Septic shock is a highly lethal condition where several pathogenic factors are involved in progressive tissue hypoperfusion.1 Fluid resuscitation is a first-line therapy to reverse hypoperfusion. However, this may induce fluid overload, particularly when administered to fluid-unresponsive patients or when inappropriate resuscitation goals are pursued. Unfortunately, despite extensive research, many uncertainties remain on the best perfusion monitoring and resuscitation target.
The complexities of persistent hyperlactatemiaRecent guidelines recommend lactate normalization as a resuscitation target. However, the rationale of lactate-guided therapy has been challenged as it may expose patients to the risk of over-resuscitation considering that the decrease in lactate levels over time is relatively slow even in survivors.2,3 In addition, lactate is a non-specific marker of hypoperfusion and several pathogenic mechanisms besides hypoperfusion may be involved. Adrenergic-driven muscle glycolysis and impaired hepatic lactate clearance are important confounding mechanisms in septic shock.1,2 Recognizing a clinical pattern of hypoperfusion-related hyperlactatemia is important since optimizing systemic blood flow in that context could improve prognosis. In contrast, pursuing additional resuscitation in non-hypoperfusion-related cases might lead to the toxicity of over-resuscitation.
Multimodal perfusion monitoring including flow-sensitive parameters such as central venous O2 saturation (ScvO2), central venous-arterial pCO2 gradient (Pcv-aCO2), and peripheral perfusion, may disclose the presence of hypoperfusion-related hyperlactatemia when any of these variables is abnormal.1,2,4 Persistent hyperlactatemia without a hypoperfusion context is associated with a better prognosis,4 and eventually this condition could be managed more conservatively.
The role of capillary refill time (CRT) assessmentCRT emerges as a rational alternative to guide septic shock resuscitation.5 The skin territory lacks auto-regulatory flow control, and therefore, sympathetic activation impairs skin perfusion during circulatory dysfunction, a phenomenon that can be evaluated by peripheral perfusion assessment. Several studies confirm that abnormal peripheral perfusion after initial6 or advanced7 resuscitation is associated with increased morbidity and mortality.6,7 The improved prognosis associated with CRT normalization, its rapid-response time to fluid loading, its relative simplicity, its availability in resource-limited settings, and its capacity to change in parallel with perfusion of physiologically relevant territories such as the hepatosplanchnic region,8 constitute strong reasons to consider CRT as target for initial septic shock resuscitation.
The ANDROMEDA-SHOCK trialANDROMEDA-SHOCK was a multicenter, randomized controlled trial comparing CRT- versus lactate-targeted resuscitation in patients with early septic shock.5 The hypothesis was that targeting CRT assessed with a standardized method, would lead to decreased mortality and organ dysfunction. The protocol mandated sequential steps starting with fluid challenges, followed by vasoactive-related interventions if necessary, until the target was reached. CRT-targeted resuscitation was associated with lower mortality (34.9% vs. 43.4%; p=0.06), beneficial effects on organ dysfunction, and less treatment intensity. The worldwide impact and immediate application of CRT-guided resuscitation makes additional research an urgent task (Table 1).
Main findings of the ANDROMEDA-SHOCK study and research agenda for CRT.
| Main findings of the ANDROMEDA-SHOCK study favoring CRT-targeted septic shock resuscitation |
| Lower mortality (34.9% vs. 43.4%; p=0.06) |
| Less organ dysfunctions at 72h (p=0.045) |
| Lower mortality in the predefined subgroup of patients with less organ dysfunctions at baseline (20.4% vs. 39.3%; p=0.03) |
| Faster improvement in organ dysfunctions during the first 72h (p<0.001) |
| Less resuscitation fluids (p=0.01) |
| Less vasopressor testing (p=0.02) |
| Some unsolved issues and challenges for a CRT-focused research agenda |
| Mechanisms of the beneficial effect of CRT-targeted resuscitation in the ANDROMEDA-SHOCK study |
| Pathophysiologic determinants of an abnormal CRT |
| Does CRT accurately represent skin blood flow? |
| Is CRT an equivalent of a vascular occlusion test to detect abnormal microvascular reactivity? |
| Is there a relationship between an abnormal CRT and adrenergic tone? |
| Does CRT respond in real-time to increments in systemic blood flow? |
| What is the impact of vasoactive agents on CRT? |
| Does normalization of CRT after a fluid challenge predict the status of hemodynamic coherence between macrocirculation and regional/microcirculatory blood flow? |
CRT, capillary refill time.
