Despite major advances in our understanding of the physiopathology of brain death (BD), there are important controversies as to which protocol is the most appropriate for organ donor management. Many recent reviews on this subject offer recommendations that are sometimes contradictory and in some cases are not applied to other critically ill patients. This article offers a review of the publications (many of them recent) with an impact upon these controversial measures and which can help to confirm, refute or open new areas of research into the most appropriate measures for the management of organ donors in BD, and which should contribute to discard certain established recommendations based on preconceived ideas, that lead to actions lacking a physiopathological basis. Aspects such as catecholamine storm management, use of vasoactive drugs, hemodynamic objectives and monitoring, assessment of the heart for donation, and general care of the donor in BD are reviewed.
A pesar de los avances en la comprensión de la fisiopatología de la muerte encefálica, existen controversias importantes sobre el protocolo más adecuado para el tratamiento del donante de órganos. En muchas revisiones recientes aparecen recomendaciones, a veces contradictorias, y a veces no aplicadas a otros pacientes críticos. Este artículo revisa publicaciones, muchas de ellas recientes, que tienen un impacto en estas medidas controvertidas y que pueden ayudar a confirmar, refutar o abrir nuevas áreas de investigación sobre las medidas más apropiadas para el tratamiento del donante y que deberían hacer olvidar algunas recomendaciones habituales basadas en ideas preconcebidas, que conducen a acciones carentes de una base fisiopatológica. Se revisan aspectos como: el control de la tormenta catecolamínica, el uso de fármacos vasoactivos y de hormonas, los objetivos hemodinámicos y su monitorización, la evaluación del corazón para donación y otros aspectos generales del tratamiento del donante en muerte encefálica.
The involvement of intensivists is one of the fundamental pillars on which the successful and worldwide referent Spanish Model of donation and transplantation is based. Intensivists are often responsible for hospital transplant coordination, are actively involved in diagnosis of brain death (BD) and their cooperation is key to increasing donation programs in circulatory arrest.1 Most transplanted organs are from donors in BD and it is in the Intensive Care Units where most of deaths occur in this situation, so intensivists have the responsibility of identifying the potential donor and its subsequent management until organ retrieval for transplantation.2,3 During and subsequent to onset of BD may occur hemodynamic, hormonal and inflammatory disorders that can cause cardiac arrest of potential donor or may alter or irreversibly damage the function of different organs before retrieval occur.4 In this period of time it is necessary to establish an active treatment in order to prevent, minimize or reverse these conditions and achieve not only a greater number of potentially transplantable organs, but also a higher quality of the same to ensure their optimal function after transplantation, a greater longevity of its function and, therefore a higher quality of life of the recipient.
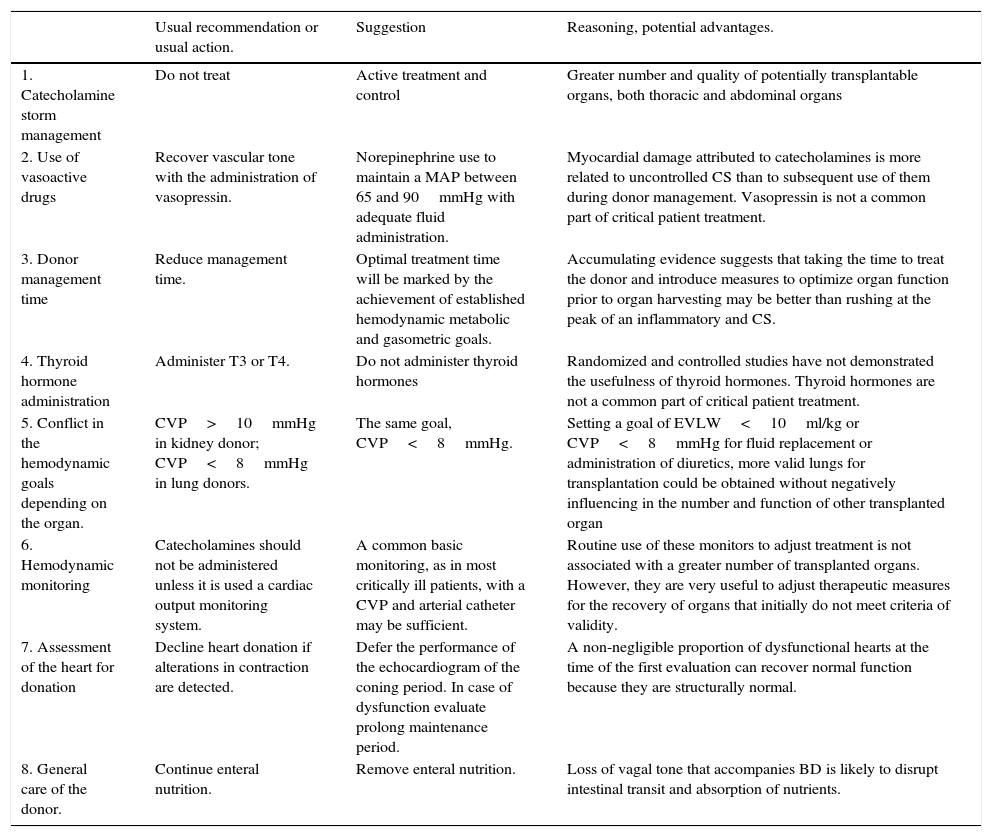
In spite of major advances in the understanding of the pathophysiology of BD, there are important controversies about which protocol is the most appropriate for organ donor management. A recent meta-analysis and systematic review of published studies, until August 2012, found no protocol or measure of proven efficacy.5 However, the conclusion of this work should not be deducted that there are no adequate and essential measures, but rather reflects the lack of well-designed studies with clearly identifiable and comparable objectives that demonstrate with scientific evidence the superiority of some measures over other. Most of the recommendations