To describe the clinical and respiratory characteristics of a cohort of 43 patients with COVID-19 after an evolutive period of 28 days.
DesignA prospective, single-center observational study was carried out.
SettingIntensive care.
PatientsPatients admitted due to COVID-19 and respiratory failure.
InterventionsNone.
VariablesAutomatic recording was made of demographic variables, severity parameters, laboratory data, assisted ventilation (HFO: high-flow oxygen therapy and IMV: invasive mechanical ventilation), oxygenation (PaO2, PaO2/FiO2) and complications. The patients were divided into three groups: survivors (G1), deceased (G2) and patients remaining under admission (G3). The chi-squared test or Fisher exact test (categorical variables) was used, along with the Mann-Whitney U-test or Wilcoxon test for analyzing the differences between medians. Statistical significance was considered for p < 0.05.
ResultsA total of 43 patients were included (G1 = 28 [65.1%], G2 = 10 [23.3%] and G3 = 5[11.6%]), with a mean age of 65 years (range 52-72), 62% males, APACHE II 18 (15-24), SOFA 6 (4-7). Arterial hypertension (30.2%) and obesity (25.6%) were the most frequent comorbidities. High-flow oxygen therapy was used in 62.7% of the patients, with failure in 85%. In turn, 95% of the patients required IMV and 85% received ventilation in prone decubitus. In the general population, initial PaO2/FiO2 improved after 7 days (165 [125-210] vs.194 [153-285]; p = 0.02), in the same way as in G1 (164 [125-197] vs. 207 [160-294]; p = 0.07), but not in G2 (163 [95-197] vs. 135 [85-177]). No bacterial coinfection was observed. The incidence of IMV-associated pneumonia was high (13 episodes/1000 days of IMV).
ConclusionsPatients with COVID-19 require early IMV, a high frequency of ventilation in prone decubitus, and have a high incidence of failed HFO. The lack of improvement of PaO2/FiO2 at 7 days could be a prognostic marker.
Describir las características clínicas y respiratorias de una cohorte de 43 pacientes con COVID-19 tras 28 días de evolución.
DiseñoProspectivo observacional en un solo centro
ÁmbitoMedicina intensiva
PacientesPacientes ingresados por COVID-19 e insuficiencia respiratoria
IntervencionesNinguna.
VariablesSe obtuvieron de forma automática variables demográficas, de gravedad, de laboratorio, de asistencia ventilatoria recibida (OAF: oxigenoterapia alto flujo y VMI: ventilación mecánica invasiva), de oxigenación (PaO2, PaO2/FiO2) y de complicaciones. Los pacientes se dividieron en 3 grupos: supervivientes(G1), fallecidos(G2) y aquellos que continuaban ingresados(G3). Se utilizó “chi” cuadrado o Fisher (variables categóricas) y “U” Mann-Whitney o Wilcoxon para analizar la diferencia entre medianas. Se consideró significativo un valor de p < 0.05.
ResultadosSe incluyeron 43 pacientes (G1 = 28[65,1%],G2 = 10[23,3%] y G3 = 5[11,6%]), edad 65(52-72) años, 62% hombres, APACHE II 18(15-24), SOFA 6(4-7), Hipertensión arterial(30,2%) y obesidad(25,6%) fueron las comorbilidades más frecuentes. La OAF fue usada en el 62,7% de pacientes, 85% fracasó. El 95% de los pacientes necesitó VMI y el 85% ventilación en prono. En la población general, la PaO2/FiO2 inicial mejoró a los 7 días (165[125-210] vs. 194[153-285], p = 0.02), al igual que en G1(164[125-197] vs. 207[160-294], p = 0.07) pero no en G2 (163[95-197] vs. 135[85-177]). No se observó co-infección bacteriana. El desarrollo de neumonía asociada a la VMI fue elevado (13 episodios/1000 días de VMI).
ConclusionesLos pacientes con Covid-19 requieren VMI precoz, elevada frecuencia de ventilación en prono y presentan alta prevalencia de fracaso a OAF. La falta de mejoría de la PaO2/FiO2 a los 7 días podría ser un marcador de pronóstico.
On 11 March 2020, the World Health Organization (WHO) declared a new pandemic as the result of the rapid spread of the SARS-CoV-2 virus outside China.1 Patients infected with SARS-CoV-2 can develop serious viral pneumonia known as COVID-19, characterized by severe respiratory failure, and which has placed a heavy burden on Spanish Intensive Care Units (ICUs) and the national healthcare system as a whole.2,3 In Europe, the first case of adult acute respiratory distress syndrome (ARDS) attributable to SARS-CoV-2 was diagnosed in Italy on 20 February 2020,4 and a little less one month later the first patient with ARDS due to COVID-19 was admitted to our ICU.
