To evaluate the incidence and risk factors for early mortality (EM) in the ICU in patients with community-acquired septic shock (CASS).
DesignA retrospective cohort study of patients with CASS admitted to the ICU (2003–2016).
SettingICU at a University Hospital in Spain.
PatientsAll consecutive patients admitted to the ICU with CASS.
InterventionsNone.
Main variables of interestCASS was defined according to the Sepsis-3 definitions. EM were defined as occurring within of 72h following ICU admission. A multinomial logistic regression analysis was performed to identify the risk factors associated with early deaths.
ResultsDuring the study period, 625 patients met the Sepsis-3 criteria and admitted with CASS. 14.4% of all patients died within the first 72h. Of 161 patients who died in the ICU, 90 (55.9%) died within the first 72h. The percentage of early and late mortality did not vary significantly during the study period. The need and adequacy of source control were significantly lower in patients with EM. In the multivariate analysis, ARDS, non-respiratory infections, bacteremia and severity at admission were variables independently associated with EM. The only factor that decreased EM was adequate source control in patients with infections amenable to source control.
ConclusionsThe incidence of EM has remained stable over time, which means that more than half of the patients who die from CASS do so within the first 72h. Infections where adequate source control can be performed have lower EM.
Evaluar la incidencia y los factores de riesgo de mortalidad precoz (MP) en la UCI en pacientes con shock séptico adquirido en la comunidad (SSAC).
DiseñoEstudio de cohorte retrospectivo de pacientes con SSAC ingresados en la UCI (2003-2016).
LugarLa UCI de un hospital universitario en España.
PacientesTodos los pacientes consecutivos ingresados en la UCI por SSAC.
IntervencionesNinguna.
Principales variables de interésEl SSAC se definió según los criterios de Sepsis-3. Una MP se consideró la que ocurría dentro de las 72h posteriores a la admisión en la UCI. Se realizó un análisis de regresión logística multinomial para identificar los factores de riesgo asociados con MP.
ResultadosDurante el período de estudio, 625 pacientes cumplieron los criterios de Sepsis-3 e ingresaron por SSAC. El 14,4% de los pacientes fallecieron en las primeras 72h. De los 161 pacientes que fallecieron en la UCI, 90 (55,9%) lo hicieron dentro de las primeras 72h. El porcentaje de mortalidad precoz y tardía no varió significativamente durante el período de estudio. La necesidad y la eficacia del control del foco de sepsis fueron significativamente menores en los pacientes con MP. En el análisis multivariado, el SDRA, las infecciones no respiratorias, la bacteriemia y la gravedad al ingreso fueron variables asociadas independientemente con una MP. El único factor que disminuyó la MP fue un control del foco de sepsis adecuado en aquellos pacientes con infecciones susceptibles de precisar un control del foco.
ConclusionesLa incidencia de MP se ha mantenido estable en el tiempo, lo que significa que más de la mitad de los pacientes que mueren por SSAC lo hacen dentro de las primeras 72h. Las infecciones donde se puede realizar un control adecuado del foco tienen una MP inferior.
Septic shock is the most severe form of sepsis and occurs as a result of both community-acquired and health care-associated infections. Pneumonia, followed by intraabdominal and urinary tract infections are the most common causes.1–4
Despite major technological advances, mortality of septic shock remains high, ranging from 20 to 50%.5–7 However, recent reports have shown decreases in mortality, associated to advances in training, better surveillance and monitoring, and prompt initiation of therapy to treat the underlying infection and support failing organs.8–12
Most studies in patients with septic shock have analyzed ICU and/or hospital mortality, but few studies have evaluated the time in which these patients die.13–15 The most recent study to analyze the time of death of patients with septic shock found that 32% of patients with septic shock died within 72h of admission to the ICU, and that these early deaths were mainly attributable to intractable multiple organ failure.13
The main objective of the present study was to estimate the annual incidence rates of mortality within 72h after ICU admission of community-acquired septic shock (CASS) through a secondary analysis of recently published data from a 14-year period.12 Secondary objectives included characterizing these patients and identifying risk factors associated with early mortality (EM) with the aim of improving survival in these patients.
Material and methodsStudy populationThis is a secondary analysis of a retrospective observational cohort of consecutive adult patients (aged>18 y) admitted to the ICU at a university hospital in Spain between January 1, 2003 and December 31, 2016 with a first-time diagnosis of CASS and no other obvious cause of shock.12 The hospital's ethics committee approved the study and waived the need for informed consent due to the observational nature of the study (Ethics Committee Corporació Sanitaria Universitaria Parc Taulí, 2018/610).
Data abstraction and definitionsTrained research physicians used a standardized, piloted data form to collect data and select patients from a prospectively registered data base of all patients admitted to the ICU with community-acquired infection during the study period. Two investigators (JV and ED) with experience in infectious diseases were responsible for maintaining the database and ensuring the application of homogeneous diagnostic criteria during the study period. Septic shock was defined according to the Sepsis-3 definitions as the presence of hypotension requiring vasopressor therapy to maintain mean blood pressure≥65mmHg during≥48h, SOFA score>2 at admission to the ICU, and serum lactate>2mmol/L after adequate fluid resuscitation.16 Patients diagnosed with sepsis but who did not meet the diagnostic requirements for septic shock according to the Sepsis-3 definitions were not included in the definitive database.
Variables collected included year of admission for septic shock, patient demographics (age, sex), and baseline comorbidities (VIH/AIDS, cancer, cirrhosis, chronic heart failure (≥ NYHA-II), chronic obstructive pulmonary disease (COPD), chronic renal failure requiring dialysis, diabetes mellitus, alcohol abuse, and immunodepression), diagnosed according to criteria reported elsewhere.12,17 Pathogens isolated from the local site and/or blood cultures within 48h of ICU admission were considered potential causes of septic shock and were classified as negative culture, gram-positive bacteria, gram-negative bacteria, anaerobes, fungi or other (Influenza, Pneumocystis jirovecii, Mycobacterium tuberculosis). We also recorded the presence of bacteremia and fungemia and the appropriateness of antimicrobial agents. Initial empirical antibiotic treatment was defined as appropriate if an antibiotic prescribed within 24h of the first encounter with the patient matched the in vitro susceptibility of a pathogen deemed to be the likely cause of infection. For episodes of septic shock with negative culture infections, the appropriateness of antibiotic therapy was not analyzed. Treatment decisions, including antibiotic prescriptions, were taken by attending physicians following protocols derived from locally adapted guidelines.
