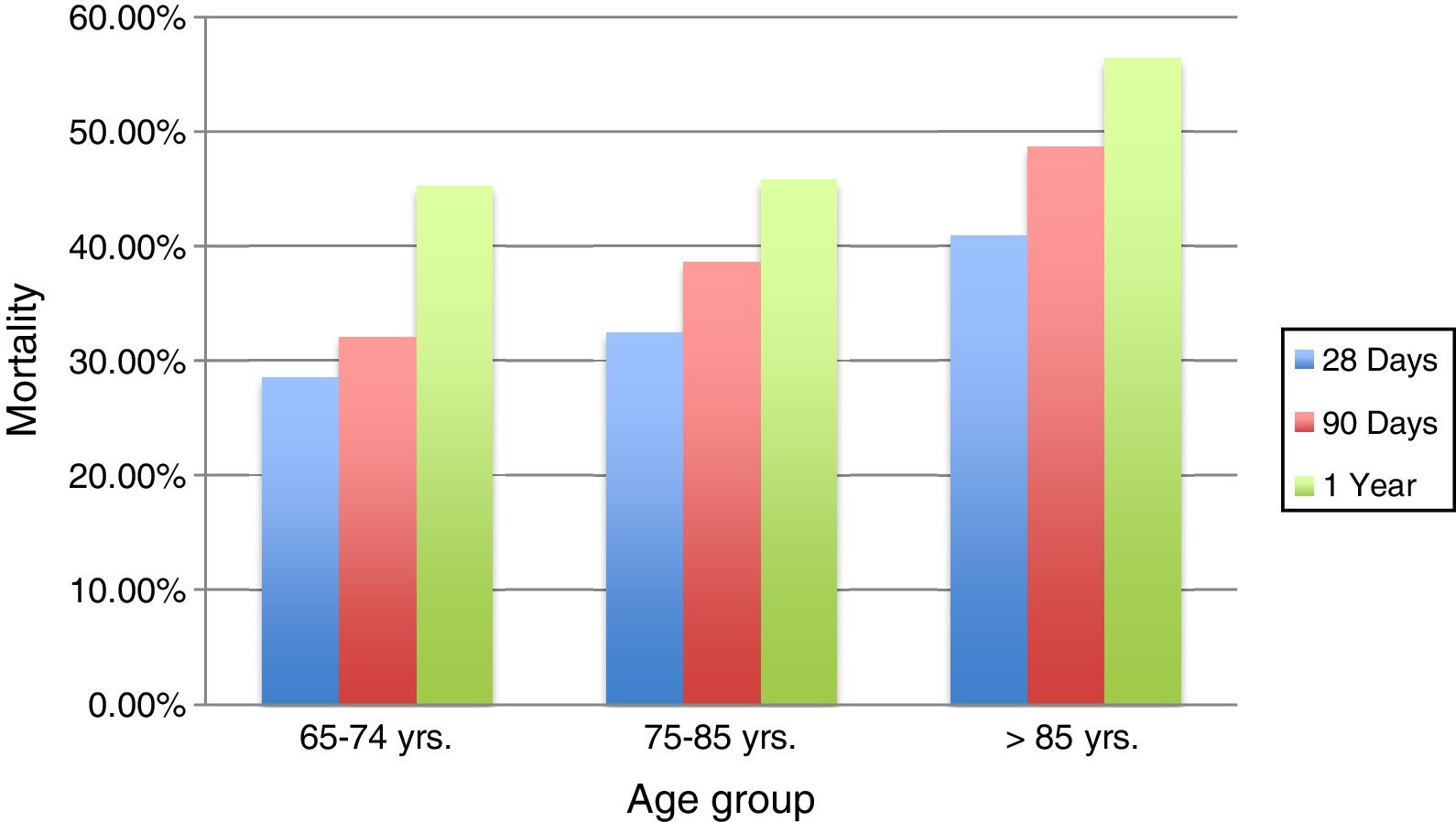To determine the differences in short- and long-term mortality in elderly septic patients with multiorgan dysfunction syndrome and establish the factors related to non-survival.
Materials and methodsA retrospective cohort study was made of 206 patients over 65 years of age with septic and septic shock criteria admitted to the ICU of Rio Hortega Hospital between January 2011 and February 2017. Study variables were obtained from electronic database records.
ResultsA total of 206 patients were included, divided into three groups of age (65–74, 75–85, >85 years). There were no significant differences in mortality according to age group after 28 days, 90 days or one year (28.6%, 32.1% and 45.2% in the 65–74 years age group; 32.5%, 38.6% and 45.8% in the 75–85 years age group, 41%, 48.7% and 56.4% in the >85 years age group). The factors related to mortality were: chronic heart failure, non-haematological cancer, liver dysfunction and central nervous system dysfunction.
ConclusionsThe results indicate that there is no significant difference in mortality among the different age groups. About 50% of the elderly patients survive a septic process. There is a close relationship between the number of affected organs and days of dysfunction, the use of interventional techniques and long-term mortality.
Determinar las diferencias en la mortalidad a corto y largo plazo en pacientes sépticos ancianos con síndrome de disfunción multiorgánica, y cuáles son los factores de riesgo determinantes de esta.
Materiales y métodosEstudio retrospectivo de cohortes de 206 pacientes mayores de 65 años con criterios de shock séptico y sepsis admitidos en la UCI del Hospital Río Hortega entre enero de 2011 y febrero de 2017. Los datos analizados se obtuvieron a través de los registros de bases de datos electrónicas de la unidad.
ResultadosSe incluyeron 206 pacientes divididos en 3 grupos de edad (65-74; 75-85; >85). No encontramos diferencias estadísticas en la mortalidad por grupo de edad al cabo de 28 días, 90 días y un año (28,6, 32,1 y 45,2% en el grupo de 65-74 años; 32,5, 38,6 y 45,8% en el grupo de 75-85 años; 41, 48,7 y 56,4% en el grupo de edad >85) Los factores relacionados con la mortalidad fueron: insuficiencia cardíaca crónica, cáncer no hematológico, disfunción hepática y disfunción del sistema nervioso central.
ConclusiónLos resultados indican que no hay una diferencia significativa en la mortalidad entre los diferentes grupos de edad. Alrededor del 50% de los pacientes ancianos sobreviven ante un proceso séptico. Existe una estrecha relación entre el número de órganos disfuncionantes, los días de disfunción, el uso de técnicas de intervención y la mortalidad a largo plazo.