are based on experimental animal models, retrospective observational studies or expert opinion and extrapolated measures, as can be otherwise, common actions on any other critically ill patient. Regardless of this scientific limitation, the treatment applied to donors is one of the factor that most influences the number and quality of transplanted organs.6,7 In many recent reviews on this subject appear recommendations, sometimes contradictory, and sometimes not applied to other critically ill patients, which may confuse the reader. This article review publications, many of them recent, which have an impact on these controversial measures and which can help to confirm, refute or open new areas of research into the most appropriate measures for treatment of organ donors in BD and what they should be done to forget some established recommendations based on preconceived ideas, that lead to actions lacking a pathophysiological basis (Table 1).
Recommendations and usual actions to reflect on.
| Usual recommendation or usual action. | Suggestion | Reasoning, potential advantages. | |
|---|---|---|---|
| 1. Catecholamine storm management | Do not treat | Active treatment and control | Greater number and quality of potentially transplantable organs, both thoracic and abdominal organs |
| 2. Use of vasoactive drugs | Recover vascular tone with the administration of vasopressin. | Norepinephrine use to maintain a MAP between 65 and 90mmHg with adequate fluid administration. | Myocardial damage attributed to catecholamines is more related to uncontrolled CS than to subsequent use of them during donor management. Vasopressin is not a common part of critical patient treatment. |
| 3. Donor management time | Reduce management time. | Optimal treatment time will be marked by the achievement of established hemodynamic metabolic and gasometric goals. | Accumulating evidence suggests that taking the time to treat the donor and introduce measures to optimize organ function prior to organ harvesting may be better than rushing at the peak of an inflammatory and CS. |
| 4. Thyroid hormone administration | Administer T3 or T4. | Do not administer thyroid hormones | Randomized and controlled studies have not demonstrated the usefulness of thyroid hormones. Thyroid hormones are not a common part of critical patient treatment. |
| 5. Conflict in the hemodynamic goals depending on the organ. | CVP>10mmHg in kidney donor; CVP<8mmHg in lung donors. | The same goal, CVP<8mmHg. | Setting a goal of EVLW<10ml/kg or CVP<8mmHg for fluid replacement or administration of diuretics, more valid lungs for transplantation could be obtained without negatively influencing in the number and function of other transplanted organ |
| 6. Hemodynamic monitoring | Catecholamines should not be administered unless it is used a cardiac output monitoring system. | A common basic monitoring, as in most critically ill patients, with a CVP and arterial catheter may be sufficient. | Routine use of these monitors to adjust treatment is not associated with a greater number of transplanted organs. However, they are very useful to adjust therapeutic measures for the recovery of organs that initially do not meet criteria of validity. |
| 7. Assessment of the heart for donation | Decline heart donation if alterations in contraction are detected. | Defer the performance of the echocardiogram of the coning period. In case of dysfunction evaluate prolong maintenance period. | A non-negligible proportion of dysfunctional hearts at the time of the first evaluation can recover normal function because they are structurally normal. |
| 8. General care of the donor. | Continue enteral nutrition. | Remove enteral nutrition. | Loss of vagal tone that accompanies BD is likely to disrupt intestinal transit and absorption of nutrients. |
BD, brain death; CS, catecholamine storm; CVP, central venous pressure; EVLW, extra-vascular lung water; MAP, mean arterial pressure; T3, triiodothyronine; T4, thyroxine.