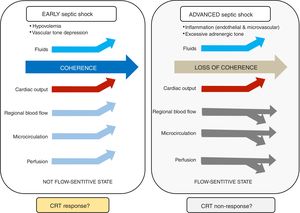
Hemodynamic coherence is a condition in which resuscitation of systemic macrohemodynamic variables results in concurrent improvement in regional and microcirculatory blood flow.9–11. Impaired vascular tone with decreased venous return and arterial hypotension are key pathogenic mechanisms in early septic shock. At this stage, fluid resuscitation (in fluid-responsive patients) and mean arterial pressure (MAP) optimization may improve macrocirculatory, regional, and microcirculatory blood flow, which is consistent with preserved hemodynamic coherence and associated with better prognosis (Figure 1).
At more advanced stages, when excessive adrenergic tone and microvascular/endothelial inflammation predominate, regional flow distribution and microcirculatory dysfunction may not respond to systemic blood flow optimization. Thus, hemodynamic coherence is lost, and efforts to further increase stroke volume or MAP by fluids or vasoactive agents might lead to fluid overload or catecholamine toxicity. This could result in worsening tissue perfusion by promoting interstitial edema, or by further deteriorating regional perfusion. How to treat patients at this stage is uncertain and a matter of future research, including the potential role of early immunomodulating therapies.
CRT: the link between macrocirculation and the microcirculation?Capillary refill time (CRT) appears as a physiologically sound target and its improvement after stroke volume optimization is a signal of tissue reperfusion in patients with septic shock. Some observations support the potential role of CRT in revealing the status of hemodynamic coherence. First, three recent studies show that patients with normal vs. abnormal CRT after fluid resuscitation exhibit a highly significant difference in mortality (ranging from 9 to 23% vs. 45 to 55%).5–7 This remarkable and consistent difference suggests, although does not prove, a preserved hemodynamic coherence in CRT normalizers (responders). Second, improvement in CRT after fluid resuscitation is associated with a parallel increase in hepatosplanchnic blood flow.8 Third, CRT showed the fastest kinetics of recovery in septic shock survivors as compared with other commonly used perfusion parameters.3 Fourth, normalization of CRT in the ANDROMEDA-shock study was associated with less organ dysfunction.5
On the other hand, an abnormal CRT not responding to increments in systemic flow might be explained by several mechanisms including a more advanced stage of septic shock with uncoupling or loss of hemodynamic coherence; an excessive adrenergic tone with regional hypoperfusion; or a more severe systemic inflammatory state with endothelial/coagulation activation/dysfunction which could lead to impairment and heterogeneity of microcirculatory flow.11 None of these mechanisms may respond to systemic flow optimization at this stage.
Based on the preceding considerations, we could propose that CRT response to a rapid flow increasing maneuver may be used as a novel “hemodynamic coherence test.” A parallel improvement in regional blood flow, microcirculation and hypoperfusion-related parameters should be expected in patients that normalize CRT, as reflection of preserved hemodynamic coherence.11
ConclusionsMultimodal perfusion monitoring might be useful to determine a hypoperfusion-context in persistent hyperlactatemia, thus promoting a physiologically-oriented septic shock resuscitation. CRT-guided septic shock resuscitation is associated with decreased mortality and organ dysfunction. CRT changes after rapid flow increasing maneuvers may identify the status of hemodynamic coherence, helping clinicians to decide on the most appropriate strategy for each stage. Further research is required to test these hypotheses.
Conflict of interestThe authors declare that they have no conflict of interest.