Sepsis and septic shock are currently two of the most important reasons for admission into intensive care units (ICU), accounting for approximately 1 in 4 admissions1 and being the second cause of death among patients admitted into non-coronary ICUs.2 In recent years, there has been an increase in the percentage of the population over 60 years old compared to the total population. In fact, in 2000 this population range represented about 10% and it is estimated to increase to up to 21% in 2050.3 One of the problems of an ageing population is the increase in the incidence and severity of diseases such as community and nosocomial sepsis as compared to younger patients.4,5 This fact is of great significance if we consider that over the next decades the population over 80 years old will double.5
Numerous studies have shown a decrease in hospital mortality,6,7 as well as a decrease in overall mortality in patients admitted into Critical Care Units.8–10 However, multiple studies published show an increase in elderly patient mortality. Nasa et al.11 recorded a 79% mortality rate in the elderly in the ICUs where they were admitted; and Tabah et al.12 studied mortality in elderly septic patients with a one-year follow-up, finding 67% mortality, approximately.
In addition, some authors have found differences between septic elderly patients and younger ones regarding the causes of admission into ICU and therapeutic intensity.13
A French study carried out between 2004 and 2006 found that only 40% of elderly patients over 80 years old who came to the emergency room in critical condition were referred to the ICU, and only half of them were accepted13; other studies report that older patients received less intensive treatments.14,15
Several authors have published data on short-term and long-term mortality in elderly or very old patients with sepsis.16,17 However, the literature available regarding both short-term and long-term mortality in a purely surgical cohort is very scarce.
The purpose of this study is to analyze mortality in ICU elderly patients at 28 days, 90 days and a year after the septic process in a postoperative cohort, according to the different age groups.
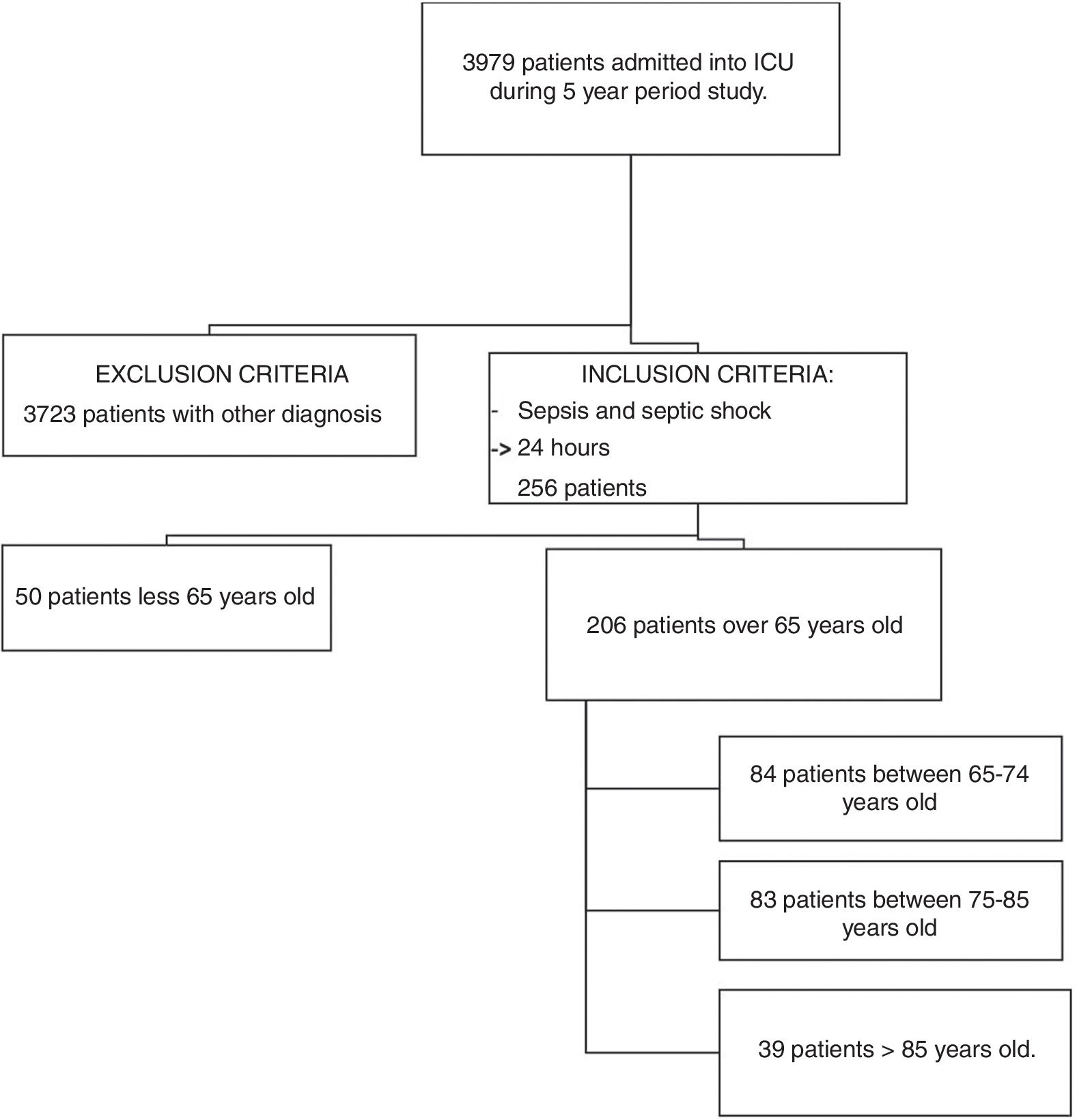
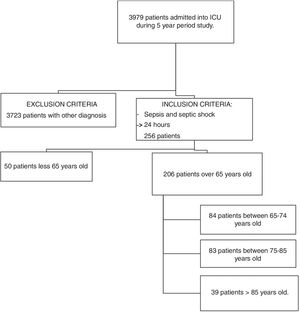
MethodsSource of information and inclusion criteriaA retrospective search for patients with sepsis or septic shock was performed. The study was approved by the local ethics committee. The informed consent of the patients included was not obtained since it is a retrospective study and the data was treated regardless of identity. The patients who met the criteria for sepsis and septic shock (Sepsis-3 definition) were over 65 years old and had been hospitalized for at least 24h in the ICU of the Hospital Universitario Rio Hortega (HURH). They were recruited using our electronic database over a period of 5 years (1 January 2011 to 31 December 2016). We divided the patients in three groups, according to age. The age groups cited by the WHO are: people under 65 years old are considered young; 65–85 years old are considered as young elders, and those older than 85 years of age are considered elderly. In our study, we have introduced an intermediate age group between 75 and 85 years old, as in other previously published works.18 Patients excluded from the study were: patients under 65 years old admitted into the ICU, patients hospitalized for less than 24h, those not diagnosed with sepsis or septic shock, and those who were transferred to another ICU for whatever reason.
