hypertension. (6) Anyone with any known disease such as endocrinopathies or diabetes.
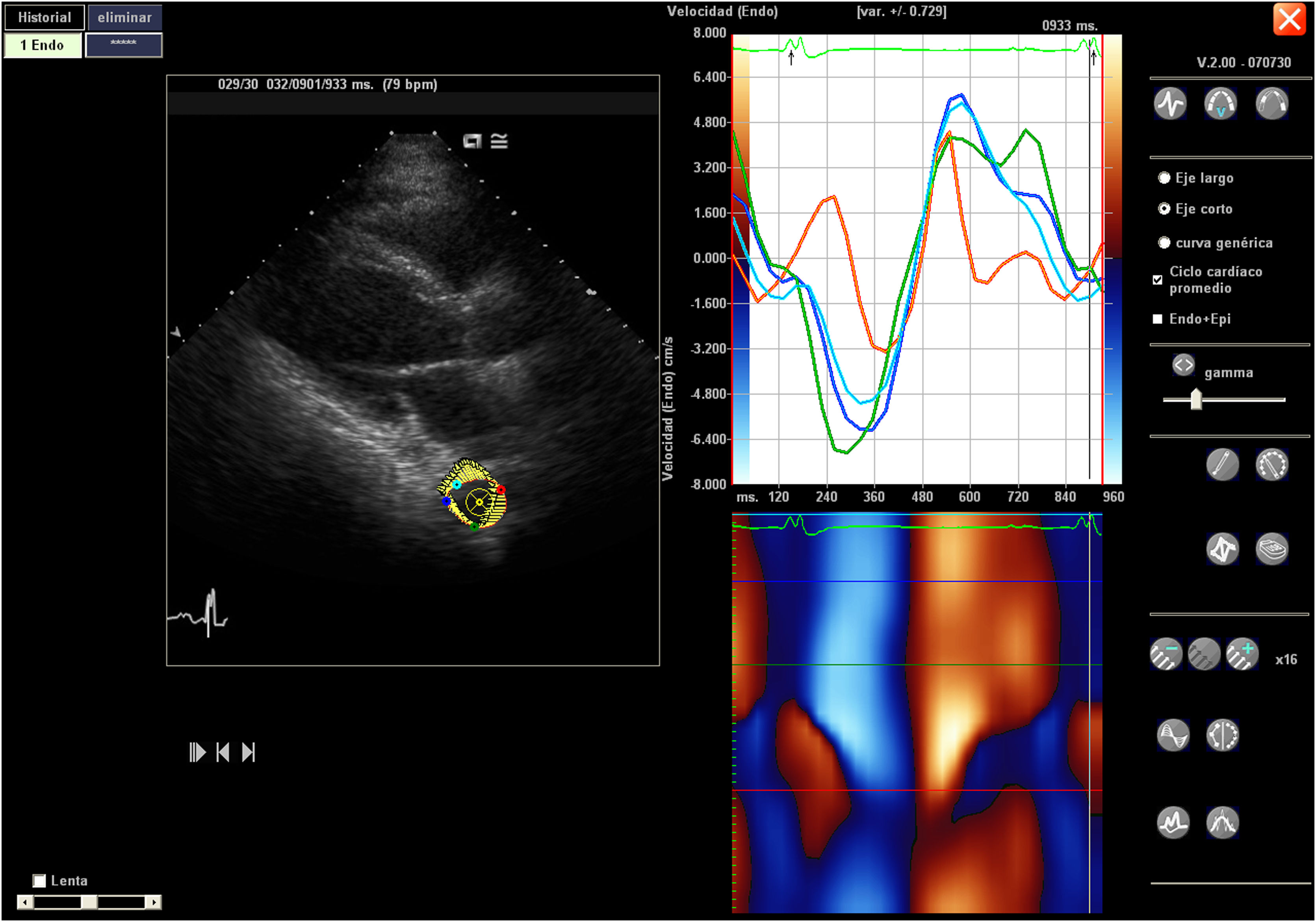
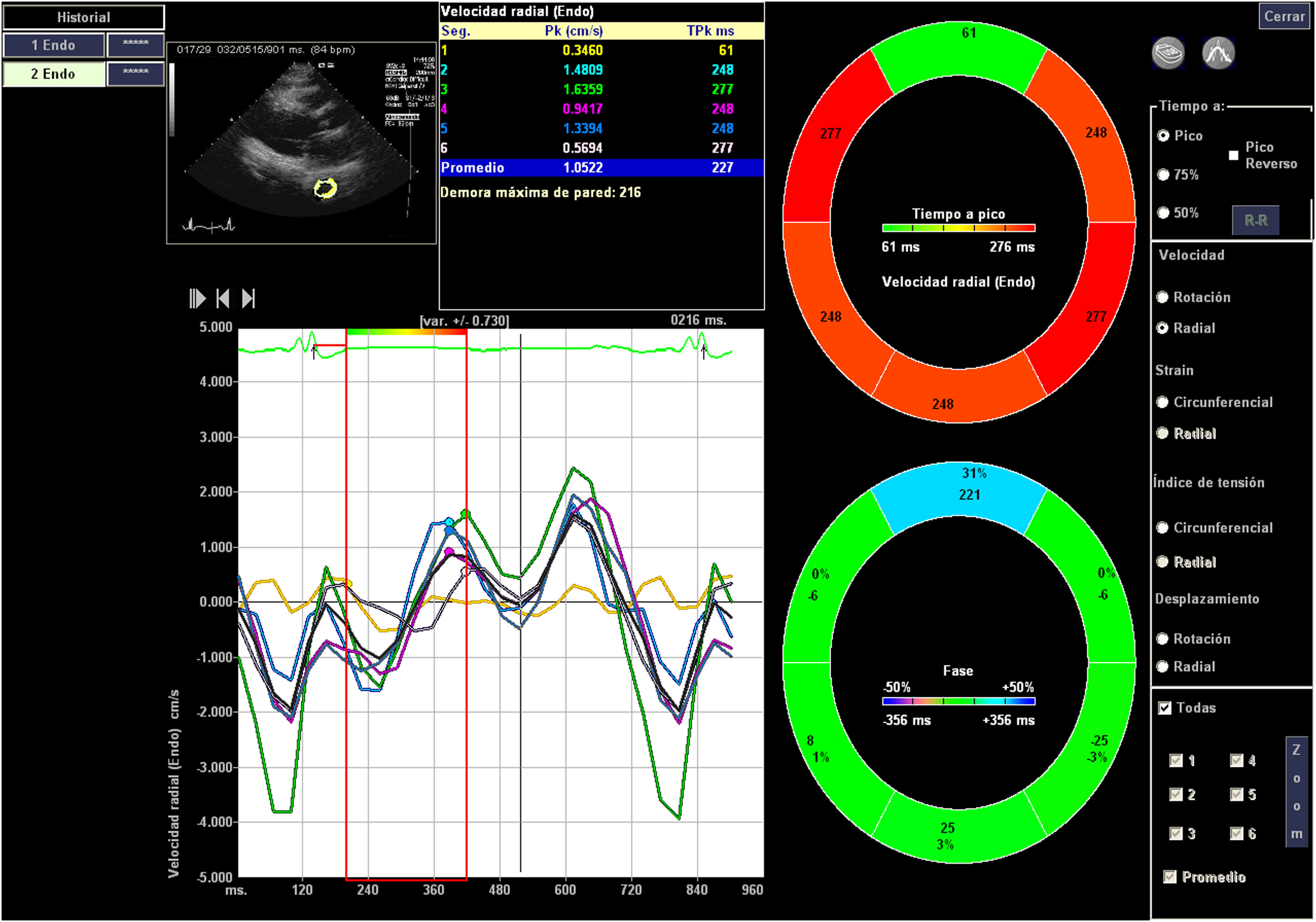
Image acquisition and processingStandard transthoracic echocardiography (ETT) was performed in the supine position and was recorded in digital DICOM format. For off-line analysis purposes, we used 2D and 3D, Speckle Tracking Hybrid Velocity Vector Imaging (VVI) analysis.11–14 Echocardiographic studies were performed with the following echocardiographic equipment available during long-term study period: Sequoia c512, Acuson, SC 2000, Siemens Medical Systems, Mountain View, CA, USA, and Epiq CX50. Transthoracic and (TTE) transesophageal echocardiography (TEE) were performed. The