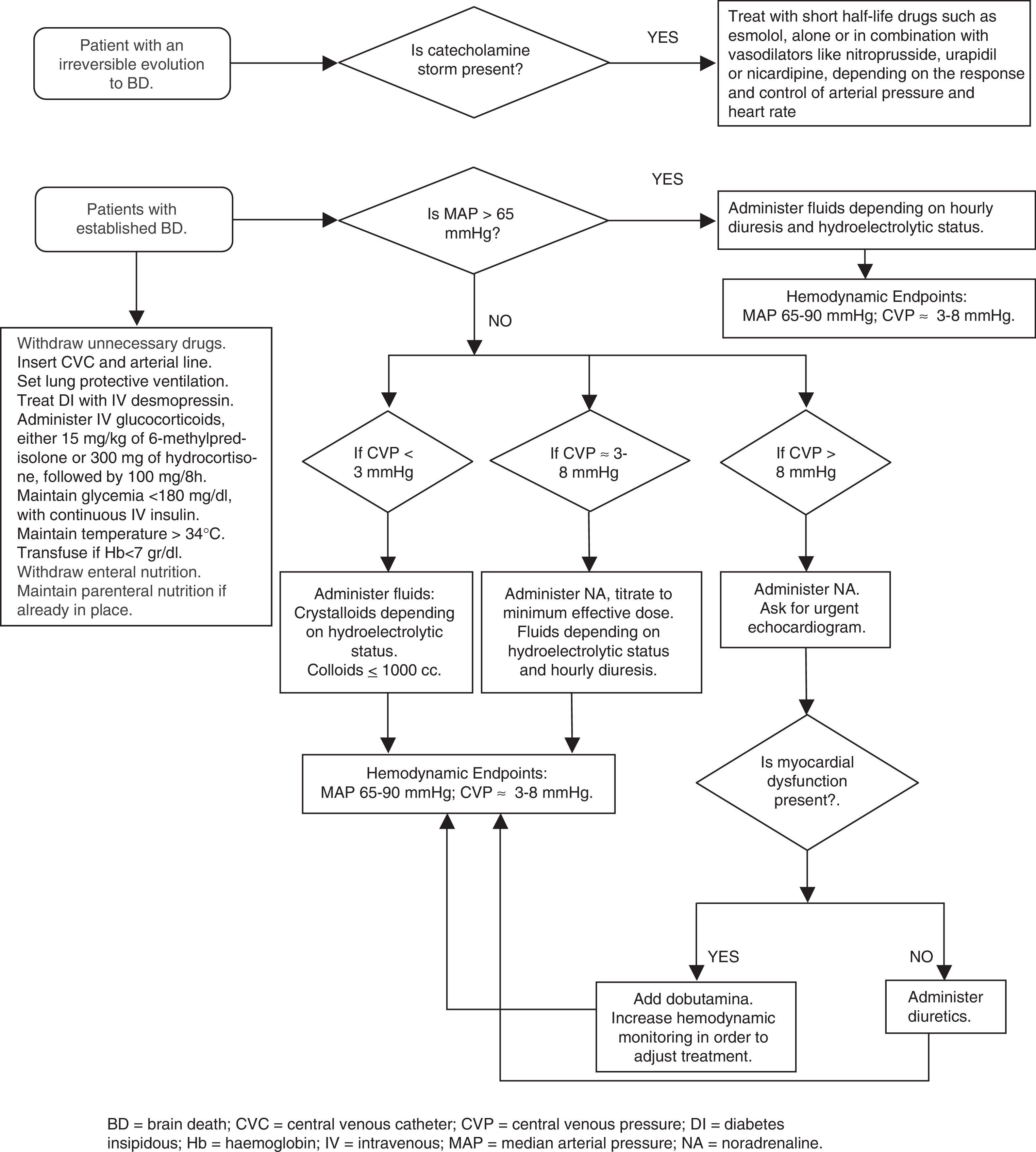
During the establishment of BD a series of hemodynamic disorders derived from the rostrocaudal evolution of cerebral ischemia occurs. When nucleus of vagus is destroyed, the sympathetic system remains unopposed, producing the so-called “catecholamine storm” (CS) characterized by arterial hypertension, tachycardia, increased cardiac output (CO) and myocardial oxygen consumption.8,9 The clinical manifestations are most important in those patients in whom the evolution toward BD has been very abrupt or rapidly progressive.10 As this phase precedes the destruction of the medullary vasomotor centers with the consequent neurogenic shock, typical of BD,11 numerous revisions or do not mention the possibility of an active treatment of CS3,12–23 or recommend not to treat to it arguing its brief duration and the subsequent risk of aggravate or difficult the control subsequent hypotension.24,25
The CS causes a serious imbalance between myocardial oxygen demand and supply, which triggers metabolic functional alterations and sometimes structural heart damage, even in young people without heart disease. In addition, the sudden increase in pulmonary vascular resistance may lead to right ventricular dysfunction and elevation of systemic vascular resistance is one of the pathogenic factors of neurogenic pulmonary edema.26 Other organs may also be affected by the intense vasoconstriction of this phase and subsequent alterations resulting from ischemia-reperfusion phenomena associated with vasoconstriction and subsequent vasodilation.
Audibert et al.27 showed that actively managing the CS in donors with the potential for cardiac donation, achieved a greater number of transplantable hearts, a better performance of the same and a greater survival of the recipient. This work confirms the findings of previous experimental studies.28 It is noteworthy that although definition of CS used by these authors to start treatment was very strict: an episode of at least 10min of systolic blood pressure (SBP) greater than 200mmHg with a sudden increase in heart rate (HR) above 140; this phenomenon was present in 63% of donors studied and had an average duration of one hour with a range between 30min to 6h.