In order to obtain long-term mortality, patients’ medical records were reviewed until 1 February 2017.
Definitions and variablesSeveral variables were taken into account such as patient demographic data, ASA classification, comorbidities, personal history, risk scales – measured by APACHE and SOFA scales and SOFA scale parameters itemized by organ everyday since admission into surgical ICU the first seven days, as well as the global number of days of organ dysfunction; presence of acute renal failure, according to ADQI.19 Another variable was reason for ICU admission, which was subdivided according to scheduled surgery, urgent surgery and medical cause, differentiating the latter between respiratory failure, cardiovascular failure and infection. The type of germ causing the infection was also collected, as well as antibiotics adequacy treatment, according to the latest antibiogram. In addition, once in the ICU, a study of the complications was carried out. We grouped the complications as follows: peritonitis, abscesses, haemorrhage, pneumonia, respiratory failure, surgical site infections, leakage, cardiovascular complications and other complications, using standardized international definitions (Supplementary Digital Content)
Infectious status of the patient: sepsis or septic shock. According to the SEPSIS-3 consensus, sepsis is defined as organ dysfunction with a score on the SOFA scale greater than 2 points, and septic shock the status in which vasopressors are required to maintain a MAP>65mmHg or there is a presence of lactic acid>2mmol/L (>18mg/dL) in the absence of hypovolemia.20 Other variables taken into account were infection according to origin, hours of treatment with vasoactive amines >0.05mcg/kg/min; presence of organic and multi-organic dysfunction and mortality during ICU admission, during hospital stay and 28 days, 90 days, and a year after the event.
This study is based on the new definitions of the SEPSIS-3 consensus and the SOFA scale, therefore, organ dysfunction is an acute change in the SOFA scale, with an increase of more than 2 points secondary to an infectious process, assuming as score 0 those patients with unreported organ failure. To define dysfunction of the different organs according to the SOFA scale, the assessment of 6 organs or systems (respiratory, haemostasis, hepatic, cardiovascular, central nervous and renal) is taken into account, with scores for each of them from 0 to 4, dysfunction being when the score is 1 or 2 points, and organ failure when it reaches 3 or 4, providing a daily score of up to 24 points when there is a simultaneous failure of the six organs mentioned.21
Objectives of the studyThe main objective was the measurement of mortality during ICU stay, in-hospital mortality at 28 days, 90 days and a year, by age group. Secondary goals were to establish a relationship between mortality and type of organ dysfunction, number of organs in dysfunction and length of dysfunction duration. All our results were obtained for the different age groups established by our team and according to the previously defined time periods.
Statistic analysisIn the description of quantitative variables, measures of key trends and distribution of frequencies for the qualitative variables were used. For the comparison between qualitative variables, the Pearson Chi-square test (χ2) was used; and for independent quantitative samples the Student's t-test or the non-parametric Mann–Whitney U test were used, as necessary. The non-parametric Kaplan–Meier method was used for the study of factor comparison and survival, graphically depicting survival curves and comparing them with the Log Rank test, then analysing mortality with the contingency table and the test Chi-square at 28 days, 90 days and a year after the event. Binomial regression analysis of variables that were significant in relation to long-term mortality (1 year) was performed to predict which factors increase the likelihood of long-term mortality in elderly patients hospitalized for sepsis. For the comparison of the continuous variables with respect to survival, in case of not fulfilling the normality assumption, Mann–Whitney U test was used. Values p<0.05 were considered statistically significant. A predictive model was subsequently estimated for each variable, by regression logistics, to control confusing factors and identify those variables associated with long-term mortality (dependent variables). The independent variables selected according to their clinical epidemiological relevance or because they have been associated in the univariate analysis were introduced in a regression model of multivariate Cox.
ResultsIn the 5-year study period, a total of 3979 patients were admitted into our ICU. After applying the inclusion and exclusion criteria, 206 patients were selected for the mortality study. Flowchart Fig. 1.
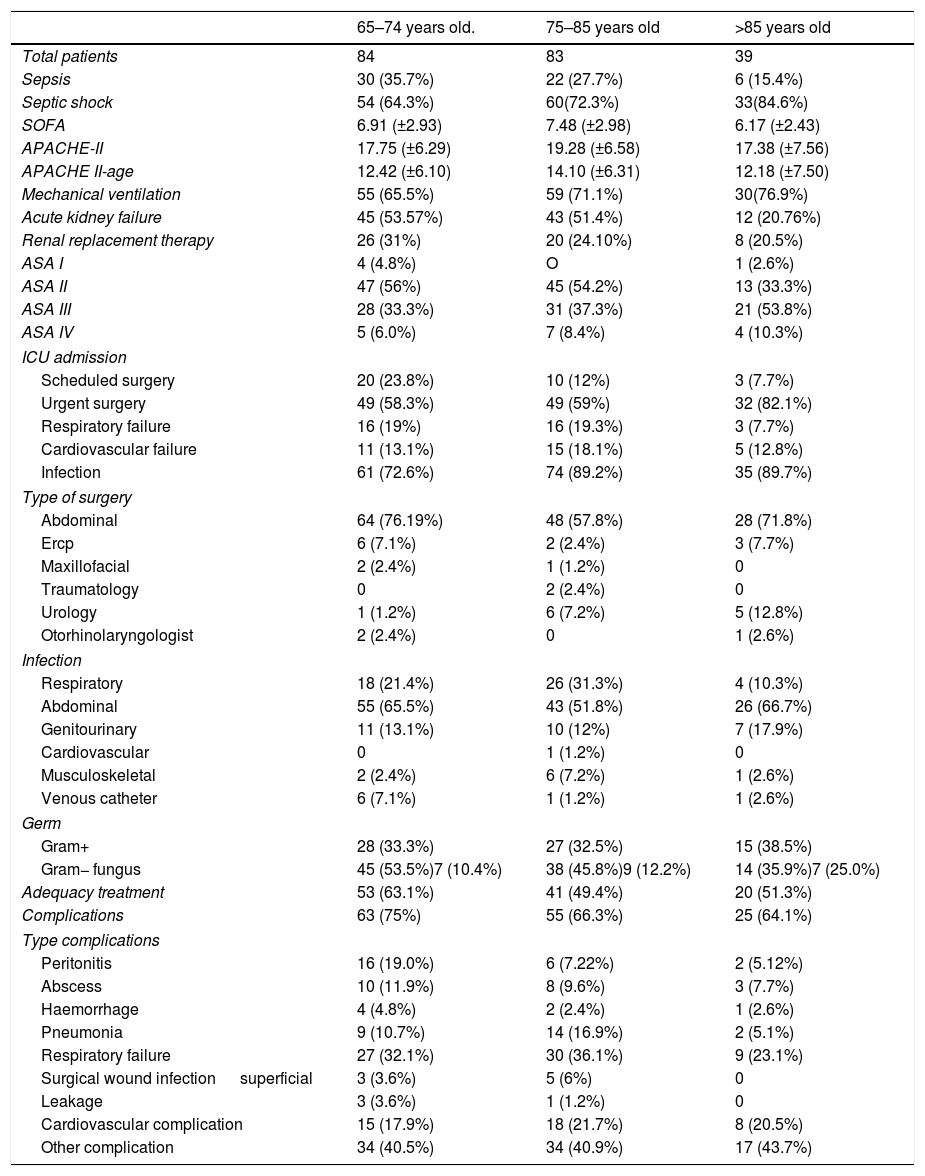
The demographic data of the patients selected is reflected in Table 1. A complete description of comorbidities among different age groups is included in the “Supplementary Digital Content Table 1”.