Based on clinical diagnosis and/or isolation of pathogens, the anatomic focus of infection considered the source of sepsis was classified as respiratory, intraabdominal (peritonitis, abscess, small-bowel obstruction, spontaneous bacterial peritonitis, Clostridium difficile-associated colitis, perforated viscus, enterocolitis or diverticulitis, ischemic bowel, pancreatitis, or other), biliary (cholecystitis or cholangitis), genitourinary (pyelonephritis, obstructive uropathy-associated urinary tract infection, or gynecologic infections), skin or soft-tissue (cellulitis, abscess, or necrotizing fasciitis), or other.
In episodes where a source control was susceptible, attending physicians decided on the most appropriate type: noninvasive (percutaneous/endoscopic) or invasive (surgical/laparoscopic). We recorded the time from hospital admission to source control and divided patients into those who received source control ≤12h, which is the upper limit of the time recommended in the latest guidelines from the Surviving Sepsis Campaign18 and those who received source control>12h following onset. Adequate source control was defined as having a percutaneous, endoscopic, laparoscopic, or open procedure to drain infected fluid collections, debride infected tissues, and control ongoing enteric or other drainage producing peritonitis and if patients improved after the intervention (disappearance of fever, normalization of white blood counts, improvement of acute phase reactants, and a decrease in doses of vasopressors required) and required no further interventions on the same source during the ICU stay. In cases where these variables could not be reviewed, the effectiveness of source control was not evaluated.
We also recorded the use of adjunctive therapies such as mechanical ventilation and renal replacement techniques as well as complications detected after admission to ICU that could be related to the natural history and/or management of septic shock such as acute kidney injury (defined as an increase in serum creatinine≥0.3mg/dL within 48h19), thrombocytopenia (defined as platelets<150,000/μL20), and acute respiratory distress syndrome (ARDS) (defined as PaO2/FiO2 <300mmHg with bilateral thoracic infiltrates in the absence of heart failure21).
Patients’ severity at admission was recorded as APACHE II22 score during the first 24h after ICU admission and SOFA score20 at admission to the ICU. Patients were followed up until death or discharge from the ICU and from the hospital.