Therefore, and considering that the published scientific evidence is scarce, we believe that CS should be actively treated with drugs of short half-life such esmolol, alone or in combination with vasodilators such as nitroprusside, urapidil or nicardipine, depending on the response and control of BP and HR. Adequate control may result in a greater number and quality of potentially transplantable organs, both thoracic and abdominal organs.
Use of vasoactive drugs in the donorAfter CS, herniation results in spinal cord sympathetic deactivation, there is a decrease in circularing catecholamines with loss of vasomotor and cardiac sympathetic tone. The release of inflammatory mediators contributes to typical vasoplegia of chronic phase of BD.9 On the way to recover vascular tone is necessary to administer vasoactive drugs.
Many authors and guides, especially anglosajons, recommend the administration of catecholamines at low doses and to recover vascular tone with the administration of vasopressin.3,8,9,12,14,15,18,22,25,29–35 They suggest that use of high doses of catecholamines is deleterious to organs, both thoracic and abdominal, especially for the heart. Recommended management protocols include vasopressin administration on the basis that this drug reduces the doses of catecholamines required for maintenance. Recently the group of Harbor-UCLA Medical Center, following a retrospective review of data from more than 10,000 donors from the Organ Procurement and Transplantation Network (OPTN) concluded that vasopressin should be universally adopted in the management of donors with BD.36,37 The authors found that in donor group receiving vasopressin more organ were retrieval for transplantation, both lungs37 and other organs.36 However, in these articles it is not mentioned whether vasopressin was used to achieve hemodynamic stability or only for treatment of diabetes insipidus (DI), on the other hand no comparative hemodynamic data were provided between the two groups and how DI was treated, or if it was treated in those who did not receive vasopressin. It is noteworthy that the group that received vasopressin was significantly younger, died more due to brain trauma and had a lower incidence of bacteremia than those who did not receive it, which could suggest a priori a potentiality for donation of more organs.