Demographic data.
| 65–74 years old. | 75–85 years old | >85 years old | |
|---|---|---|---|
| Total patients | 84 | 83 | 39 |
| Sepsis | 30 (35.7%) | 22 (27.7%) | 6 (15.4%) |
| Septic shock | 54 (64.3%) | 60(72.3%) | 33(84.6%) |
| SOFA | 6.91 (±2.93) | 7.48 (±2.98) | 6.17 (±2.43) |
| APACHE-II | 17.75 (±6.29) | 19.28 (±6.58) | 17.38 (±7.56) |
| APACHE II-age | 12.42 (±6.10) | 14.10 (±6.31) | 12.18 (±7.50) |
| Mechanical ventilation | 55 (65.5%) | 59 (71.1%) | 30(76.9%) |
| Acute kidney failure | 45 (53.57%) | 43 (51.4%) | 12 (20.76%) |
| Renal replacement therapy | 26 (31%) | 20 (24.10%) | 8 (20.5%) |
| ASA I | 4 (4.8%) | O | 1 (2.6%) |
| ASA II | 47 (56%) | 45 (54.2%) | 13 (33.3%) |
| ASA III | 28 (33.3%) | 31 (37.3%) | 21 (53.8%) |
| ASA IV | 5 (6.0%) | 7 (8.4%) | 4 (10.3%) |
| ICU admission | |||
| Scheduled surgery | 20 (23.8%) | 10 (12%) | 3 (7.7%) |
| Urgent surgery | 49 (58.3%) | 49 (59%) | 32 (82.1%) |
| Respiratory failure | 16 (19%) | 16 (19.3%) | 3 (7.7%) |
| Cardiovascular failure | 11 (13.1%) | 15 (18.1%) | 5 (12.8%) |
| Infection | 61 (72.6%) | 74 (89.2%) | 35 (89.7%) |
| Type of surgery | |||
| Abdominal | 64 (76.19%) | 48 (57.8%) | 28 (71.8%) |
| Ercp | 6 (7.1%) | 2 (2.4%) | 3 (7.7%) |
| Maxillofacial | 2 (2.4%) | 1 (1.2%) | 0 |
| Traumatology | 0 | 2 (2.4%) | 0 |
| Urology | 1 (1.2%) | 6 (7.2%) | 5 (12.8%) |
| Otorhinolaryngologist | 2 (2.4%) | 0 | 1 (2.6%) |
| Infection | |||
| Respiratory | 18 (21.4%) | 26 (31.3%) | 4 (10.3%) |
| Abdominal | 55 (65.5%) | 43 (51.8%) | 26 (66.7%) |
| Genitourinary | 11 (13.1%) | 10 (12%) | 7 (17.9%) |
| Cardiovascular | 0 | 1 (1.2%) | 0 |
| Musculoskeletal | 2 (2.4%) | 6 (7.2%) | 1 (2.6%) |
| Venous catheter | 6 (7.1%) | 1 (1.2%) | 1 (2.6%) |
| Germ | |||
| Gram+ | 28 (33.3%) | 27 (32.5%) | 15 (38.5%) |
| Gram− fungus | 45 (53.5%)7 (10.4%) | 38 (45.8%)9 (12.2%) | 14 (35.9%)7 (25.0%) |
| Adequacy treatment | 53 (63.1%) | 41 (49.4%) | 20 (51.3%) |
| Complications | 63 (75%) | 55 (66.3%) | 25 (64.1%) |
| Type complications | |||
| Peritonitis | 16 (19.0%) | 6 (7.22%) | 2 (5.12%) |
| Abscess | 10 (11.9%) | 8 (9.6%) | 3 (7.7%) |
| Haemorrhage | 4 (4.8%) | 2 (2.4%) | 1 (2.6%) |
| Pneumonia | 9 (10.7%) | 14 (16.9%) | 2 (5.1%) |
| Respiratory failure | 27 (32.1%) | 30 (36.1%) | 9 (23.1%) |
| Surgical wound infection superficial | 3 (3.6%) | 5 (6%) | 0 |
| Leakage | 3 (3.6%) | 1 (1.2%) | 0 |
| Cardiovascular complication | 15 (17.9%) | 18 (21.7%) | 8 (20.5%) |
| Other complication | 34 (40.5%) | 34 (40.9%) | 17 (43.7%) |
SOFA: Sequential Organ Failure Assessment; ASA: American Society Anaesthesia Physical Status. APACHE-II: Acute Physiology And Chronic Health Evaluation II. ICU: Intensive Care Unit. ERCP.: Endoscopic Retrograde Cholangio Pancreatography.