Based on the criteria used in a previous study in critically ill patients with septic shock,13 we defined deaths occurring within 72h after ICU admission as early and those occurring after this timepoint as late. Patients who received limited therapeutic effort during their stay in the ICU were excluded from the study.
Statistical analysisContinuous variables are expressed as means±standard deviations and categorical variables as frequencies and percentages. To compare the characteristics of patients who died early (≤3 days from ICU admission) with those of patients who survived>3 days, we used Student's t-tests for continuous variables and chi-square tests or Fisher's exact tests for categorical variables, as appropriate. Variables associated (p<0.1) with early ICU death in univariate analyses were included in a multivariate logistic models. The final logistic model obtained by a backward elimination procedure contained only statistically significant variables. Values of p<0.05 were considered statistically significant. Commercially available software (IBM® SPSS® Statistics for Windows, Version 25.0. Arkmon, NY) was used for all analyses.
ResultsDuring the study period, 625 patients were admitted to the ICU with CASS. The most common comorbidities were diabetes (29.1%) and cancer (20%), and 24% were immunocompromised. Infection was documented by a positive culture in 92.2% of patients. Blood cultures were positive in 52.5% of the patients. The most common site of infection causing sepsis was the lung (27%). The most frequent organ dysfunction associated with septic shock was acute kidney injury, which occurred in 555 (88.8%) patients, 132 (23.7%) of whom required continuous renal replacement. Thrombocytopenia was present in 390 (62.5%) patients. A total of 410 (65.7%) patients required invasive mechanical ventilation; ARDS was diagnosed in 142 (22.8%) patients.
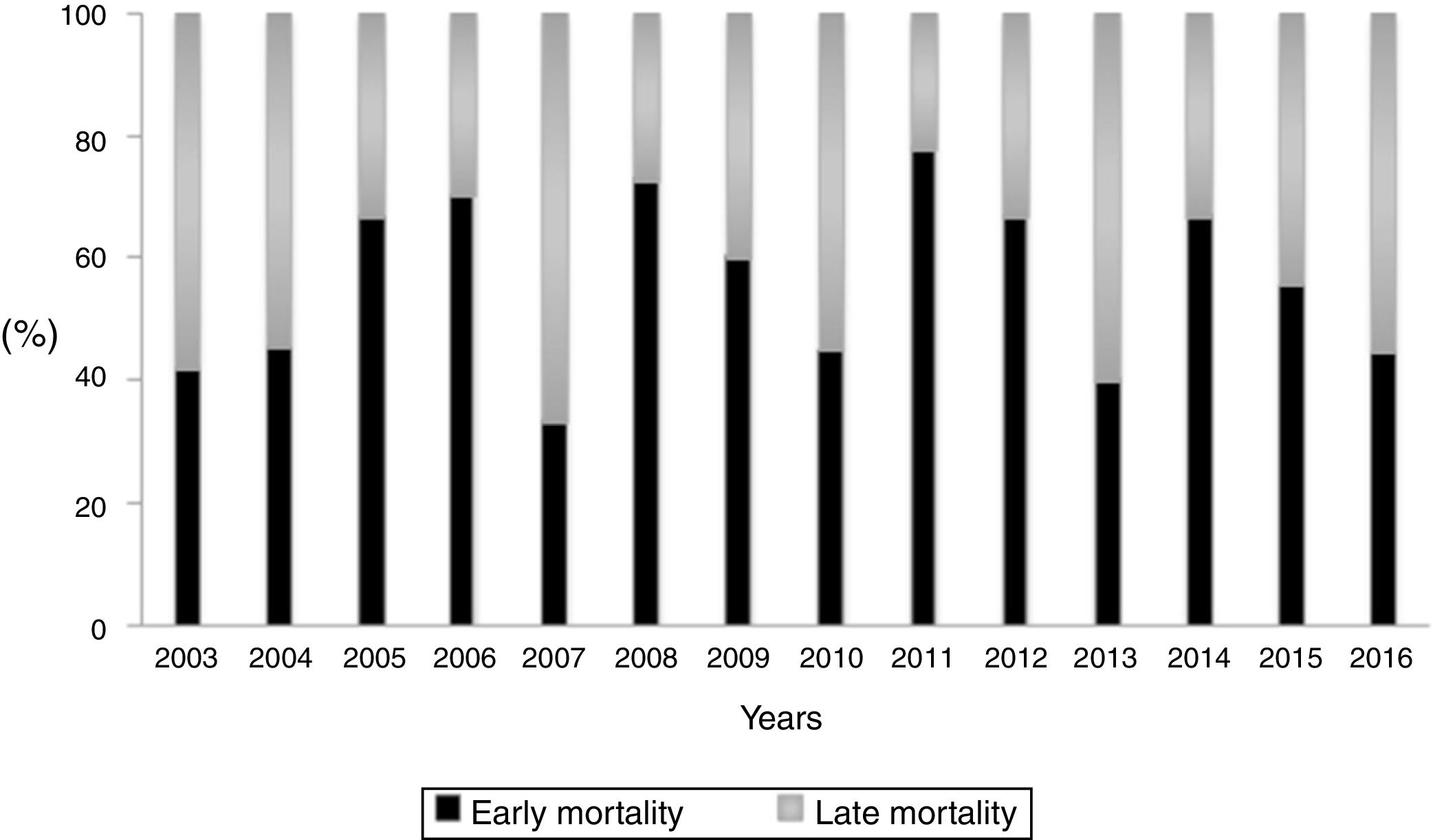
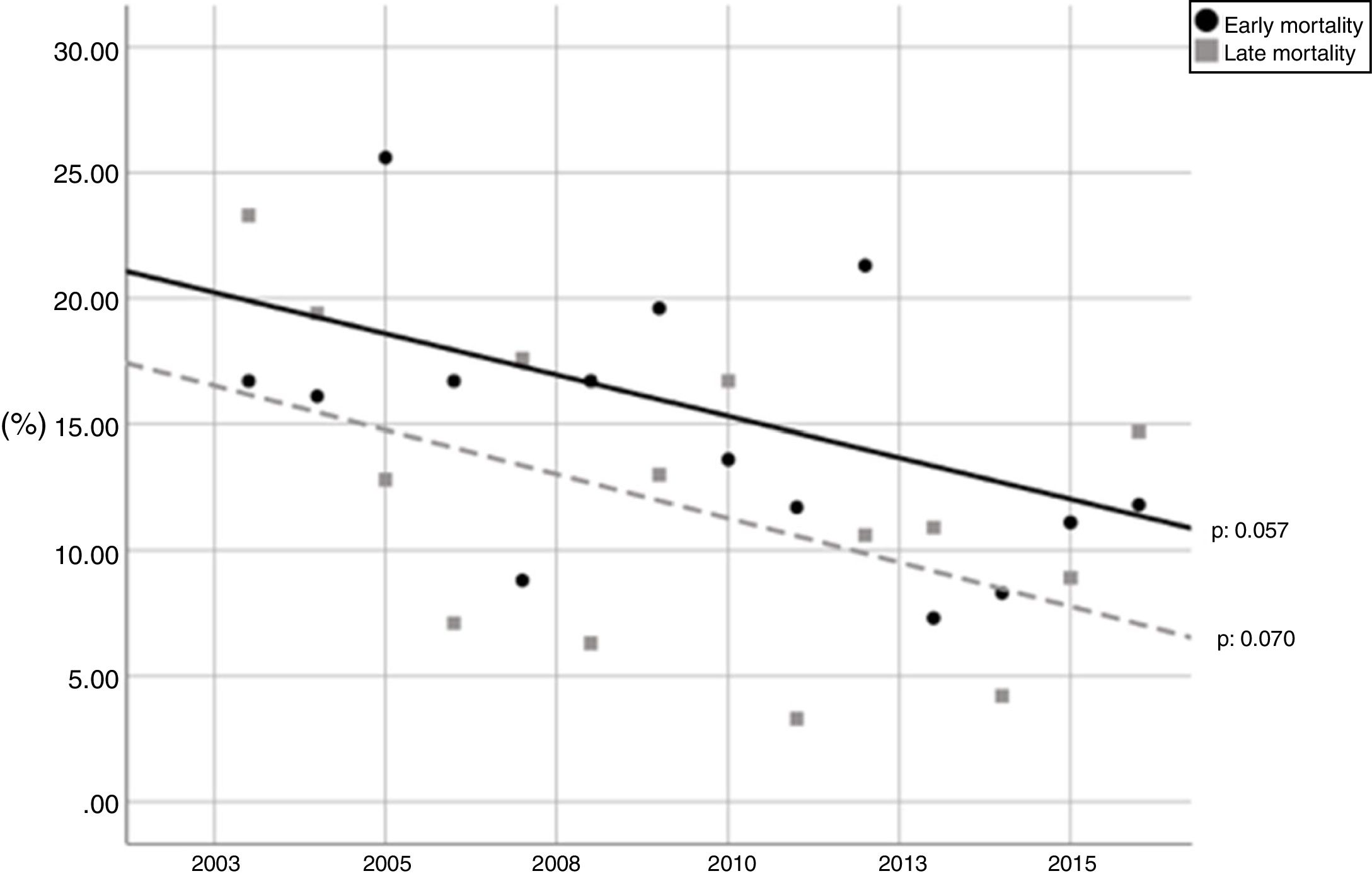
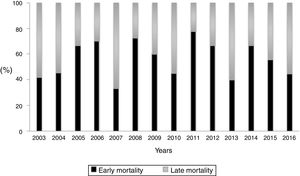
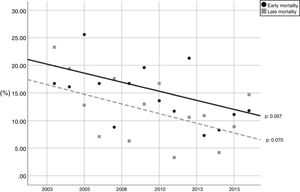
During the study period, the mean mortality related to septic shock was 25.8% in the ICU and 29.6% in the hospital. The mean EM related to septic shock was 14.4%. The percentages of early and late septic shock related deaths did not vary significantly over the years (Fig. 1). There was a trend toward decreased rates of both early and late mortality over the study period (Fig. 2).
A total of 161 patients died in the ICU, 90 (55.9%) within the first 72h of ICU admission; all these deaths were attributed to intractable multiple organ failure related to the primary infection. Of the 90 patients who died within the first 72h, 49 (54.4%) died in the first 24h, 21 (23.3%) in the second 24h, and 20 (22.2%) in the third 24h (p<0.001).