In the review of the literature no comparative study between catecholamines and vasopressin is found in the treatment of organ donor suggesting advantages with this drug. The definition of “high doses of catecholamines” used by these authors is confusing, for some high doses means the use of dopamine at dose higher of 10μg/kg/min12,14,29,35 and for others it is the use of norepinephrine at dose higher of 0.05μg/kg/min,3,34 or 0.2μg/kg/min18 or even 0.5μg/kg/min.22 The concept of catecholamines indistinctly includes dopamine, norepinephrine, phenylephrine, epinephrine and dobutamine which are obviously not comparable in their hemodynamic effects. In addition, there are no clear studies demonstrating the deleterious effect of use of catecholamines during donor management. The most referenced study which suggests that use of norepinephrine is associated with right ventricular dysfunction is one performed only during anesthesic period of cardiac retrieval, with a sophisticated and complex method of calculating the rate of ventricular contractility.38 The use of vasopressin has been shown to reduce dose of catecholamines required for hemodynamic stability although this “saver” effect has not been reflected in any improvement of transplanted organs.
On the other hand, there are publications that show that use of norepinephrine is one of the variables associated with survival of liver graft,39 in addition the maintenance of an adequate coronary perfusion pressure (CPP) is key for recovery of myocardial contraction,40 being norepinephrine the drug of choice.41
The loss of sympathetic tone at vascular and cardiac level should be counteracted by exogenous administration of catecholamines with effect α-agonist and β1-agonist, such as dopamine or, preferably, norepinephrine. The goal is to maintain a MAP between 65 and 90mmHg with adequate fluid administration. It is impossible to define the ideal or maximum dose, as this will depend on the residual level of donor vascular tone, vascular reactivity and pharmacokinetic variability of all drugs in patients, in this case of organ donors.42 Myocardial damage attributed to catecholamines is more related to uncontrolled CS than to subsequent use of catecholamines during donor management.10 Norepinephrine, not vasopressin, is the drug recommended in many other European guidelines for the organ donor management.43–45 It is understandable that in ICUs where vasopressin is used for control of DI or for treatment of septic shock, it is also used for hemodynamic stabilization of organ donor, but it is necessary to remember that in most comparative studies between vasopressin and norepinephrine for the management of septic shock, which from the hemodynamic and inflammatory point of view the alterations that are presented are very similar to that of BD, no vasopressin advantage has been observed in different aspects studied,46,47 Norepinephrinee being the recommended drug.48
Donor management timeOn many occasions once the authorization for organ retrieval has been obtained, the period until donor transfer to operating room becomes a race against time to reduce maintenance times. Some authors recommend reducing the period of maintenance to avoid heart, pulmonary or other organs deterioration.9,12,32
Accumulating evidence suggests that taking the time to treat the donor and introduce measures to optimize organ function prior to organ harvesting may be better than rushing at the peak of an inflammatory and CS. Studies have shown that less than 20% of donors meet the ideal analytical and hemodynamic criteria at time of initiation of donation process,49 since their achievement implies obtaining a greater number of organs per donor and a better performing of them.49–51 These goals can be achieved hours later, after adequate donor management.49 On the other hand, there are studies that demonstrate better functionality of donor organs with longer maintenance times. Kunzendorf et al.52 in an analysis of 1106 kidney transplants, showed that kidney grafts harvested from donors with longer duration of BD (>470min) exhibited a significantly higher incidence of primary graft function and a significantly better graft survival rate in comparison to kidneys from donors with a shorter duration of BD (<470min). Nijboer et al.53 in an analysis of 20,773 renal transplant recipients found that duration of BD was one of the factors associated with improved graft function, longer duration of BD decreased the risk for delayed graft function and 1- and 3-year graft failure. Venkateswaran et al.54 in an observational study found that donor hearts with times from coning above the median value of 11h had significantly better contractility. Active treatment of potential lung donor with optimal fluid management, recruitment maneuvers and bronchoscopy allows the recover of lungs that are initially discarded for transplantation, doubling or even tripling the number of optimal lungs.55
The period until the transfer of donor to the operating room should not be considered a maintenance time but a time of treatment necessary to recover or to reverse the damages associated with cerebral herniation. This fact becomes more significant in those donors who have suffered an uncontrolled CS, in which case a longer treatment time may allow the functional recovery of potentially damaged organs during cerebral herniation. The goal is not only to obtain a donation but to obtain a greater number of optimal organs for transplantation and with a better functionality that guarantees its correct performing. There will be cases for medical reasons, shock or uncontrolled hypoxia, or other reasons, whether logistical or wishes of the donor family, in which this time must be shortened, but as a general rule treatment time of 12–15h may be the most appropriate, but qualified according to how the brain herniation has occurred and the organs considered suitable for donation. The optimal treatment time will be marked by the achievement of established hemodynamic, metabolic and gasometric parameters, during this treatment period are necessary a frequent re-evaluation to demonstrate improvement in organ function toward defined targets. Once organ function is optimized, surgical procurement procedures should be arranged.