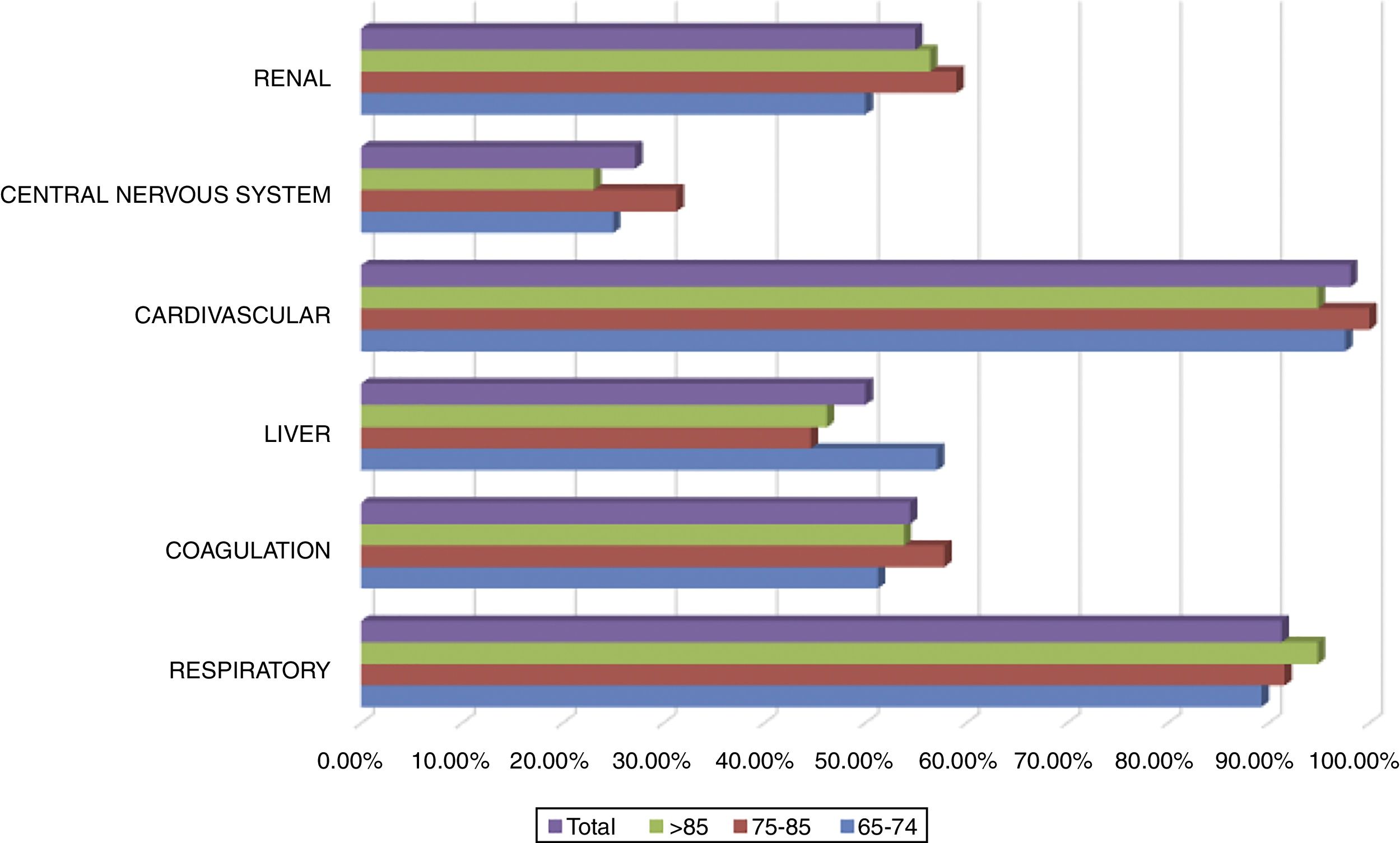
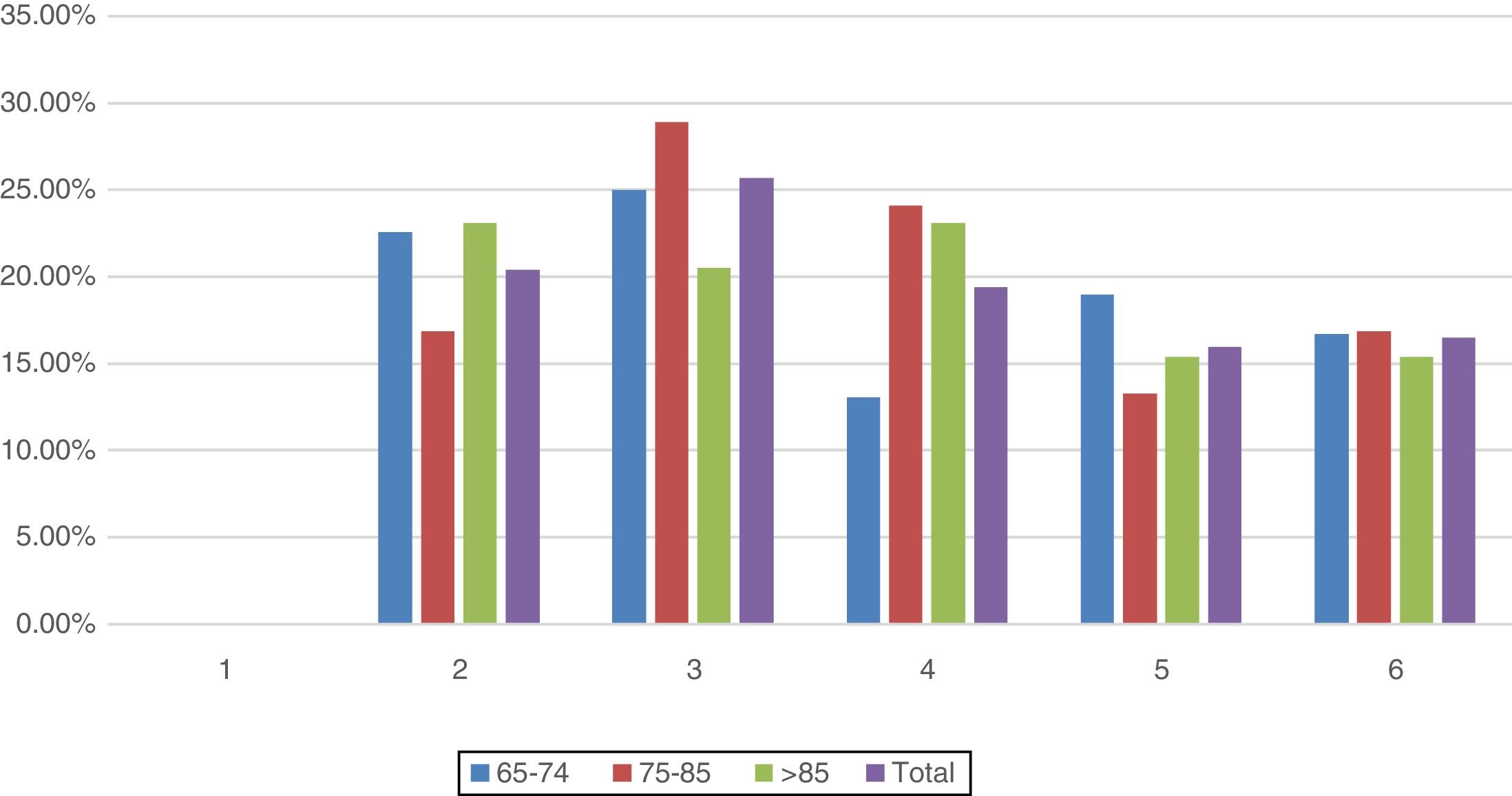
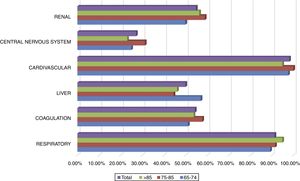
All patients included in the study had cardiovascular dysfunction during the procedure, requiring an infusion of norepinephrine with a mean of 74.8±73.6 (4–504)h and a mean maximum dose of 0.26±0.32 (0.05–2.30)mcg/kg/min. The second organ concerning frequency of dysfunction was the respiratory system in 91.3% of the cases. A complete description of dysfunction frequencies among age groups is provided in Fig. 2. No significant difference between age groups and organ dysfunction was found (p>0.149), Fig. 2.