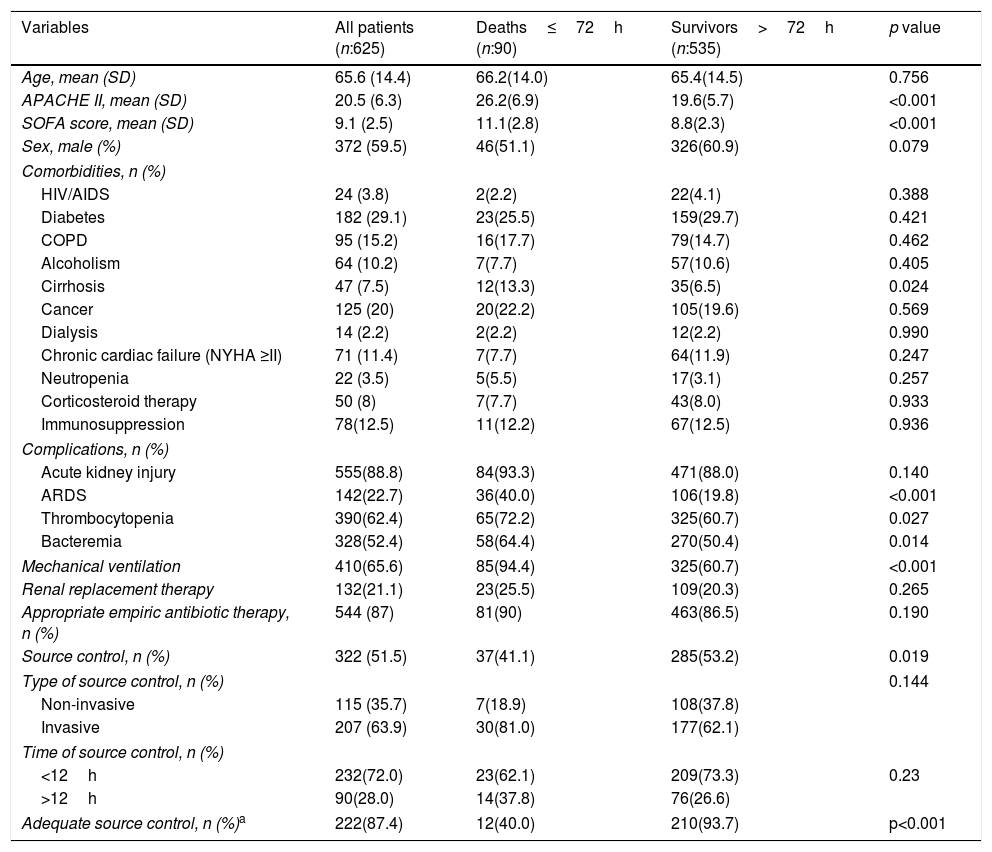
Table 1 compares the characteristics of the patients who died in the first 72h versus those of the patients who survived more than 72h. Apache II and SOFA at admission were higher in patients who died in the first 72h. Among comorbidities, only cirrhosis was significantly more frequent in the EM group. ARDS, thrombocytopenia, mechanical ventilation, and bacteremia were also more predominant in EM group.
Characteristics of patients with early mortality and survivors more than 72h.
| Variables | All patients (n:625) | Deaths≤72h (n:90) | Survivors>72h (n:535) | p value |
|---|---|---|---|---|
| Age, mean (SD) | 65.6 (14.4) | 66.2(14.0) | 65.4(14.5) | 0.756 |
| APACHE II, mean (SD) | 20.5 (6.3) | 26.2(6.9) | 19.6(5.7) | <0.001 |
| SOFA score, mean (SD) | 9.1 (2.5) | 11.1(2.8) | 8.8(2.3) | <0.001 |
| Sex, male (%) | 372 (59.5) | 46(51.1) | 326(60.9) | 0.079 |
| Comorbidities, n (%) | ||||
| HIV/AIDS | 24 (3.8) | 2(2.2) | 22(4.1) | 0.388 |
| Diabetes | 182 (29.1) | 23(25.5) | 159(29.7) | 0.421 |
| COPD | 95 (15.2) | 16(17.7) | 79(14.7) | 0.462 |
| Alcoholism | 64 (10.2) | 7(7.7) | 57(10.6) | 0.405 |
| Cirrhosis | 47 (7.5) | 12(13.3) | 35(6.5) | 0.024 |
| Cancer | 125 (20) | 20(22.2) | 105(19.6) | 0.569 |
| Dialysis | 14 (2.2) | 2(2.2) | 12(2.2) | 0.990 |
| Chronic cardiac failure (NYHA ≥II) | 71 (11.4) | 7(7.7) | 64(11.9) | 0.247 |
| Neutropenia | 22 (3.5) | 5(5.5) | 17(3.1) | 0.257 |
| Corticosteroid therapy | 50 (8) | 7(7.7) | 43(8.0) | 0.933 |
| Immunosuppression | 78(12.5) | 11(12.2) | 67(12.5) | 0.936 |
| Complications, n (%) | ||||
| Acute kidney injury | 555(88.8) | 84(93.3) | 471(88.0) | 0.140 |
| ARDS | 142(22.7) | 36(40.0) | 106(19.8) | <0.001 |
| Thrombocytopenia | 390(62.4) | 65(72.2) | 325(60.7) | 0.027 |
| Bacteremia | 328(52.4) | 58(64.4) | 270(50.4) | 0.014 |
| Mechanical ventilation | 410(65.6) | 85(94.4) | 325(60.7) | <0.001 |
| Renal replacement therapy | 132(21.1) | 23(25.5) | 109(20.3) | 0.265 |
| Appropriate empiric antibiotic therapy, n (%) | 544 (87) | 81(90) | 463(86.5) | 0.190 |
| Source control, n (%) | 322 (51.5) | 37(41.1) | 285(53.2) | 0.019 |
| Type of source control, n (%) | 0.144 | |||
| Non-invasive | 115 (35.7) | 7(18.9) | 108(37.8) | |
| Invasive | 207 (63.9) | 30(81.0) | 177(62.1) | |
| Time of source control, n (%) | ||||
| <12h | 232(72.0) | 23(62.1) | 209(73.3) | 0.23 |
| >12h | 90(28.0) | 14(37.8) | 76(26.6) | |
| Adequate source control, n (%)a | 222(87.4) | 12(40.0) | 210(93.7) | p<0.001 |
254 patients who source control could be evaluated.
SD=standard deviation; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sequential Organ Failure Assessment; HIV=human immunodeficiency virus; AIDS=acquired immune deficiency syndrome; NYHA=New York Heart Association; ARDS=acute respiratory distress syndrome.
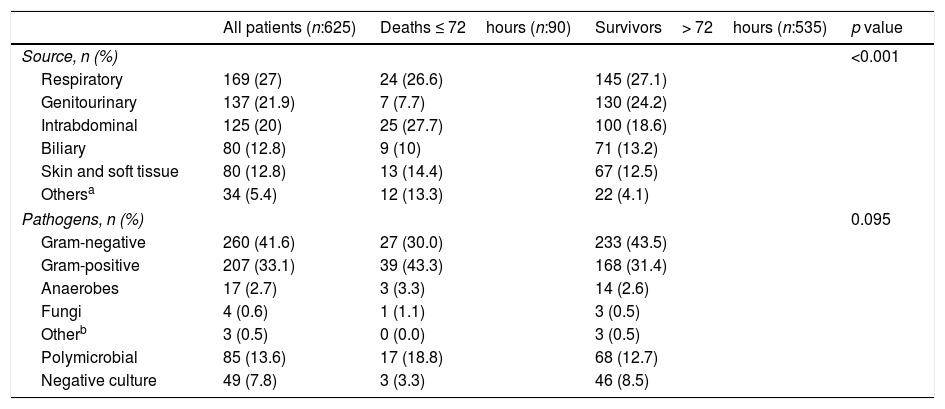
Table 2 reports the anatomic sites of infection considered the source of sepsis and microbiology findings in the two groups. The sources of infection differed significantly between the groups and the proportion of patients who underwent source control was significantly lower in the EM group (41% vs. 53.2% in the group of patients that survived>72h, p=0.019). Intraabdominal infections, skin and soft-tissue infections, and other sources that did not fit into the main predefined categories (mainly, endocarditis, meningitis, and unknown origin) were more common in the EM group, and the incidence of biliary and genitourinary infections was lower in this group. We observed no significant differences between the two groups in the pathogens responsible for the infections. There was no difference in the rate of appropriate antibiotic treatment between the two groups.