Hormone replacementIn BD the interruption of blood flow to the hypothalamus and hypophysis occurs affecting the hormone production. In only a small percentage of donors this function can be maintained by persistence of flow through the inferior hypophysial artery branch of external carotid. For this reason significant hormonal changes occur that in some cases, like the loss of production of ADH, it is forced to replace it, either with the hormone itself or with its synthetic derivatives, such as desmopressin.35 However, hormone replacement to normalize other hormonal alterations is more discussed.
Observational studies in BD donors have almost always found a decrease in the free plasma triiodothyronine (T3) concentration, but changes in the serum concentration of other hormone levels, such as thyroid-stimulating hormone (TSH) and thyroxine (T4), are variable. The most common finding is the low value of T3, but with higher values of rT3. In general, peripheral conversion of T4–T3, the physiologically active hormone, decreases in an inflammatory environment or with administration of catecholamines or glucocorticoids. Majority of guidelines and some authors recommend the administration of intravenous or oral thyroid hormones, either T3 or T4, in all donors8,14,15,17,20,31,32,34,56 or at least in donors with ventricular dysfunction12,25,29,30 or with important hemodynamic alterations.3,18,22,25,57 These recommendations are based on retrospective studies and non-comparative studies.58
Most studies performed with greater scientific and methodological rigor, randomized controlled, have not demonstrated the usefulness of thyroid hormones in different aspects evaluated either in cell metabolism,59 hemodynamic stability,60 increase of myocardial contraction61 or increasing the number of organs valid for transplantation.49 Recent systematic reviews agree that the utility of these hormones in the management of organ donor has not been demonstrated.62 Thyroid hormones are not part of treatment of critically ill patients who have alterations similar to those found in BD, such as myocardial stunning that occurs in other severe brain injuries such as subarachnoid hemorrhage or head trauma etc. Thyroid disorders associated with BD can be classified into the so-called “euthyroid sick syndrome of critically ill patient”, where the administration of these hormones has not shown benefits.
Also steroid administration in the donor is a common recommendation. A recent systematic review concludes that although most observational studies support the use of glucocorticoids, there are many confounding factors to reach a definitive conclusion about its usefulness.63 Glucocorticoids can be administered to achieve greater hemodynamic stability and/or to reduce inflammation and its repercussion on organs to be transplanted. In BD adrenal insufficiency is observed, more than 75% of donors has decreased levels of cortisol or poor response to ACTH stimulation test, which can contribute to hemodynamic instability.64 In CORTICOME, a recent study multicenter, prospective, randomized, were included brain-dead patients to administration or not of 50mg of hydrocortisone followed by a continuous infusión of 10mg/h until organ harvesting. It showed that the treated group required lower doses of norepinephrine and less time to achieve hemodynamic stability, although this finding was not reflected in a higher number of transplanted organs.64 This study has confirmed the effectiveness of steroids as part of vassopresor management to achieve hemodynamic stability donor as previously suggested.65
An elevation of pro-inflammatory cytokines such as TNF-α, interleukin (IL)-1β, IL-2R, IL-6 and IL-8 occurs during BD. The phenomena of ischemia and reperfusion as well as the loss of functionality of the vagus could explain this fact. This inflammatory milieu can lead to organic damage or cellular immunological changes that may induce an increase in the incidence of rejection phenomena.66 Kuecueck et al.,67 showed that donor treatment with steroids led to significantly decreased tissue and serum expression of proinflammatory cytokines. Kotsch et al.,68 in a prospective randomized study of the administration of methylprednisolone at an initial dose of 250mg and infusion of 100mg/h, in 100 liver donors showed that in treated donors there was a significant decrease in cytokines and the expression of adhesion molecules of hepatocytes. The liver recipients of treated donors had fewer episodes of acute rejection. These findings have not been confirmed in a subsequent, prospective, randomized, blinded study in 83 donors who received 1 gram of methylprednisolone.69 These same authors, in another publication of the same study, but this time with data from kidney donors, 269 randomized, showed that the treated donors presented a reduction in inflammatory markers, both in renal and systemic tissue biopsies, although this finding did not lead to the receptors to a decrease in the incidence and duration of early graft failure.70 Unlike the study of Kotsch et al. in which steroids were administered at the time of obtaining family authorization, in these two studies, in which the maintenance time was not specified, administration of steroids was between 3 and 6h before organs removal.