MortalityThe average hospital mortality was 35.4% (73 patients), considering the deceased during ICU stay of 41 patients, which represents 19.9% of the total 206 patients admitted into the ICU.
A total of 67 patients (32.5%) died 28 days after the event, 79 patients (38.3%) at 90 days, 85 patients (41.3%) at 6 months and 98 patients (47.6%) after a year.
To assess mortality by age, patients were divided into 3 age groups. Group 1 from 65 to 74 years old, Group 2 from 75 to 85 years old and Group 3 over 85 years old.
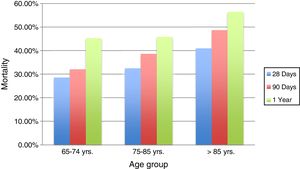
The mortality rate for patients in group 1 was 28.57% during the following 28 days post septic process, compared with 32.5% for group 2 and 41% for group 3 (p=0.39). Also, a mortality rate of 32.1% was determined at 90 days for group 1, being higher (38.6%) in group 2, and 48.7% for group 3 (p=0.263). 45.2% of patients under 74 years old (group 1) passed away in the first year post infection, similar to those between 75 and 85 years old (group 2), with a mortality rate of 45.8%, showing a slightly higher mortality in those very elderly patients over 85 years old (group 3) in the first year after the event (56.4%) (p=0.470).
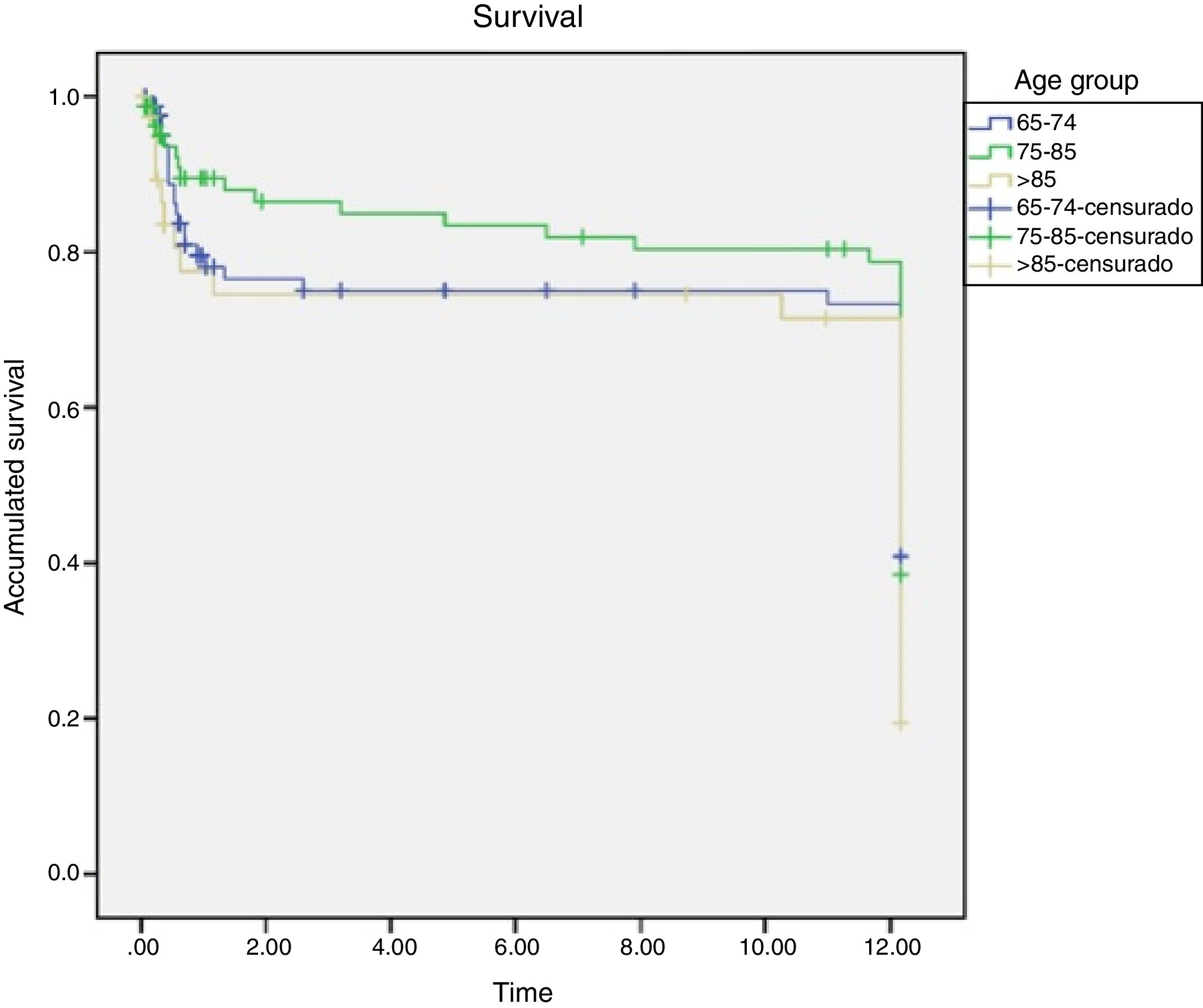
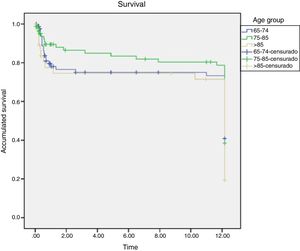
The results indicate that although the percentage is always higher in the oldest group, no significant difference in mortality was found among them, Figs. 3 and 4.