Source of infection and pathogens isolated in patients with early mortality and survivors more than 72hours.
| All patients (n:625) | Deaths ≤ 72hours (n:90) | Survivors> 72hours (n:535) | p value | |
|---|---|---|---|---|
| Source, n (%) | <0.001 | |||
| Respiratory | 169 (27) | 24 (26.6) | 145 (27.1) | |
| Genitourinary | 137 (21.9) | 7 (7.7) | 130 (24.2) | |
| Intrabdominal | 125 (20) | 25 (27.7) | 100 (18.6) | |
| Biliary | 80 (12.8) | 9 (10) | 71 (13.2) | |
| Skin and soft tissue | 80 (12.8) | 13 (14.4) | 67 (12.5) | |
| Othersa | 34 (5.4) | 12 (13.3) | 22 (4.1) | |
| Pathogens, n (%) | 0.095 | |||
| Gram-negative | 260 (41.6) | 27 (30.0) | 233 (43.5) | |
| Gram-positive | 207 (33.1) | 39 (43.3) | 168 (31.4) | |
| Anaerobes | 17 (2.7) | 3 (3.3) | 14 (2.6) | |
| Fungi | 4 (0.6) | 1 (1.1) | 3 (0.5) | |
| Otherb | 3 (0.5) | 0 (0.0) | 3 (0.5) | |
| Polymicrobial | 85 (13.6) | 17 (18.8) | 68 (12.7) | |
| Negative culture | 49 (7.8) | 3 (3.3) | 46 (8.5) | |
Source-control interventions were significantly less common in the EM group [37/90 (41.1%) patients vs. 285/585 (53.2%) patients who survived≥72h, p=0.019]. This difference was mainly due to a lower incidence of biliary and genitourinary tract infections in the EM group (17.7% vs. 37.4%, p<0.001). In the 254 cases in which the adequacy of source control could be evaluated, adequate source control was in a smaller proportion of patients in the EM group [12/30 (40%) vs. 210/224 (93.7%) in patients who survived≥72h, p<0.001]. Moreover, the proportion of patients who received adequate source control for different source categories was significantly lower in the EM group than in the group of patients who survived≥72h: for intraabdominal infections (38.4% vs. 92.3%; p<0.001), for skin and soft-tissue infections (22.2% vs. 80%; p<0.001), and for genitourinary infections (0% vs. 100%; p<0.001), except in biliary infections where all 5 patients in the EM group received effective source control but died nevertheless. The proportion of patients receiving source control within 12h of hospital admission did not differ between groups.
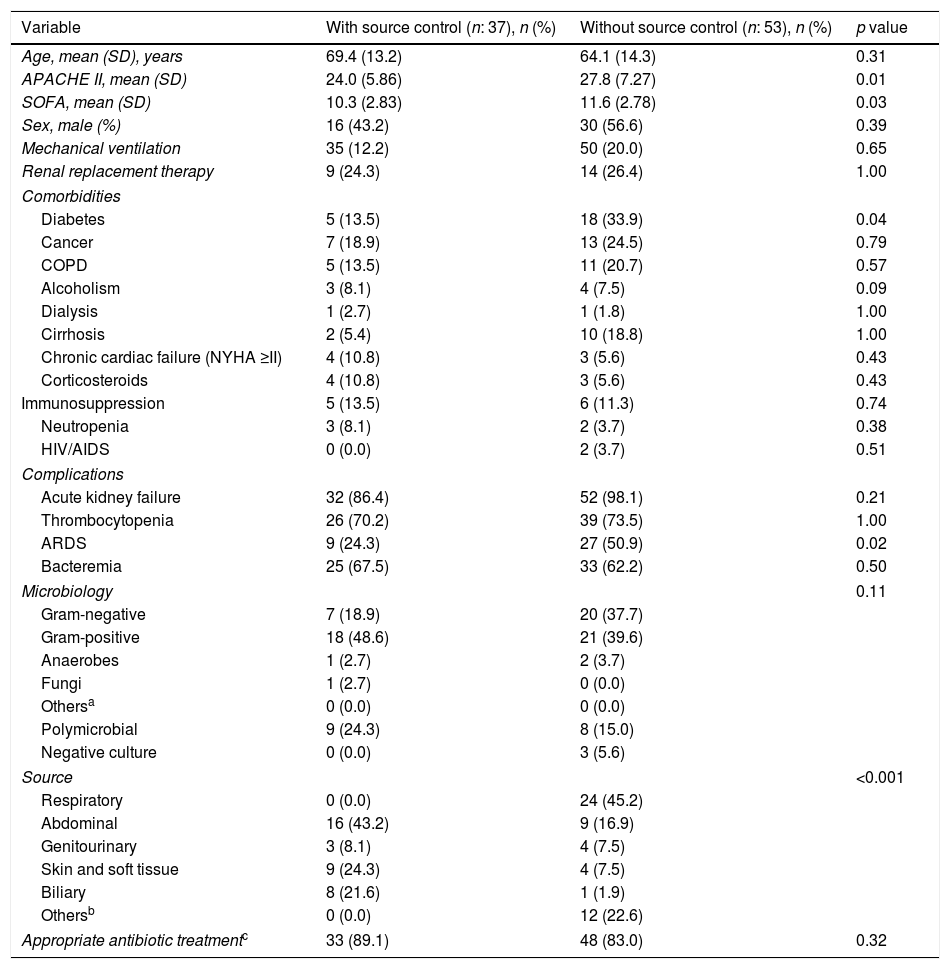
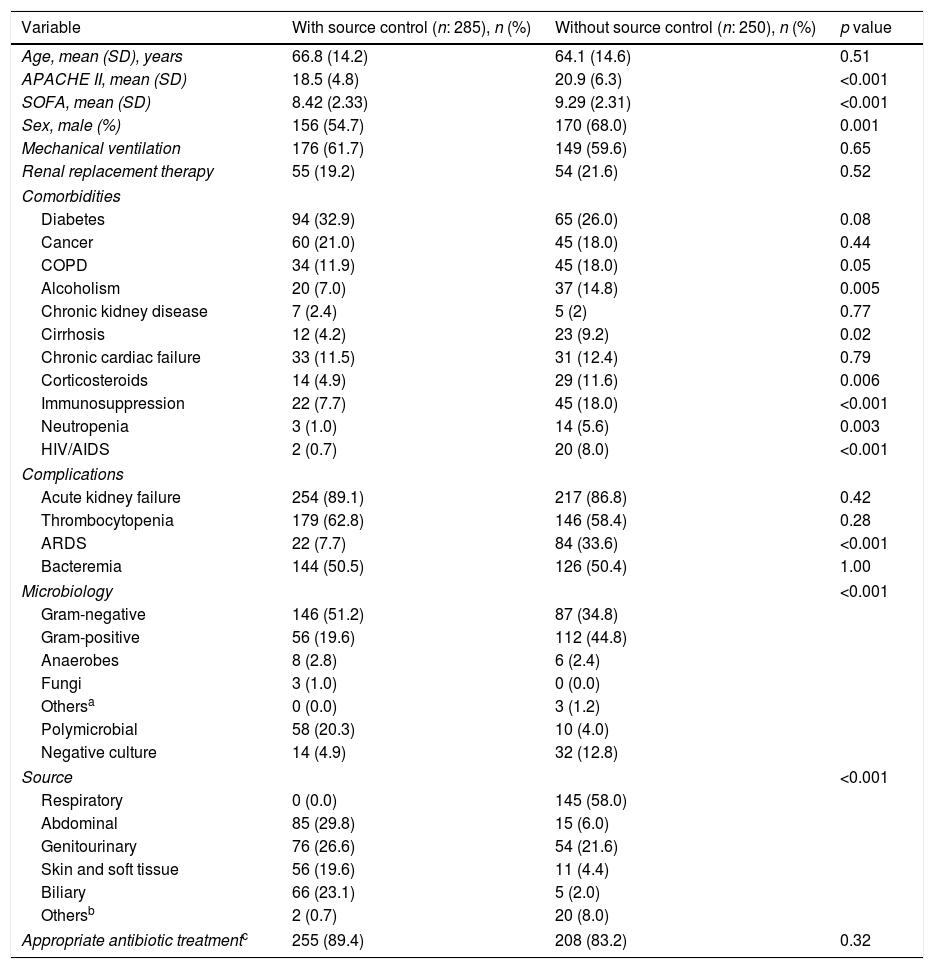