Until further studies are conducted and because of the harmful impact of inflammatory activity related to brain death in the organs to be transplanted, we recommend to administer glucocorticoid as soon as possible. Although the usually recommended dose is 15mg/kg of 6-methylprednisolone, other steroids such as hydrocortisone and other doses may be reasonable. Dhar et al.,71 in a comparative study of 60 donors with a historical series of 72 donors who received 15mg/kg of 6-methylprednisolone, found that administration of a regimen of 300mg IV of hydrocortisone, followed by 100mg each 8h resulted in a similar hemodynamic stability to that of the historical group, a same number of transplanted organs and a similar incidence of early failure or graft survival per year.
Conflict in the hemodynamic goals depending on the organ to be transplantedThere are conflicts in management priorities during organ donor management depending on whether the lungs, heart or kidneys were obtained. Traditionally, aggressive fluid resuscitation and management were thought to result in improved procurement of kidneys, while a conservative fluid replacement strategy benefited lung procurement. This theoretical antagonism of goals is still reflected in some recent recommendation.9,12,15,25,32
Miñambres et al.72 showed that a restrictive fluid administration, not exceeding 8mmHg of central venous pressure (CVP), more potential lung donors were obtained without repercussion in the incidence of renal graft failure. These data have been confirmed in a multicenter Spanish study, which EVLW setting a goal of <10ml/kg or CVP<8mmHg for fluid replacement or administration of diuretics, more valid lungs for transplantation were obtained without negatively influencing in the number and function of other transplanted organs.73
Hemodynamic monitoring in the BD donorSome authors recommend careful monitoring of potential organ donor, considering that catecholamines should not be administered, particularly in the heart or lung donor, unless it was monitored with a cardiac output (CO) monitoring system.23 Many guidelines recommend closer monitoring with Swan-Ganz or PiCCO catheter, if dopamine is required at doses higher than 10μg/kg/min.14,15,23,57
A recent multi-center study, the Monitor Trial,74 randomized to routine monitoring (277 donors) versus continuous CO monitoring using LiDCO technology (279 donors) did not demonstrate superiority of this type of monitoring either in the number of organ transplanted per donor neither in survival of the recipient, nor in the use of vasopressor drugs. Donors who were monitored with LiDCO received more fluids.
Usually most of donors, due to pathophysiology of BD, will need catecholamines. A common basic monitoring, as in most critically ill patients, with a central venous catheter for measurement of CVP and administration of vasoactive drugs, as well as an arterial catheter may be sufficient. The variability of pulse in the arterial wave can also guide the fluid therapy. In cases where the recovery of organs considered initially invalid, such as heart or lung, or in which hemodynamic or gasometric deterioration is not controlled with the usual measures, it is reasonable to escalate the monitoring with more directed technology, either with echocardiography, PiCCO, LiDCO or Swan-Ganz catheter.
Assessment of the heart for donationVentricular dysfunction prior to cardiac retrieval is one of the most important factors associated with graft failure, about 30% of potential heart donors are discarded for this reason. In most cases, this dysfunction is detected with the first echocardiogram performed, without adequately assessing the experience of the operator performing the test, the conditions of the donor at the time of test or the time elapsed between brain death and echocardiogram. Ventricular dysfunction associated with BD is included within the causes of stunned myocardium and, therefore, may be potentially reversible, as has been demonstrated in other conditions, such as subarachnoid hemorrhage. The performance of echocardiogram in non-ideal conditions or very early after the onset of BD can detect the phase of “myocardial stunning” and show global or segmental alterations that, theoretically, would force to discard the donor.