echocardiographic variables studied have been determined both online and offline, using echocardiographic programs Syngo software, Siemens® U.S., compatible with all DICOM formats of the different echocardiographic equipment and Tomtec Arena (https://www.tomtec.de/products/tomtec-arena).
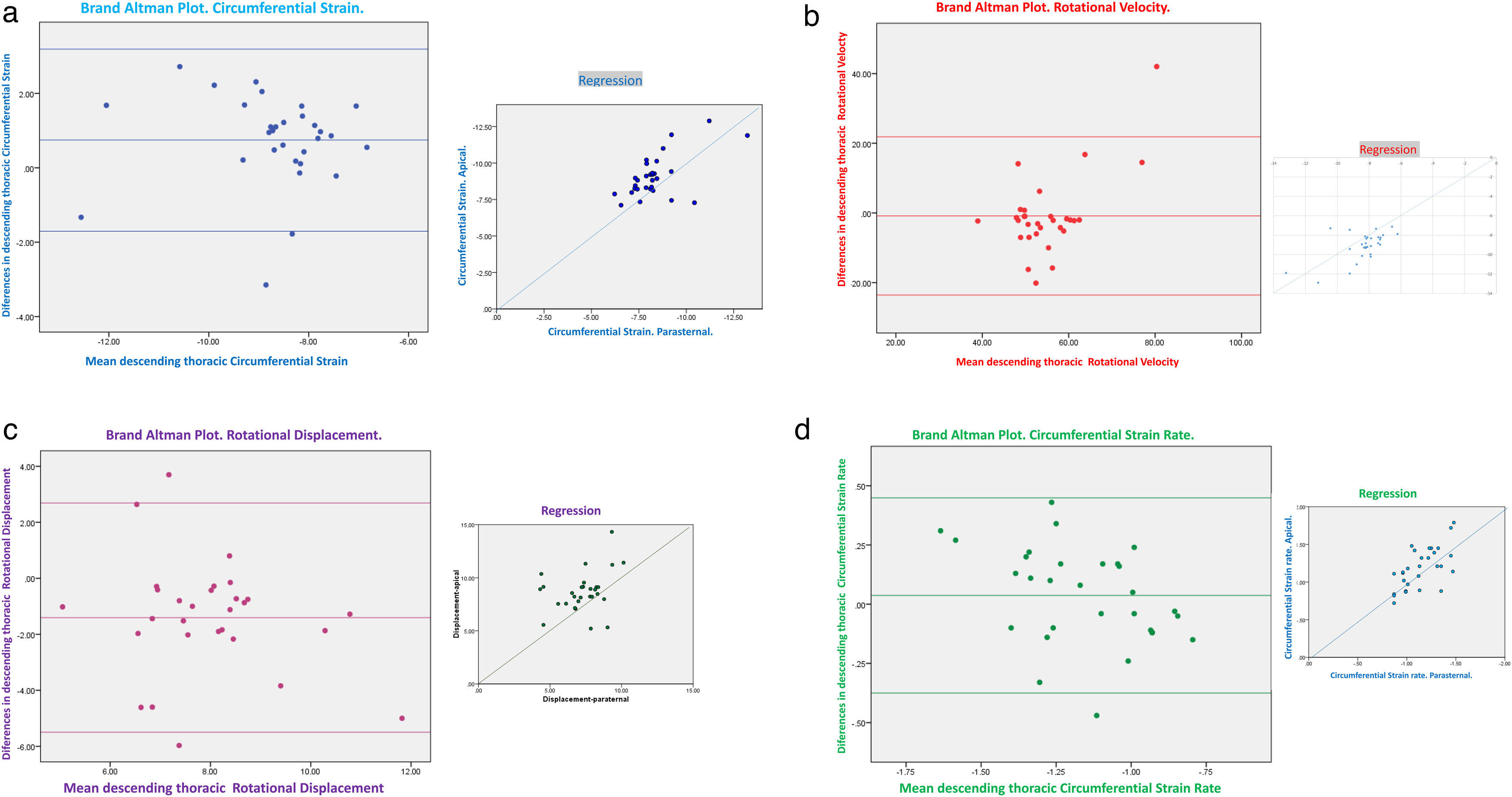
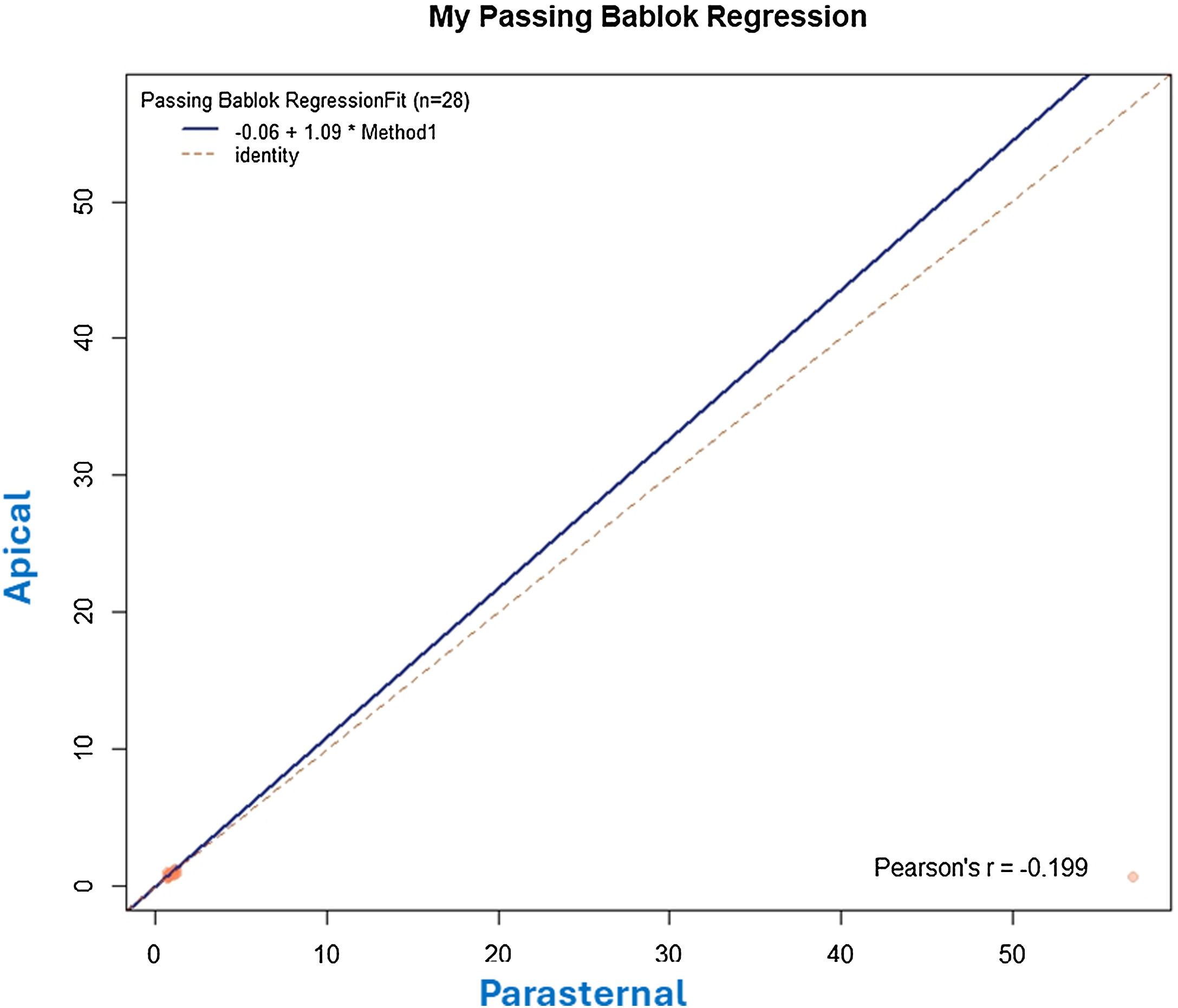
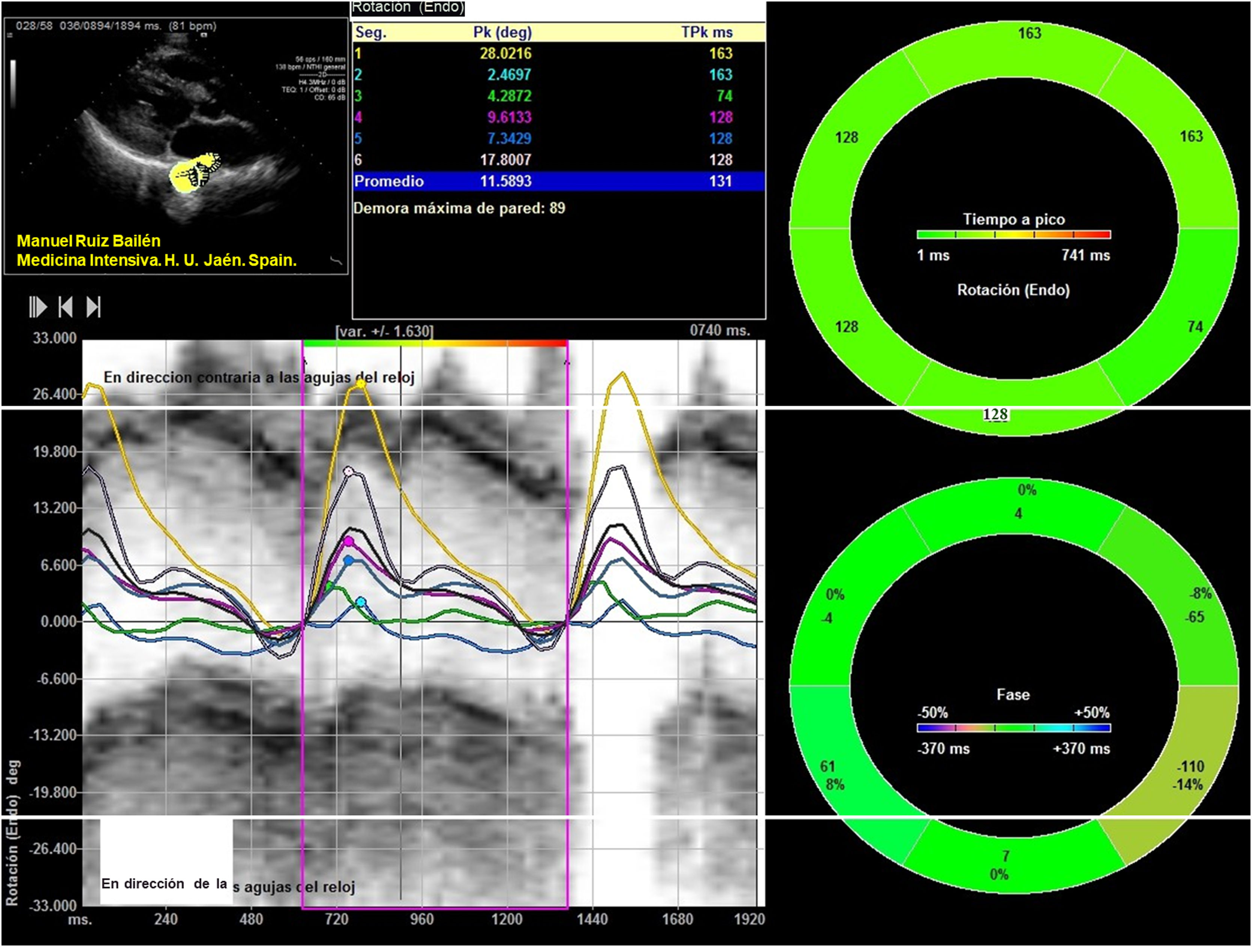
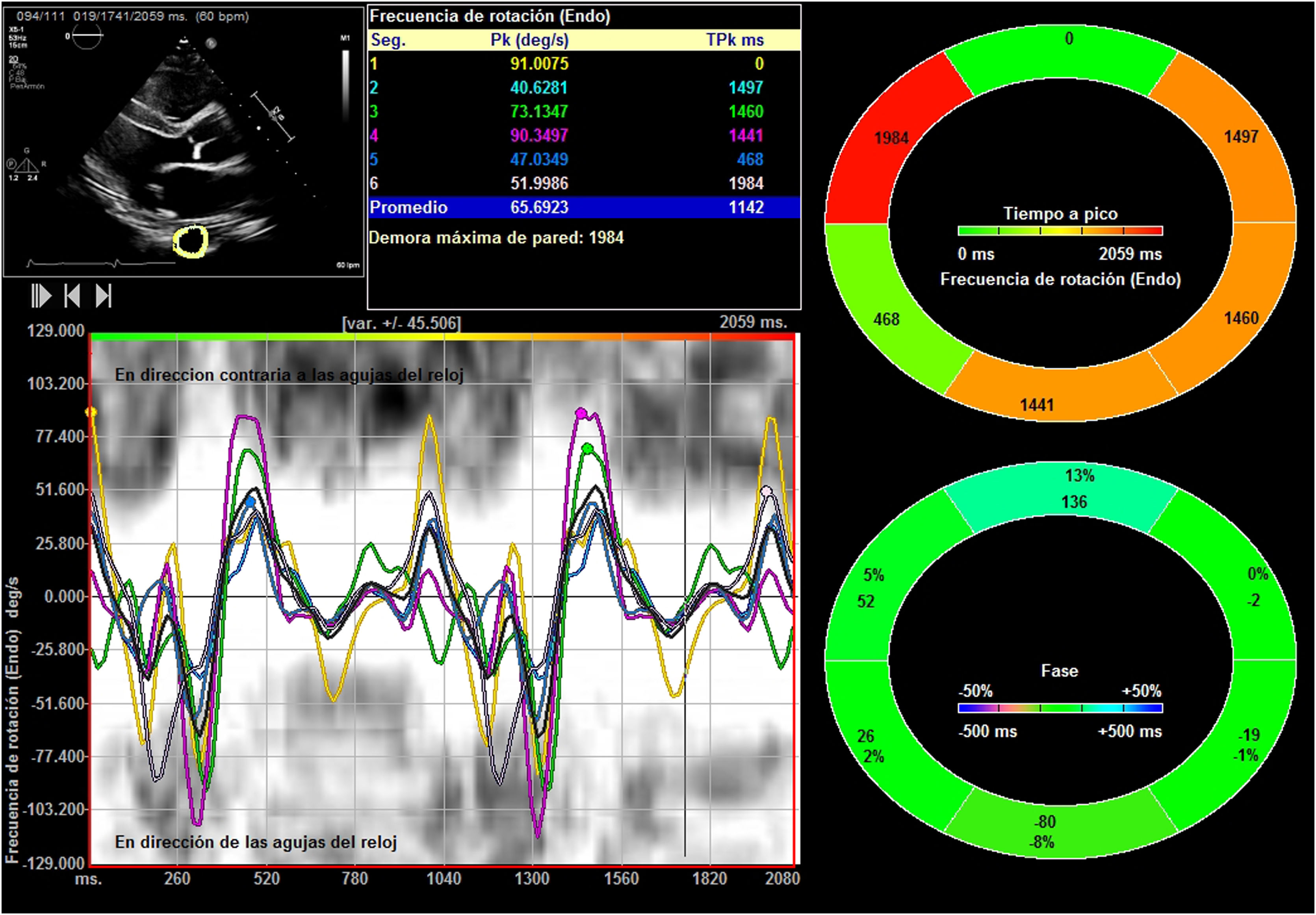
The apical 4-chamber orientation was used for the acquisition of left ventricular functional data. The descending thoracic aorta was evaluated in the parasternal long-axis projection. We zoomed in on the descending thoracic aorta, and the frame rate was set as high as possible (70–120 f/s) with