Tables 3 and 4 compare the characteristics of patients with EM and survivors over 72h depending on whether they need a source control or not. Patients without source control have a significantly higher severity on admission, incidence of ARDS and more comorbidities, mainly immunosuppression, alcoholism and cirrhosis. Abdominal, biliary and skin and soft tissue infections are more frequent in the group of patients where a source control was performed, both in patients with EM and in those who survive more than 72h. The opposite occurs with respiratory infections.
Patients with early mortality (≤72h) (n:90).
| Variable | With source control (n: 37), n (%) | Without source control (n: 53), n (%) | p value |
|---|---|---|---|
| Age, mean (SD), years | 69.4 (13.2) | 64.1 (14.3) | 0.31 |
| APACHE II, mean (SD) | 24.0 (5.86) | 27.8 (7.27) | 0.01 |
| SOFA, mean (SD) | 10.3 (2.83) | 11.6 (2.78) | 0.03 |
| Sex, male (%) | 16 (43.2) | 30 (56.6) | 0.39 |
| Mechanical ventilation | 35 (12.2) | 50 (20.0) | 0.65 |
| Renal replacement therapy | 9 (24.3) | 14 (26.4) | 1.00 |
| Comorbidities | |||
| Diabetes | 5 (13.5) | 18 (33.9) | 0.04 |
| Cancer | 7 (18.9) | 13 (24.5) | 0.79 |
| COPD | 5 (13.5) | 11 (20.7) | 0.57 |
| Alcoholism | 3 (8.1) | 4 (7.5) | 0.09 |
| Dialysis | 1 (2.7) | 1 (1.8) | 1.00 |
| Cirrhosis | 2 (5.4) | 10 (18.8) | 1.00 |
| Chronic cardiac failure (NYHA ≥II) | 4 (10.8) | 3 (5.6) | 0.43 |
| Corticosteroids | 4 (10.8) | 3 (5.6) | 0.43 |
| Immunosuppression | 5 (13.5) | 6 (11.3) | 0.74 |
| Neutropenia | 3 (8.1) | 2 (3.7) | 0.38 |
| HIV/AIDS | 0 (0.0) | 2 (3.7) | 0.51 |
| Complications | |||
| Acute kidney failure | 32 (86.4) | 52 (98.1) | 0.21 |
| Thrombocytopenia | 26 (70.2) | 39 (73.5) | 1.00 |
| ARDS | 9 (24.3) | 27 (50.9) | 0.02 |
| Bacteremia | 25 (67.5) | 33 (62.2) | 0.50 |
| Microbiology | 0.11 | ||
| Gram-negative | 7 (18.9) | 20 (37.7) | |
| Gram-positive | 18 (48.6) | 21 (39.6) | |
| Anaerobes | 1 (2.7) | 2 (3.7) | |
| Fungi | 1 (2.7) | 0 (0.0) | |
| Othersa | 0 (0.0) | 0 (0.0) | |
| Polymicrobial | 9 (24.3) | 8 (15.0) | |
| Negative culture | 0 (0.0) | 3 (5.6) | |
| Source | <0.001 | ||
| Respiratory | 0 (0.0) | 24 (45.2) | |
| Abdominal | 16 (43.2) | 9 (16.9) | |
| Genitourinary | 3 (8.1) | 4 (7.5) | |
| Skin and soft tissue | 9 (24.3) | 4 (7.5) | |
| Biliary | 8 (21.6) | 1 (1.9) | |
| Othersb | 0 (0.0) | 12 (22.6) | |
| Appropriate antibiotic treatmentc | 33 (89.1) | 48 (83.0) | 0.32 |
SD=standard deviation; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sequential Organ Failure Assessment; HIV=human immunodeficiency virus; AIDS=acquired immune deficiency syndrome; NYHA=New York Heart Association; ARDS=acute respiratory distress syndrome.
