Patients with severe COVID-19 related acute respiratory distress syndrome (ARDS) may benefit from V-V ECMO support. However, this technique remains associated with frequent complications and high mortality.1 The early identification of patients with V-V ECMO who are likely to survive on ICU discharge therefore seems of major interest.
Endocan is a circulating proteoglycan secreted by the pulmonary vasculature under inflammatory conditions such as ARDS.2 Recent data suggest that monitoring of blood endocan during 1st week following intensive care unit (ICU) admission may correlate with the severity of ARDS in Covid-19.3
Thus, we aimed in this study to assess whether plasma endocan measurements performed on the day of V-V ECMO implantation (D0) and repeated seven days later (D7) may be effective in predicting mortality on ICU discharge.
This study was conducted in a 50-bed mixed ICU, from October 2020 to June 2021. We included all consecutive COVID-19 patients undergoing V-V ECMO implantation and with available results of plasma endocan measured on day of ECMO implantation.
Endocan was measured weekly on EDTA plasma in the central laboratory of our hospital using the ENDOMARK H1 ELISA kit (Biothelis, France) in COVID-19 patients admitted in our ICU as part as the routine assessment of prognosis in ARDS,4–6 along with CRP and fibrinogen. This research was examined by our local IRB, and approved under the number HP 22/01. Because of the retrospective observational design, written informed consent was not required.
Patients’ characteristics were obtained at D0. We also retrospectively collected values on D0 and D7 for plasma endocan (endocanD0 and endocanD7), CRP (CRPD0 and CRPD7) and fibrinogen (fibrinogenD0 and fibrinogenD7). Variations between D0 and D7 were respectively calculated for endocan (endocanD0–D7), CRP (CRPD0–D7) and fibrinogen (fibrinogenD0–D7) as following: (value on D7−value on D0)/value on D0.
Categorical variables were expressed as numbers (percentages) and compared using Fisher's exact test, given small sample sizes. Skewed continuous variables were presented as median (interquartile range) and compared using Mann-Whitney U test.
Decision tree based on CART algorithm was generated using the rpart R package with default settings, using endocanD0, endocanD7, endocanD0–D7, CRPD0, CRPD7, CRPD0–D7, fibrinogenD0, fibrinogenD7 and fibrinogenD0–D7 as covariates.
All statistical tests were two-tailed, and p values <0.05 were considered statistically significant. Statistical analysis was performed using R version 3.6 (R foundation for statistical analysis, Austria).
Eleven patients undergoing V-V ECMO implantation for COVID-19 related ARDS were included in this study, of whom 5 (45%) were discharged alive from ICU. All patients underwent invasive mechanical ventilation (MV) during their stay in ICU, although invasive MV was initiated right before ECMO implantation and maintained less than 24h for 2 patients from the survivors group.
Compared to survivors, non-survivors had higher endocanD7 (median [IQR]=12.9 [6.1; 19]ng/ml vs 4.3 [3.9; 5.6]ng/ml; p=0.017), and greater increase in endocanD0–D7 (median [IQR]=+208 [−1; +612] % vs −49 [−65; +52]%; p=0.03). Conversely, no difference was found between non-survivors and survivors for endocanD0 (median [IQR]=4.7 [3; 5.7]ng/ml vs 7.9 [3.8; 12.2]ng/ml; p=0.25), CRPD0 (median [IQR]=94 [68; 200]mg/l vs 118 [26; 150]mg/l; p=0.66), CRPD7 (median [IQR]=68 [50; 77]mg/l vs 39 [35; 123]mg/l; p=0.41), CRPD0–D7 (median [IQR]=−17 [−72; −5]% vs 4 [−75; +484]%; p=0.54), fibrinogenD0 (median [IQR]=7.1 [4.9; 8.1]g/l vs 6.1 [3.9; 6.8]g/l; p=0.33), fibrinogenD7 (median [IQR]=5.1 [4.3; 5.6]g/l vs 4.6 [2.7; 5.1]g/l; p=0.23), and fibrinogenD0−D7 (median [IQR]=−26 [−43; +9] % vs −28 [−50; +15]%; p=1) (Table 1).