multiple focal points. VVI analysis was performed with a single cardiac cycle (a single heartbeat). All images were optimized with gain, compression, and dynamic range. The studies were recorded in high quality digital format. A blind, off-line analysis was conducted using the Syngo software, Siemens 2013®. U.S and Tomtec Arena® software. Separate studies were conducted to evaluate their degree of intraobserver and interobserver agreement. Figs. 1 and 2. The mean value was taken from three measurements in which the descending thoracic aorta was seen very adequately in the parasternal long-axis projection. Images with extreme values, discordant values or in which the image marked by the VVI were not adequate were rejected as outliers.
We evaluated the usual echocardiographic parameters as recommended by the American Society of Echocardiography: quantification of LVEF by 2D and 3D, Tricuspid annular plane systolic excursion (TAPSE) and the isovolumetric acceleration (IVA) calculated as the ratio of Tissue Doppler derived peak myocardial velocity during isovolumetric contraction divided by the acceleration time. The estimation of pulmonary capillary wedge pressure (PCWP) was performed using the Nagueh15 and Kuecherer equations.16 The following were also evaluated: parameters derived from speckle tracking, such as strain (S), Strain Rate (SR), longitudinal and radial displacement, and longitudinal and radial velocities in the left and the right ventricle. In the descending thoracic aorta, we calculated fractional shortening area and the following speckle-tracking VVI values as the aorta rotational and radial velocities, the aorta circumferential strain and strain rate and the thoracic aorta rotational and radial displacement. The region of interest was manually traced. Videos 1 and 2. We only assessed systolic peaks, although diastolic peaks could also have been assessed. The projection used was the parasternal long-axis, although these parameters were also used and evaluated in the apical 4-chamber projection in 30 patients. Between both projections, a concordance test has been performed to evaluate the degree of agreement between these values of the VVI speckle tracking of the descending thoracic aortic artery.