No significant differences were found with respect to mortality when comparing patients with sepsis or septic shock in the study groups in any of the predefined times, “Supplementary Digital Content Table 2”
There were no significant differences in mortality between sexes. The relationship between the presence of comorbidities and mortality in patients with sepsis and septic shock was analyzed, with only a statistically significant relationship between mortality and the presence of Dyslipidemia at 90 days, chronic heart condition at 28 days and a year, non-haematological cancer at 90 days and a year, and short-term cerebrovascular disease (28 days) (Supplementary Digital Content Table 3). No significant differences were found when analysing mortality according to the number of comorbidities (p=0.079).
However, a higher mortality was found in patients who needed to be treated with interventional measures such as invasive and non-invasive mechanical ventilation, and/or with renal replacement therapy in all the time periods analyzed (p=0.000). (Supplementary Digital Content Table 5).
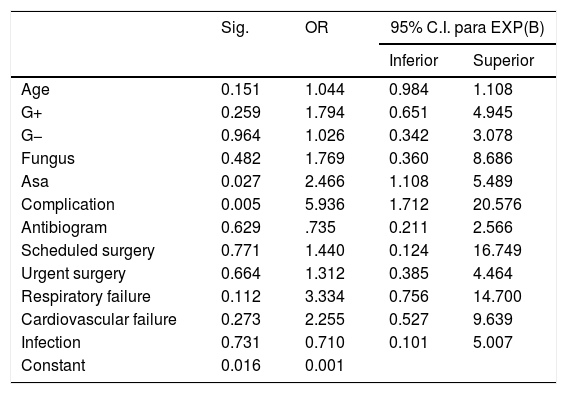
In the logistic regression study using pre-admission variables, mortality was only independently associated with ASA score (OR=2.46; CI 95%: 1.108–5.489, p=0.027) and the presence of complications (OR=5.936; CI 95%: 1.712–20.576, p=0.005). Although it was not an independently risk factor associated with mortality, cardiovascular and respiratory failure measured by SOFA scale, type of germ (Gram+, Gram− and fungus infection) were related with mortality. Age was not an independent risk factor associated with mortality, Table 2.
Binomial regression of significant variables.
| Sig. | OR | 95% C.I. para EXP(B) | ||
|---|---|---|---|---|
| Inferior | Superior | |||
| Age | 0.151 | 1.044 | 0.984 | 1.108 |
| G+ | 0.259 | 1.794 | 0.651 | 4.945 |
| G− | 0.964 | 1.026 | 0.342 | 3.078 |
| Fungus | 0.482 | 1.769 | 0.360 | 8.686 |
| Asa | 0.027 | 2.466 | 1.108 | 5.489 |
| Complication | 0.005 | 5.936 | 1.712 | 20.576 |
| Antibiogram | 0.629 | .735 | 0.211 | 2.566 |
| Scheduled surgery | 0.771 | 1.440 | 0.124 | 16.749 |
| Urgent surgery | 0.664 | 1.312 | 0.385 | 4.464 |
| Respiratory failure | 0.112 | 3.334 | 0.756 | 14.700 |
| Cardiovascular failure | 0.273 | 2.255 | 0.527 | 9.639 |
| Infection | 0.731 | 0.710 | 0.101 | 5.007 |
| Constant | 0.016 | 0.001 | ||
The number of days the patient remained in organ dysfunction was studied, resulting in an average of 9.4±8.1 days.
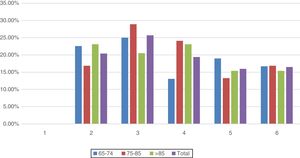
Failure of 2 organs occurred in 20.4% of the patients and failure of 3 organs in 25.7%. 19.4% of the patients presented failure of a maximum of 4 organs, followed by 16% of patients with failure of 5 organs, while 16.5% had failure of all 6 organs. A comparison by age groups and number of organs in jeopardy showed no significant differences (χ2(10)=0.599) (Fig. 5).
An association between the presence of organ dysfunction and mortality in the overall cohort was found, “Supplementary Digital Content Table 4”.
Analysing the different organs with dysfunction, by period of time and age group, there was a statistical significance only between pulmonary dysfunction and long-term mortality (1 year) in group 1, with 48.7% mortality a year from the septic process (p=0.05), “Supplementary Digital Content Tables 4.1, 4.2 and 4.3”.
A univariate and multivariate regression analysis determined that the risk factors associated with increased mortality among patients over 65 years old were the presence of chronic cardiac condition and non-haematological cancer, prior to the process, and liver dysfunction. The presence of chronic heart condition increases the risk of mortality 4.35 times; non-haematological cancer multiplies the risk of mortality by 4.98, while presenting liver or central nervous system dysfunction increased the risk of mortality by 3.70 and 3.65, respectively, “Supplementary Digital Content Table 5”.
DiscussionThis post-surgical cohort study shows the relationship between mortality by age groups and the presence of multi-organic dysfunction during a septic process.
Overall, evaluating mortality in our study and considering all patients together, according to the time of onset of the septic process, 32.5% died at 28 days, 38.3% at 90 days, 47.6% after a year. This shows a lower mortality rate compared to other studies so far.
When analysing mortality by age group, it should be noted that the mean age obtained is higher for dying patients than for those who survive, and that age differences are higher in mortality at 28 days and at 90 days of the septic process, however, the differences did not reach statistical significance per year between patients who died and those who survived. Moreover, age was not an independent risk factor associated with mortality in the logistic regression analysis and only ASA and presence of complications were independently related with mortality. ASA score increases the likelihood of dying multiplied by 2.466 and the presence of complications increases by six the probability of mortality.