The echocardiogram should be performed once hydroelectrolytic and hemodynamic stabilization of the donor has been achieved, with at least 65mmHg of mean arterial pressure and adequate intravascular volume replacement. Szabo et al.40 demonstrated that maintenance of coronary perfusion pressure is the most important factor in reversing ventricular dysfunction. As far as possible, their performing should be deferred with respect to the onset of brain death, a fact that is more relevant in donors who have had uncontrolled CS. Probably to wait an hour between the diagnosis of BD and performing echocardiogram, as some authors recommend,23 is very little margin being recommended to wait at least 4h. If ventricular dysfunction is observed a second echocardiogram can be performed hours later, it can detect the normalization of contraction, as other authors have shown; Zaroff et al.75 demonstrated the recoverability of ventricular function in 75% of the cases studied in serial echocardiograms. In their work, 13 of the 16 heart donors initially excluded due to ventricular dysfunction recovered ventricular function within a variable period of time and, subsequently, their hearts were successfully transplanted. In the study by Venkateswaran et al.54 previously mentioned, the best contraction was observed in donors with more than 11h of evolution after BD. As recovery of ventricular function does not occur in 100% of cases to justify a long wait with the purpose that the contraction recovers, other tests could be performed that may guide this potential recovery. Thus, increased contraction following a dobutamine stimulation test could identify the contractile reserve of the dysfunctioning zones and distinguish the stunned myocardium from necrotic myocardium. Also, serial determination of enzymatic markers of myocardial damage may serve to make a decisión. Elevated levels of troponin T or troponin I, associated with alterations of contraction, may suggest a theoretically non-reversible structural damage at a prudent time. However, the finding of normal or near-normal troponins could lead to minimal structural damage and therefore justify some waiting times to detect the possible recovery of contraction.76
General care of the donorProbably in this aspect is where there is more agreement in the majority of the authors. Aspects such as the withdrawal of unnecessary drugs, e.g. anticonvulsants, osmotics, etc., and the continuation of other drugs, such as antibiotics and prophylactic measures of deep vein thrombosis or upper gastrointestinal bleeding, are common recommendations. Uncontrolled hyperglycemia has been shown to be a risk factor for renal and pancreatic graft malfunction. Maintaining glycemia below 180mg/dl, with continuous insulin administration, as recommended in other critically ill patients, is associated with a greater number of valid and better functioning organs, as suggested by the recent study that analyzed data from 1611 donors.77
There is also a majority agreement on the need to establish a protective ventilation to avoid acute lung injury that may induce mechanical ventilation in an inflammatory environment as in BD and to maintain the measures of prophylaxis of pneumonia associated with mechanical ventilation. Macías et al.78 in a multicenter, randomized study of 118 potential lung donors showed that the use of this type of ventilation doubled the number of transplanted lungs from 27% to 54% without affecting the 6-month survival of recipients.
Maintenance of normothermia is a common recommendation. However, a recent study of 370 donors, randomized to normothermia (36.5°–37.5°) or hypothermia (34°–35°), shows a lower incidence of graft function delay in kidneys from donors with hypothermia, (28.2% vs 39.2%), this benefit increases in kidneys from donors with expanded criteria.79 The study was stopped for clear benefit before recruiting the number of preestablished donors. This interesting work, however, presents some deficiencies and should only be considered as a hypothesis to be confirmed in other studies. For example, it only evaluates the incidence of delayed function but not other variables, such as the final evolution of the grafts. In addition, it does not evaluate the time of maintenance of the donor, which, as mentioned previously, may have an influence on the posterior renal function, probably the donors in hypothermia had a longer maintenance time, since at the usual time it would be necessary to add the necessary one to obtain the hypothermia from the normothermia that is required for the diagnosis of brain death. On the other hand, they do not use perfusion machines that may reduce the incidence of delayed donor graft function with expanded criteria.
The usual recommendation to maintain enteral nutrition,35 if it was previously instituted in the organ donor is also debatable. There are no studies to support this theoretical recommendation to maintain reserves of liver glycogen, and potential benefit in liver transplantation and intestinal tropism.80 Loss of vagal tone that accompanies BD is likely to disrupt intestinal transit and absorption of nutrients, and therefore, no benefits from maintenance of nutrition
ConclusionsThere are still many controversies in management of organ donor. The recent publication of randomized controlled trials has helped to unify criteria in some of them, but in other respects there are still sometimes conflicting recommendations. Many treatment elements are not specific to brain death so they should be based on the usual management in any other critically ill patient, and, in this case, taken in account the brainstem death pathophysiology (Fig. 1).
Conflict of interestsThe authors declare no conflict of interest with the content of the manuscript and no funding has been received for their preparation.