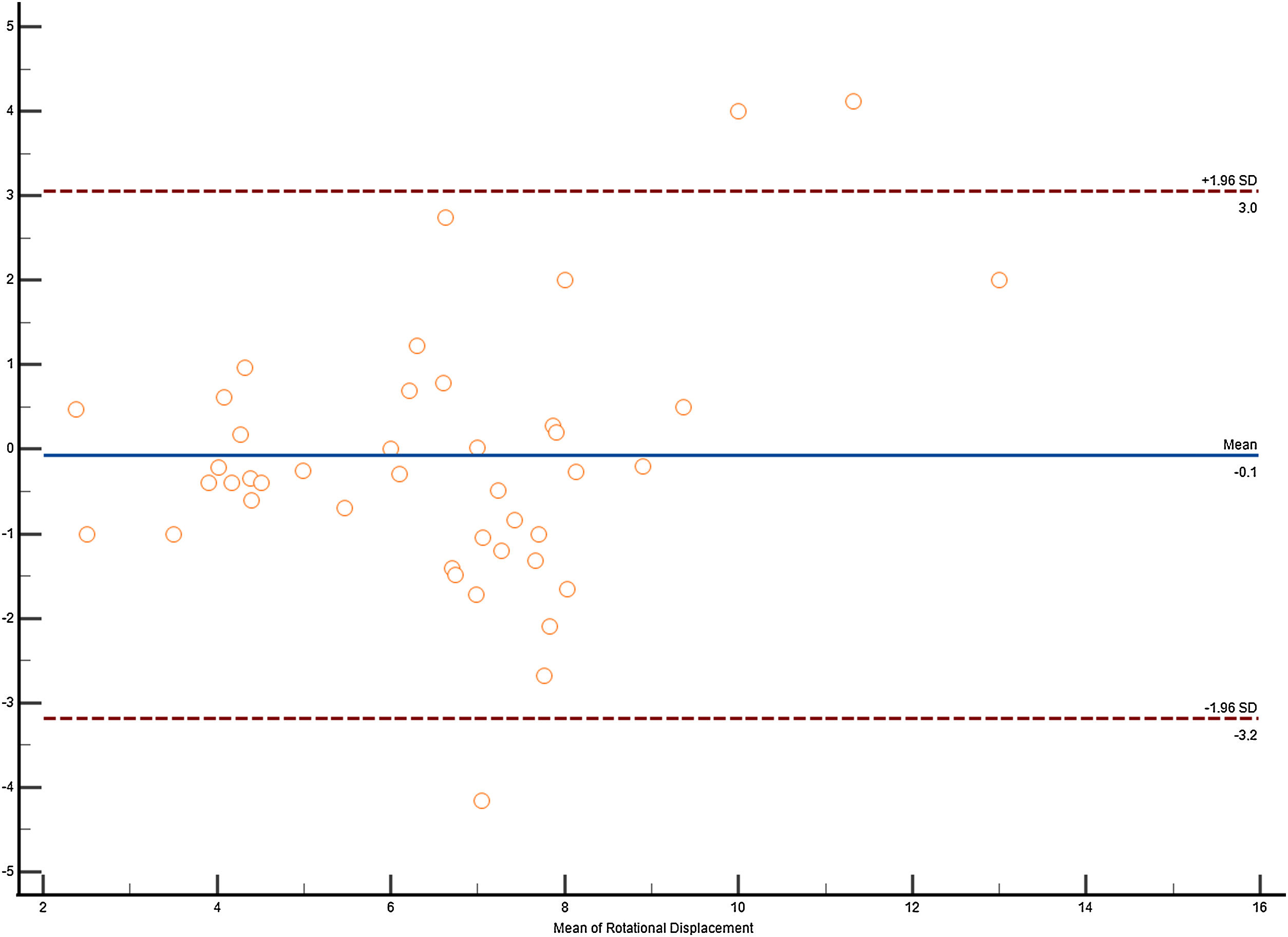
Statistical analysisA descriptive analysis of quantitative variables was performed. The R2 coefficients of determination were evaluated by linear regression between the cardiological parameters and the values found by Speckle Tracking of the descending thoracic aorta. Degree of agreement between the results for strain, strain rate, velocities and displacement value obtained in the parasternal long axis and the apical four-chamber planes was evaluated using a Bablok regression and a Bland-Atman plot. Intraobserver and interoperator agreement was evaluated. Results were presented using means and standard deviations. A p value <0.05 was considered statistically significant. MedCalc® Statistical Software version 22.030 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2024) and IBM SPSS for Windows, version 30.0; SPSS Inc., Chicago, Illinois, USA were used.
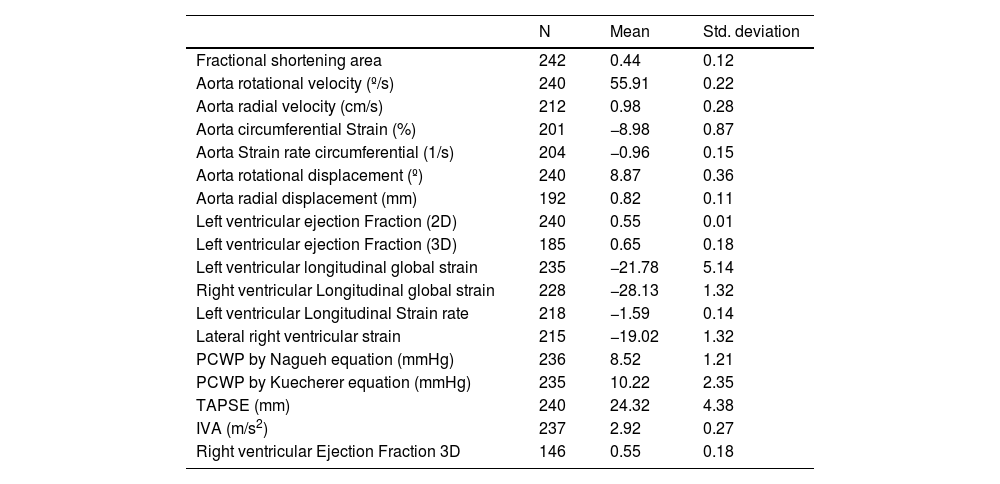
Results242 analyses with good acoustic quality in healthy people were included in this study (102 women). The patients mean age was 42.35±16.37 years years, their mean height 175.88±16.87cm (men) and 163.87±14.71cm (women) and the mean weight was 71.22±14.45 Kg. Descending thoracic circumferential strain, circumferential strain rate, rotational velocities and displacement were evaluated in thirty people, in the apical 4-chamber and in the parasternal long-axis view. Both projections presented significant degree of agreement. Comparing results derived from the two methodologies we considered that the apical 4-chamber provides the best echocardiographic view (window), centered and zooming in the descending thoracic aorta, although it generally yields higher values than in the parasternal window (Figs. 1 and 2) (Video 1–6). The mean LVEF was 0.55±0.01 using 2D echo and 0.65±0.18 for 3D echo with a median heart rate of 71.44±21.11 beats per minute. Global left ventricular longitudinal strain was (−21.78±5.14) (Table 1). The DTA rotational velocity was 55.91±0.22º/s, the DTA radial velocity was 0.98±0.28cm/s, the circumferential strain of the DTA was −8.98±0.87%, while the circumferential strain rate was (−0.96±0.15/s). The rotational displacement of the DTA was determined to 8.87±0.36º, and the radial displacement was 0.82±0.11mm (Figs. 3–6).