Patients survivors>72h (n:535).
| Variable | With source control (n: 285), n (%) | Without source control (n: 250), n (%) | p value |
|---|---|---|---|
| Age, mean (SD), years | 66.8 (14.2) | 64.1 (14.6) | 0.51 |
| APACHE II, mean (SD) | 18.5 (4.8) | 20.9 (6.3) | <0.001 |
| SOFA, mean (SD) | 8.42 (2.33) | 9.29 (2.31) | <0.001 |
| Sex, male (%) | 156 (54.7) | 170 (68.0) | 0.001 |
| Mechanical ventilation | 176 (61.7) | 149 (59.6) | 0.65 |
| Renal replacement therapy | 55 (19.2) | 54 (21.6) | 0.52 |
| Comorbidities | |||
| Diabetes | 94 (32.9) | 65 (26.0) | 0.08 |
| Cancer | 60 (21.0) | 45 (18.0) | 0.44 |
| COPD | 34 (11.9) | 45 (18.0) | 0.05 |
| Alcoholism | 20 (7.0) | 37 (14.8) | 0.005 |
| Chronic kidney disease | 7 (2.4) | 5 (2) | 0.77 |
| Cirrhosis | 12 (4.2) | 23 (9.2) | 0.02 |
| Chronic cardiac failure | 33 (11.5) | 31 (12.4) | 0.79 |
| Corticosteroids | 14 (4.9) | 29 (11.6) | 0.006 |
| Immunosuppression | 22 (7.7) | 45 (18.0) | <0.001 |
| Neutropenia | 3 (1.0) | 14 (5.6) | 0.003 |
| HIV/AIDS | 2 (0.7) | 20 (8.0) | <0.001 |
| Complications | |||
| Acute kidney failure | 254 (89.1) | 217 (86.8) | 0.42 |
| Thrombocytopenia | 179 (62.8) | 146 (58.4) | 0.28 |
| ARDS | 22 (7.7) | 84 (33.6) | <0.001 |
| Bacteremia | 144 (50.5) | 126 (50.4) | 1.00 |
| Microbiology | <0.001 | ||
| Gram-negative | 146 (51.2) | 87 (34.8) | |
| Gram-positive | 56 (19.6) | 112 (44.8) | |
| Anaerobes | 8 (2.8) | 6 (2.4) | |
| Fungi | 3 (1.0) | 0 (0.0) | |
| Othersa | 0 (0.0) | 3 (1.2) | |
| Polymicrobial | 58 (20.3) | 10 (4.0) | |
| Negative culture | 14 (4.9) | 32 (12.8) | |
| Source | <0.001 | ||
| Respiratory | 0 (0.0) | 145 (58.0) | |
| Abdominal | 85 (29.8) | 15 (6.0) | |
| Genitourinary | 76 (26.6) | 54 (21.6) | |
| Skin and soft tissue | 56 (19.6) | 11 (4.4) | |
| Biliary | 66 (23.1) | 5 (2.0) | |
| Othersb | 2 (0.7) | 20 (8.0) | |
| Appropriate antibiotic treatmentc | 255 (89.4) | 208 (83.2) | 0.32 |
SD=standard deviation; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sequential Organ Failure Assessment; HIV=human immunodeficiency virus; AIDS=acquired immune deficiency syndrome; NYHA=New York Heart Association; ARDS=acute respiratory distress syndrome.
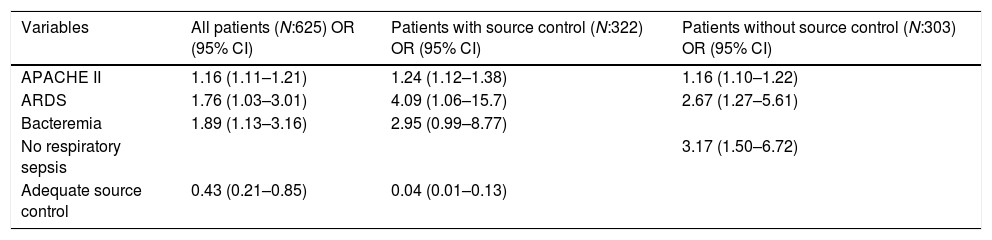
Table 5 reports variables significantly associated with EM mortality in the multivariate analyses. Variables independently associated with worse prognosis in the first 72h of ICU admission adjusted for age, comorbidities, and severity at ICU admission were APACHE II, ARDS, bacteremia, and effective source control was independently associated with better survival.
Multivariate analysis for independent factors associated with death within 72h of ICU admission.
| Variables | All patients (N:625) OR (95% CI) | Patients with source control (N:322) OR (95% CI) | Patients without source control (N:303) OR (95% CI) |
|---|---|---|---|
| APACHE II | 1.16 (1.11–1.21) | 1.24 (1.12–1.38) | 1.16 (1.10–1.22) |
| ARDS | 1.76 (1.03–3.01) | 4.09 (1.06–15.7) | 2.67 (1.27–5.61) |
| Bacteremia | 1.89 (1.13–3.16) | 2.95 (0.99–8.77) | |
| No respiratory sepsis | 3.17 (1.50–6.72) | ||
| Adequate source control | 0.43 (0.21–0.85) | 0.04 (0.01–0.13) |
We repeated a multivariate analysis in the group of patients without SC, and the APACHE II, ARDS and origin of sepsis other than respiratory source were the variables independently associated with an early poor prognosis, while the multivariate analysis in the group of patients susceptible to source control, showed that ARDS, bacteremia, and APACHE II were the variables associated with EM. However, adequate source control was associated with a better survival rate in the first 72h (Table 5).
DiscussionDespite the trend toward reduced ICU mortality in patients with CASS during the period observed by the present study, half of all the patients who die in the ICU due to septic shock continue to die within the first 72h of admission and this has remained stable over time. Patients with infections of non-respiratory origin which are not susceptible to source control or those where source control is necessary but inadequate, have a higher probability of EM secondary to CASS.
Overall, 14.4% of all patients admitted with CASS died during the first 72h (more than half of these died in the first 24h). Patients who died within the first 72h accounted for 55.9% of all deaths due to CASS in the ICU. The only factor that could be modified to decrease EM was adequate source control in the subgroup of patients with infections amenable to source control.
In the 1990s, a French multicenter study found that 27% of patients with documented severe sepsis or septic shock died within 48h of onset of severe sepsis.14 Ever since, the few studies that have analyzed the time of death of patients with severe sepsis or septic shock have reported lower EM rates similar to the 14.4% found in our study. In 2008, Spanish researchers published the results of a multicenter observational study of cohort including both severe sepsis and septic shock patients in two three-month periods in 2002, reporting that 19.3% had died within 72h after onset of severe sepsis.15 Ten years later, in a new multicenter study to analyze the influence of Surviving Sepsis Campaign, the Spanish group found mortality in the first 48h had decreased from 14.8% to 7%.23 In a French multicenter study in 2004 (EPISEPSIS)24 analyzing the epidemiology and prognosis of severe sepsis, mortality within 72h after diagnosis was 13.3%. In an observational study in 2015 analyzing the timing and causes of death in patients with septic shock, Daviaud et al.13 observed a 72-hour mortality rate of 14.3%, and multiple organ failure accounted for most early deaths. More recently, another French study analyzing the dose of vasopressors and the risk of early mortality in 370 patients with septic shock reported 13% mortality within 72h following vasopressor initiation.25
The present study analyzed early mortality over a 14-year period in which the early identification and treatment of patients with septic shock have improved.18,26 Despite these improvements, the proportion of deaths directly attributable to septic shock and progressive multiorgan failure occurring within 72h of ICU admission remained stable over time. Moreover, 54.4% of early deaths occurred during the first 24h, suggesting possible delays in identifying patients with septic shock and admitting them to the ICU, fulminant sepsis or inability to perform proper source control which renders therapeutic efforts ineffective. Unfortunately, we have no data about the duration of symptoms before patients reach the hospital.