Characteristics of patients and biomarkers in survivors and non-survivors.
| Survivors | p value | ||
|---|---|---|---|
| Yes (n=5) | No (n=6) | ||
| Demographical data | |||
| Sex, male | 4 (80%) | 5 (83%) | 1 |
| Age, years | 48 [33; 62] | 57 (48; 63) | 0.43 |
| BMI, kg/m2 | 37 [32; 40] | 34 [28; 42] | 0.66 |
| Severity score on ECMO implantation | |||
| SOFA | 9 [6; 13] | 9 [8; 11] | 0.93 |
| RESP | 1 [−6; 2.5] | −4 [−6; −2] | 0.43 |
| Comorbidities | |||
| Diabetes mellitus | 2 (40%) | 1 (17%) | 0.55 |
| Chronic respiratory failure | 0 (0%) | 0 (0%) | 1 |
| COPD | 0 (0%) | 2 (33%) | 0.45 |
| Coronary disease | 1 (20%) | 1 (17%) | 1 |
| Immunocompromised patients | 0 (0%) | 0 (0%) | 1 |
| Characteristics of ECMO | |||
| Time from onset of symptoms to ECMO, days | 21 [10.5; 33.5] | 18 [13.5; 24] | 0.71 |
| Time from ICU admission to ECMO, days | 13 [6; 28] | 11 [6; 17] | 0.58 |
| Time from invasive MV to ECMO, days | 3 [0.5; 20] | 9.5 [3; 13] | 0.41 |
| Invasive MV initiated on the day of ECMO implantation and maintained for less than 24h | 2 (40%) | 0 (0%) | 0.18 |
| RPM on day of ECMO implantation, ×1000 | 3.9 [2.5; 4.6] | 3.7 [3.4; 4.1] | 0.93 |
| ECMO blood flow on day of ECMO implantation | 4.8 [3.9; 7.1] | 5.2 [4.6; 6.3] | 0.85 |
| Outcomes | |||
| Duration of ECMO, days | 18 [12; 34] | 27 [19; 33] | 0.2 |
| Length of stay in hospital, days | 53 [35; 79] | 36 [31; 49] | 0.31 |
| Length of invasive MV, days | 13 [5; 43] | 26 [34; 48.5] | 0.25 |
| Biomarkers | |||
| Endocan at D0, ng/ml | 7.9 [3.8; 12.2] | 4.7 [3; 5.7] | 0.25 |
| Endocan at D7, ng/ml | 4.3 [3.9; 5.6] | 12.9 [6.1; 19] | 0.017 |
| Variation of endocan between D0 and D7, % | −49 [−65; 52] | 208 [−1; 612] | 0.03 |
| CRP at D0, mg/l | 118 [26; 150] | 94 [68; 200] | 0.66 |
| CRP at D7, mg/l | 39 [35; 123] | 68 [50; 77] | 0.41 |
| Variation of CRP between D0 and D7, % | 4 [−75; 484] | −17 [−72; −5] | 0.54 |
| Fibrinogen at D0, g/l | 6.1 [3.9; 6.8] | 7.1 [4.9; 8.1] | 0.33 |
| Fibrinogen at D7, g/l | 4.6 [2.7; 5.1] | 5.1 [4.3; 5.6] | 0.23 |
| Variation of fibrinogen between D0 and D7, % | −28 [−50; 15] | −26 [−43; 9] | 1 |
Data are presented as number (%) or median [IQR]. BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; D0: day of ECMO implantation; D7: day 7 following ECMO implantation; ECMO: Extra Corporeal Membrane Oxygenation; ICU: Intensive Care Unit; MV: Mechanical Ventilation; RESP: Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; RPM: Rounds Per Minute; SOFA: Sequential Organ Failure Assessment; VAP: Ventilator Associated Pneumonia.