Altman-Bland plot.
(a) Alman–Bland plot for descending thoracic aorta Circumferential strain.
(b) Alman–Bland plot for descending thoracic aorta Rotational velocity.
(c) Alman–Bland plot for descending thoracic aorta Rotational displacement.
(d) Alman–Bland plot for descending thoracic aorta circumferential strain rate.
Descriptive data of the descending thoracic aorta and both ventricles.
| N | Mean | Std. deviation | |
|---|---|---|---|
| Fractional shortening area | 242 | 0.44 | 0.12 |
| Aorta rotational velocity (º/s) | 240 | 55.91 | 0.22 |
| Aorta radial velocity (cm/s) | 212 | 0.98 | 0.28 |
| Aorta circumferential Strain (%) | 201 | −8.98 | 0.87 |
| Aorta Strain rate circumferential (1/s) | 204 | −0.96 | 0.15 |
| Aorta rotational displacement (º) | 240 | 8.87 | 0.36 |
| Aorta radial displacement (mm) | 192 | 0.82 | 0.11 |
| Left ventricular ejection Fraction (2D) | 240 | 0.55 | 0.01 |
| Left ventricular ejection Fraction (3D) | 185 | 0.65 | 0.18 |
| Left ventricular longitudinal global strain | 235 | −21.78 | 5.14 |
| Right ventricular Longitudinal global strain | 228 | −28.13 | 1.32 |
| Left ventricular Longitudinal Strain rate | 218 | −1.59 | 0.14 |
| Lateral right ventricular strain | 215 | −19.02 | 1.32 |
| PCWP by Nagueh equation (mmHg) | 236 | 8.52 | 1.21 |
| PCWP by Kuecherer equation (mmHg) | 235 | 10.22 | 2.35 |
| TAPSE (mm) | 240 | 24.32 | 4.38 |
| IVA (m/s2) | 237 | 2.92 | 0.27 |
| Right ventricular Ejection Fraction 3D | 146 | 0.55 | 0.18 |
Linear regression showed and showed association between the rotational displacement of the DTA with left ventricular longitudinal strain and right ventricular longitudinal strain, (R2 0.43, p value 0.038 and R2 0.28, p value 0.042 respectively) and PCWP estimated by Nagueh (R2 0.21, p value <0.001) and Kuecherer's equation (R2 0.23, p value <0.001).
Rotational velocities of the DTA are associated with left ventricular longitudinal strain, and right ventricular longitudinal strain, (R2 0.44, p value 0.021 and R2 0.36, p value 0.02 respectively). We found no relationship between these associations when adjusted for age or body mass index.
Intraoperator agreement was adequate for all parameters evaluated in the thoracic aorta, except for circumferential strain and strain rate. Similar findings were obtained for interoperator agreement (Figs. 7 and 8).
DiscussionIt has been shown that aortic artery has functions that seriously impact and alter cardiovascular physiology and clinical semiology. These functions could be affected by sex, or by aldosterone inhibition, which could decrease aortic elasticity.17,18 Aortic artery lesions or simply atherosclerosis can also alter its functions.7,19
Age plays a significant role, as it causes changes in the aorta, increasing its stiffness, diameter, taper and impedance. In general, any cardiovascular pathology can modify the behavior of the aorta, which in turn can have an impact on cardiovascular behavior. These effects of changes in aortic properties and function on left ventricular contraction have been known since 2008 as ventricular-vascular interaction.9,19–21
The development of heart failure after aortic repair could confirm this reciprocal interaction.21 Previous reports evaluating aortic diameters and aortic strain have evidenced the improvement of aortic artery elasticity with the administration of levosimendan in animal models,22 as well as after renal transplantation in patients.23 These interactions are currently being investigated with computational models.24
The most important finding of this study is that it generates the hypothesis that Speckle Tracking values, can be measured in the normal population. However, we could ask ourselves a series of questions about our findings, the first being, what is their validity when performed with software not designed for the aorta? The second question would be what would be the possible applicability of this technique? Speckle tracking is an undeniably useful tool that has advanced our knowledge of cardiac physiology.11
We used Speckle Tracking designed for the cardiac cavities and traced the borders of the thoracic aorta along the short axis of the left ventricle, assessing the systolic peak of each parameter. Obviously, it is not a specific software to assess the aorta, but we have detected some patterns and values which obviously cannot be considered as reference values due to the low population and because it has not been performed with a specific software. Working with a tool designed for another purpose makes it difficult to validate the results. We performed the study with a single cardiac cycle with a high frame rate and zooming. Sometimes the vector velocity analysis does not recognize the edges of the thoracic aorta, so the endoaortic border must be repeated. Another important limitation in its applicability is that intraoperator and interoperator agreement is not observed for strain or circumferential strain rate, although it is observed for all other parameters. Systolic peaks and diastolic peaks could be evaluated, however only systolic peaks have been evaluated. However, if this technological tool were improved, diastolic peaks could be evaluated, and more information could be obtained.