Although the percentage of patients who die early in our study is similar to other published studies, the distribution of patients dying between early and late is different. Indeed, in our study, 90 of the 166 patients who died in the ICU (55.9%) died early. In contrast, in the Daviaud study13 that analyzes the time that patients die in septic shock, they find only a 32% of early deaths. In other older studies, death within 48–72h accounted for 27%17 and 40%15 of ICU deaths secondary to septic shock. These differences could be partially explained by differences in the study populations. Considering the difficulty of applying the definitions of Sepsis-3 in a retrospective study and the possible biases in the analysis that this entails, the present study only included patients with community-acquired infections if they fulfilled the criteria outlined in the Sepsis-3 definitions.16 Other studies14,15 have included patients with both community-acquired and healthcare-associated infections as well as some patients who had severe sepsis without septic shock and used other criteria for a septic shock diagnosis. Although our study and Daviud et al.’s study13 both analyzed only patients with septic shock, there are other important differences between populations. Whereas all of our patients had community-acquired infections, more than half the patients in their study had healthcare-associated infections, and the incidence of bacteremia in their early mortality group (20%) was much lower than in ours (64.4%). Bruin-Buisson et al.14 found that bacteremia was associated with an increased risk of early mortality in patients with severe sepsis and septic shock, and our multivariate analysis of risk factors found that bacteremia was independently associated with EM, corroborating these findings.
On the other hand, the incidence of cirrhosis in the group of patients with EM in our study was higher than in those who survived for more than 72hours (13.3% vs. 6.5%, p=0.024); of these, 41% had septic shock of abdominal origin, 58.3% had bacteremia, and 91% had acute kidney injury, all of which are associated with high mortality. Although the prognosis of patients with septic shock and cirrhosis has improved in recent years,27 our results show that cirrhosis continues to increase the risk of poor outcome in patients with septic shock.
An important finding in our study was that the proportion of patients who underwent source control was significantly lower in the EM group (41% vs. 53.2% in the group of patients that survived>72h, p=0.019). This difference is partly explained by the lower frequency of urinary and biliary tract infections in the group with EM which usually require source control. These results would confirm that septic shock due to infections not amenable to source control is associated with poor prognosis.28 On the other hand, although delaying source control increases mortality,29 we found no significant difference in the percentage of patients undergoing early (<12h) source control between the EM group (62.1%) and the group who survived more than 72h (73.3%).
It is important to note not only that the EM group had a lower proportion of patients who needed source control, but also that source control was inadquate in 60% of those who underwent source control interventions, mainly in urinary, abdominal and skin and soft tissue infections. In the multivariate analysis of risk factors for EM, adequate source control was an independent protective factor among the patients with infections amenable to source control, while in the group that did not require source control, non-respiratory infections (73% of cases) were associated with higher EM. These results support Kumar et al.’s30 theory that the total microbial load is what drives shock in septic shock, so reducing the microbial load early through effective source control and appropriate antimicrobial treatment is crucial for survival. Another study that analyzed autopsy findings in surgical patients who died of sepsis/septic shock found a persistent septic focus in approximately 75%,31 indicating that ineffective measures to eliminate the infection decrease the chances of survival.
Finally, among the organ failures associated with septic shock, ARDS was more common in patients who died early (40% vs. 19.8% in the group of survivors over 72h, p<0.001); furthermore, in the multivariate analysis ARDS was independently associated with increased risk of dying early. Zilberberg et al.32 reported that sepsis as the origin of ARDS was independently associated with worse prognosis. The greater proportion of patients with inadequate source control in the EM group could also explain the higher incidence of ARDS in this group. In an experimental model of peritonitis in rabbits, Matute-Bello G et al.33 showed that the ability to contain the infection at the primary site appear to be an important factor affecting the severity of septic shock and distant organ injury. Inadequate source control and failure to reduce the bacterial load at the site of infection could result in a more severe systemic response that could explain the refractoriness of septic shock and the persistency of lung injury.
This study has several limitations. It was a retrospective study, done at a single center, so the results cannot be extrapolated to other populations in hospitals with different characteristics. Moreover, although we adjusted for a number of predisposing patient factors, there may be other confounding factors that we did not measure. We analyzed only clinical and microbiological data, and we cannot rule out the possibility that other variables such as lactate level or lactate clearance might have affected our results. Furthermore, although nearly 90% of patients in both groups received appropriate empirical antibiotic treatment, we could not assess the time to initiation of antibiotic treatment, so we cannot rule out the possibility of greater delays in the early mortality group. Unfortunately, we have no data about the duration of symptoms before patients reach the hospital. We must also consider the definition of the adequacy of source control used in our study, which may lead to bias in the interpretation of the results. However, it is necessary to consider the difficulty of evaluating the response to source control in critically ill patients, as has been previously stated by other authors.34,35 However, our study also has its strengths. The data come from a homogeneous database collected over a long period of time with strict quality-control measures to ensure validity; thus, we were able to analyze a large uniform cohort of patients with septic shock according to Sepsis-3 definitions who were monitored until death or hospital discharge.
ConclusionsOur study confirms a trend over time in decreasing the ICU-mortality of patients admitted to the ICU with septic shock. However, mortality within the first 72h occurs in more than half of all patients who die in the ICU, mainly in those patients with non-respiratory infections who do not need source control or in patients with infections amenable to source control but this is inadequate.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionJordi Vallès, responsible for the design of the study, review of patient data and preparation of the article.
Andrey Rodriguez, Jaume Mestre, Consuelo Guía and Ana Navas, contributed to the acquisition of patient data included in the study.
Emili Diaz, Ana Ochagavia, Melcior Martinez, Guillem Gruartmoner, Candelaria de Haro and Jaume Mesquida review and elaboration of the document.
Joan Carles Oliva, review and statistical analysis of the study.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors confirm that they have no conflicts of interest in the elaboration of this article.
We thank Ana Villagrá MD for reviewing the manuscript.