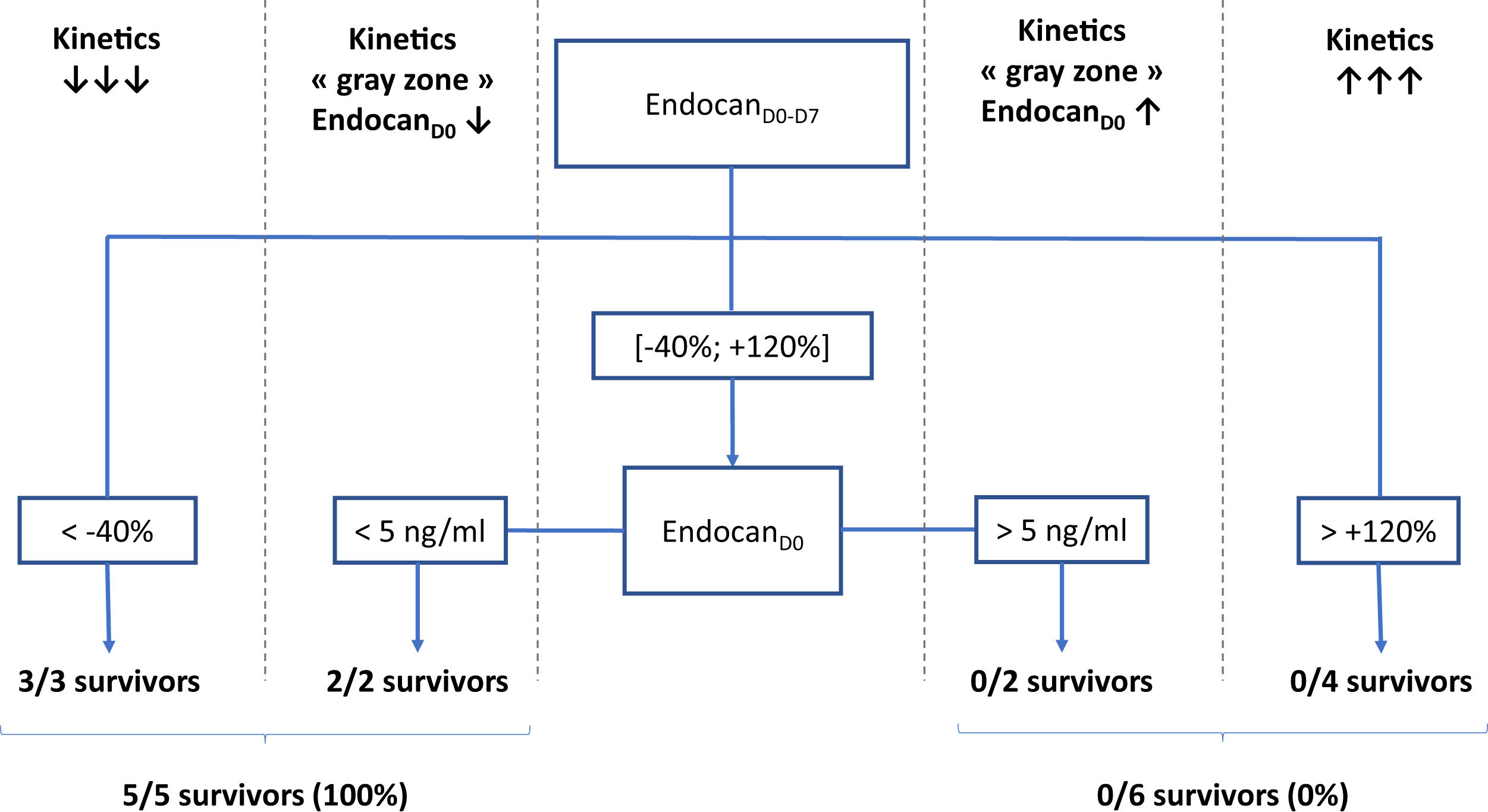
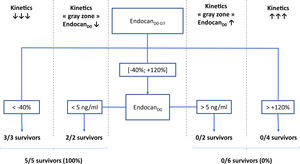
According to a decision tree based on a CART algorithm, a 2-nodes model combining endocanD0–D7 with static value of endocanD0 was able to correctly identify all survivors and non-survivors. Indeed, according to this decision tree, 3/3 patients with endocanD0−D7<−40% survived, while 4/4 patients with endocanD0−D7>+120% died. Regarding patients with endocanD0−D7 in the gray zone ranging between −40% and +120%, 2/2 survivors had endocanD0<5ng/ml, while 2/2 non-survivors had endocanD0>5ng/ml (Fig. 1). These cut-offs were inferred from the CART algorithm, and are not supported by any statistical test.