Despite all these limitations this tool together with tissue imaging or cardiac MRI could advance our understanding of aortic function could be useful and other authors have certainly used non-specific software to explore this hypothesis.25–36 The assessment of the abdominal descending aorta time velocity integral, not evaluated in this study, could also be associated with hemodynamic values and with the perfusion status and hemodynamic situation.
The applicability of aortic evaluation by speckle tracking could be very diverse. We first wanted to evaluate the values shown by this hybrid Speckle Tracking by VVI and try to associate it with the strain of the left ventricle. Its values could contribute to determining the healthy state of the aorta and even guide health professionals when assessing anatomical aortic lesions. Interestingly, we have found that the values that best correlate are not precisely the strain, nor the strain rate; but the rotational velocities and rotational displacement, which have the greatest association with cardiological values, both left and right systolic function and curiously with the estimation of the PCWP. This could help us to better treat our patients in heart failure or even septic shock or assess their physiological response to stress. One possible application of this tool is that during resuscitation of a hypovolemic septic patient with normal diastolic and systolic functions and with an elastic aorta, the patient would have at least greatly increased rotational velocities and rotational displacement. However, in the presence of myocardial dysfunction or atherosclerosis, these values or aortic deformity would be much lower. For this reason, we understand that the values obtained may have a pattern, and that in the future this technique could be applied to the aorta, to detect heart failure semiology or even diastolic dysfunction, or simply anatomical and functional alterations of the aorta itself. The development of a specific technology to evaluate the aorta could help, for example, to differentiate true light from false light in doubtful cases of aortic dissection or facilitate its diagnosis in untrained explorers (Videos 3 and 4). In the present study we have analyzed the baseline values of the descending thoracic aorta. Obviously, these values should be confirmed in a follow up study with software designed specifically for the aorta. Our initial data however clearly shows that the analysis of the aorta using this technology can provide us with a large amount of novel information on the aorta’s pathophysiology and potentially prevent the development of acute aortic syndrome in patients susceptible to them. It is hence considered a powerful new tool taking advantage of established technology. Our future aim is consequently to evaluate whether these values change in patients with different pathologies and to try to show cut-off points through which we can assess the degree of correlation of the aorta with heart failure.
Another interesting finding is the concordance detected between parasternal and apical projections, which helps their performance. This was most visible in apical 4 chamber views, where slightly higher values are obtained than in the parasternal window, a window that we have used to obtain the data Figure of the Blant–Atman plot. We have not been able to see all the parameters in all patients, so, interestingly, the ones that are best seen with this technique are rotational displacement and velocities, which are the ones that correlate the most with cardiological parameters. Probably the parameter we found most interesting, as it is the one that correlates most with the rest of the values, is not strain but rotational displacement, which has been evaluated by other experimental techniques and has been found to be more affected in aortic annulus lesions.34
We have selected those who had a very good echocardiographic window, which could generate a systematic bias that could explain this good concordance. However, we felt more comfortable with the apical 4-chamber window, a window that seemed to us to be more efficient in drawing the endoaortic border.
We used a small sample of young patients, with non-specific software, so the findings of this study should be interpreted with caution. The software used had been designed for left ventricular parameters, so we assumed that it was suitable for measuring velocities, strain and displacement. The reference values with more specific programs could probably be different, but this study clearly shows that VVI analysis can be a new and very useful tool to study the aorta being able to evaluate its possible areas with lower velocity, deformity or motility due to fibrosis or atherosclerosis.37 Study focused on the descending aorta artery we believe that it is feasible that the ascending aorta could be studied in the parasternal long axis35 and even the aortic arch in the suprasternal projection.
Despite all the limitations that this manuscript presents, our results strongly suggest that Speckle Tracking can be used in the aorta, providing a new dimension of information. Using the identified reference values, aortic pathologies or cardiovascular pathological interactions could be better understood and used to explain the semiology of heart failure. Speckle Tracking applied in intensive care medicine38 appears to be an effective tool. Also, in the aorta could also be used to see the impact of the aortic prosthesis repair on the patient (Videos 3 and 4).
In summary, the use of this technology in the aorta constitutes a completely new line of research, with the aim to evaluate aortic function in a reproducible manner.
ConclusionsSpeckle Tracking could be used to study the descending thoracic aorta, and its values could suggest or rule out the presence of cardiovascular pathology.
CRediT authorship contribution statementRuiz-Bailén, Manuel. MD, PhD 1,2. Principal investigator, author of the manuscript, database, statistical study, author of echocardiography, Martín-Hidalgo, Javier. MD 1. co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Clau Terre, Fernando co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Martínez-Gámez, Javier co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Ramos Cuadra, José Ángel. MD. PhD4 co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Lohman, Johannes Dagomar. MD5 co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Manetsberger, Julia. PhD1 co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
Lavilla Lerma, María Leyre. PhD 1 co-author of the manuscript, reviewer of the manuscript, collaborate in the preparation of the manuscript, translation.
FundingPAIDI CTS 606, Andalusian Health Service Project no, “PI-0585-2012 Ecocardiografía en Medicina Crítica. Detección de la disfunción Miocárdica del Paciente Crítico e interacción entre la ecocardiografía y la ventilación mecánica”.
The authors declare no conflict of interest related to this article.
The following are Supplementary data to this article:
The video shows a VVI study of descending thoracic aorta included in the study. The thoracic aorta is first selected, observed in 3D, and then the VVI is applied. In the final part of the video it can be seen how the endoaortic velocity follows a pattern similar to tissue Doppler, with protosystolic, systolic and diastolic waves. Similarly, the anatomical color curved mode could be studied