The literature confirms a high mortality rate among elderly septic patients. A study11 conducted in elderly patients over 80 years old showed 79% mortality versus our findings, of just 19.9% mortality rate. Mortality at 28 days without determining age range was 42%, according to the EPISS study,22 higher than the 32.5% mortality found in our ICU. Also, mortality in elderly septic patients with a one-year follow-up was approximately 67%,12 versus 51% of patients who died during the first year after the onset of the septic process in our study.
In the study of 821 patients of 61 years old mean age, Pandharipande et al.23 obtained 31% mortality at 90 days and 38% a year after the septic event. Biston et al.18 also published mortality data from patients with shock, differentiating them into 3 age groups: under 75, between 75 and 84 years old, and over 85 years of age, classified as non-elderly, elderly and very old, respectively. Mortality for those under 75 years old was 44% at 28 days, 49% at 6 months and 66% after a year. For the elderly group, mortality at 28 days was 64%, 79% at 6 months and 84% at 1 year. And for the very elderly group the mortality figures at 28 days were 75%, 92% at 6 months and 97% one year after the event. These figures are higher than our results.
In our study, there is a very low percentage of mortality in patients over 85 years old with acute renal failure. This might be due to a smaller number of patients in this group, compared to other studies published to date, such as the one by Suarez-de-la-Rica et al., in which mortality in elderly critical patients is directly related to the use of extracorporeal clearance techniques.24
Nevertheless, mortality figures one year after the sepsis event tend to vary. As seen by Flaatten et al.25 who compared 11 different studies published in the last 15 years, where long-term mortality varies from 40% to 70%.
In studies published to date, differences in survival have been found between patients who developed Multi-organic Dysfucntion Syndrome (MDS) and those who did not, with mortality even five times higher for those with MDS. Moreno et al. found that the presence of MDS was associated with a 45% mortality rate in ICUs, compared to 6.2% of patients who did not develop multi-organ dysfunction.26,27
It is also worth noting that 80% of ICU mortality is attributable to MDS as cited by Cook,28 and that there is a death risk of approximately 50% when the patient evolves to MDS.29
In our case, all patients presented MDS, resulting in an overall in-hospital mortality of 35.4%, of which only 19.9% died in the ICU. This difference in mortality between the previous work and ours could be explained by the changes in the management of septic patients, where age is no longer a factor to avoid taking interventionist measures.
As Flaatten et al.25 explains, nowadays, very old patients do not receive the same amount of critical care as younger ones, but in our daily activity we consider the severity of the process but not the age of the patients, in order to pursue a treatment. Perhaps it is an explanation to a lower mortality rate. The literature documents that intensity of treatment is lower and duration is shorter,14 in fact, the data collected in the Norwegian Critical Care Registry between 2006 and 2009 indicate that the group of very elderly patients over 85 years old received up to 15% less ventilatory support than younger ones.30
The lower intensity of treatment in the very elderly is somewhat contradictory,16 since these patients have fewer physiological reserves and, therefore, they need greater therapeutic support and other interventionist measures, avoiding, in any case, medical or therapeutic overloads that do not improve the patient's survival. In a recent cohort of very critically ill elderly surgical patients admitted in the ICU, Suarez de la Rica et al.24 observed that non survivors had a very lengthy stay in the ICU as well as in the ward, questioning important decisions about end-of-life care in this group of patients.
It should be borne in mind that elderly patients who survive an ICU stay suffer more long-term sequels, not only an increase in long-term mortality, as mentioned in this paper, but also functional, cognitive alterations and a worse quality of life.31 As Flaatten et al. mentioned, frailty among elderly patients admitted into the ICU is a significant factor for a shorter-term survival.32 In addition, in a recent prospective study with ICU patients over 72 years old, Gordo et al. found that pre-admission functional status is an independent factor associated with mortality and very poor functional status at hospital discharge.33 These alterations persist over time, as Iwashyna et al. describes in a study of septic process survivors, which shows that neither functional nor cognitive status improves months after hospital discharge.34 In fact, recent studies suggest that admission of elderly patients in ICU may not improve survival but worsen their quality of life.24,35,36 These negative psychological repercussions are well recognized nowadays, as is the duration of psychological morbidity as well.37
Indeed, the group of Guidet et al.38 published an article proposing a protocol in which septic elderly patients should systematically enter the ICU. Nevertheless, given the high mortality rates in this age group, the results have been quite unsatisfactory, which means that a systematic admission does not reduce mortality at 6 months.
Unfortunately, there is not enough data available regarding frailty and quality of life of our patients before ICU admission and the influence of these important factors on mortality cannot be evaluated in our cohort.
However, and in line with our findings, a recent publication by Karakus et al.17 including 216,196 patients from 31 different ICUs showed a decrease in mortality in recent years, compared to a previous study period, even in patients with greater severity in disease, due to a therapeutic intensification in the ICU in the last years.
LimitationsThere are certain limitations that this work presents, in order to adequately analyze the results obtained: It is a retrospective study, with a limited number of patients included. To be a single centre and the fact that only post-surgical patients were selected could also contribute to produce a bias. Another important limitation of the study is the lack of data about the pre ICU admission functional status and the frailty of the patients included in the study, which could have an important impact on mortality.
ConclusionsIn recent years there has been a breakthrough and a great development in support measures, associated with the emergence of new therapies and a greater and more detailed knowledge of pathologies such as sepsis and septic shock. Owing to these factors, the characteristics of critically ill patients have also changed, becoming an ageing population,39 which makes the treatment of critically ill elderly patients a controversial subject regarding therapeutic intensity. In our study, no differences in mortality were found between the elderly and the younger, with a lower mortality rate in elderly patients. This may be due to the therapeutic criteria, which does not define therapeutic intensity according to the age of the patients, but rather to the severity of the disease suffered.
Contribution of authorsA P-G designed the study, recruited the data, and drafted the manuscript. D A-C, O LM, S M-A, J R-F and J LM recruited the data and collaborate in drafting the manuscript. C A designed the study, recruited the data and drafted the manuscript. All the authors read the manuscript and fully approve it.
FundingNo financial support was required for this study.
Conflict of interestsThe authors declare that they have no conflicts of interest.
To our colleagues staff of Rio Hortega Hospital Anaesthesia and Intensive Care Unit, and to Dr Javier Pérez Fernandez. To Ms Marcela Solis for the English revision of the manuscript.