Decision tree for prediction of survival on ICU discharge. EndocanD0, endocanD7, endocanD0–D7, CRPD0, CRPD7, CRPD0–D7, fibrinogenD0, fibrinogenD7 and fibrinogenD0–D7 were tested as covariates for survival in ICU prediction model. D0: day of ECMO implantation. D7: day 7 following ECMO implantation; D0–D7: variation between D0 and D7, calculated as (value on D7−value on D0)/value on D0.
We hereby report results from a pilot study exploring the potential usefulness of endocan in patients with Covid-19 related ARDS undergoing V-V ECMO. Compared to survivors, non-survivors from our cohort were older, with greater RESP scores and longer time from invasive MV to ECMO implantation, which was in line with previously published data.
Data from this pilot study suggest that among patients undergoing V-V ECMO for COVID-19 related ARDS, survival on ICU discharge would be associated with significant fall in plasma endocan measured 1 week after ECMO implantation. Indeed, subjects experiencing marked fall in plasma endocan were all discharged alive from ICU, while those with greatest rises did not survive. Further, it seems that patients with intermediate variations of plasma endocan might be segregated into 2 groups according to initial levels of plasma endocan: subjects with baseline values lower than 5ng/ml who survived, in contrast with those exhibiting initial values higher than 5ng/ml who were not discharged alive from ICU.
These results are in line with previous data regarding the biological role of endocan in critically ill subjects. Indeed, secretion of endocan is upregulated by pro-inflammatory cytokines,7 and endocan has been widely reported as a marker of pulmonary endothelial stress.2 Furthermore, endocan is known as an inhibitor of leukocyte recruitment, therefore regulating lung inflammation.8 Consistent data suggest that low circulating levels of endocan at the early phase of lung aggression, followed by secondary rise in its plasmatic values, would reflect insufficient initial protection against lung inflammation, being thus associated with poor outcomes.5,9–11 Interestingly, both patients from our cohort who underwent ECMO implantation with a duration of invasive MV<24h experienced marked decreases of plasma endocan on D7, respectively found at −69% and −47%. Further studies are needed to investigate the significance of these results.
Authors’ contributionsAT, SA and SBL designed the study. AT wrote the manuscript. FBL, CA, FD and YT contributed to data collection. SA and SBL participated in its critical revision. All authors read and approved the final manuscript.
FundingNone declared.
Conflict of interestThe authors listed on this publication have no conflict of interest.
None.
Erika Parmentier-Decrucq, Julien Poissy, Sylvain Dubucquoi, Pauline Boddaert, Morgan Caplan, Julien Goutay, Arthur Durand, Benoit Graffin, Myrtille Gaudel, Charles Detollenaere, Ines Gueguen, Marine Van Ceunebroek, Romain Tortuyaux, Ouriel Saura, Ahmed El Kalioubie, Raphael Favory, Patrick Girardie, Marion Houard, Emmanuelle Jaillette, Mercedes Jourdain, Geoffrey Ledoux, Daniel Mathieu, Anne Sophie Moreau, Saad Nseir, Thierry Onimus, Sebastien Preau, Laurent Robriquet, Anahita Rouze, Sophie Six, Jerome Soquet, Valentin Loobuyck, Agnes Mugnier, André Vincentelli.