The admission of patients with COVID-19 to intensive care varies markedly from one country to another, with prevalences ranging from 9% in Italy4 to 32% in China.5 According to data published by the National Epidemiological Surveillance Network of the Instituto de Salud Carlos III,6 on 16 of April 2020, of the total 59,094 hospitalized patients in Spain, 4390 were admitted to the ICU – this representing a proportion of 7.4%. Taking into account that both the characteristics of the patients admitted to the ICU due to COVID-19 and the care received – and hence the resulting crude mortality rate – can differ considerably among different centers7 and countries,3,5,7–10 the present study was carried out to describe the clinical and respiratory characteristics of a series of consecutive patients with severe COVID-19 in a Spanish tertiary hospital, differentiating the subjects according to ICU outcome after 28 days.
Material and methodsA prospective, observational cohort study was made, including all consecutive patients admitted to the Department of Intensive Care Medicine (DICM) from 14 March to 16 April 2020 with a confirmed diagnosis of SARS-CoV-2 infection, based on RT-PCR testing of nasopharyngeal swab and/or bronchial aspirate samples according to the criteria of the WHO.11 The RT-PCR tests were made in the reference laboratory (Hospital Clinic de Barcelona) up until 24 March 2020, after which testing was made in our laboratory, which was designated as reference laboratory for the province of Tarragona.
The study was approved by the Clinical Research Ethics Committee of Hospital Universitario de Tarragona Joan XXIII (# CEIM: 066/2020), and informed consent for secondary use of the automatically compiled data was verbally requested from the patients or their direct relatives, with due reporting of the fact in the electronic case history.
The present study was carried out using information stored in the database of the clinical information system (CIS) of our center (Centricity Critical Care® [CCC], General Electric). The data are compiled in the CIS on a routine basis through manual registries, automated capture from devices and automated integration with the laboratory and the Hospital Information System (SAP) of our center. Depending on the source and type of information, the latter is entered in different tables in the CIS database. Each table contains at least one field or attribute that relates it to another table within the system (relational schema) – thus allowing integration of all the data through extract, transform and load (ETL) processes. The ETL process that allowed generation of the cohort from the raw database tables of the CIS was fully implemented using free software (Python 3.0, Jupyter Notebook and Docker).
Study variablesDemographic data were collected, together with severity parameters (APACHE II SCALE), level of organ dysfunction upon admission (SOFA score) and comorbidities. Clinical variables (mean blood pressure [MBP], heart rate [HR] and respiratory frequency [RF]) and variables related to ventilatory support and oxygenation (need for invasive mechanical ventilation [IMV], high-flow oxygen therapy [HFO], arterial oxygen pressure [PaO2], arterial carbon dioxide pressure [PaCO2], fractional inspired oxygen [FiO2], arterial pH, PaO2/FiO2 ratio, peak pressure [Pmax], plateau pressure (PPl), positive end-expiratory pressure [PEEP]) were also recorded. In addition, we considered laboratory test parameters such as hemoglobin concentration, leukocyte count, lactate, C-reactive protein (CRP) and procalcitonin (PCT). All variables were recorded upon admission and after 7 days for comparative purposes.
The indication of orotracheal intubation (OTI), HFO or mechanical ventilation (MV) was established by the physician in charge of the patient. There was no specific respiratory management protocol for COVID-19. Due to the recommendation not to use noninvasive ventilation (NIV) or HFO because of the risk of aerosol generation,12 NIV was disadvised on the basis of internal consensus, and HFO was indicated in the context of a limited availability of respirators or as a strategy to delay and avoid MV. All the patients with failed HFO were intubated and subsequently received MV. No patients with limitations of life support were considered. The assessment of patient admission to the ICU was carried out in abidance with the ethical recommendations of the SEMICYUC.13
The patients were divided into three groups according to outcome after 28 days: survivors (group 1); non-survivors (group 2), and patients still in the ICU after 28 days (group 3).
Principal definitions- •
Severe SARS-CoV-2 pneumonia: defined according to the criteria of the Spanish Ministry of Health14 as a consistent clinical condition, with fever (body temperature > 38 °C), cough, sore throat, muscle pain and flu symptoms plus RT-PCR testing positive for SARS-CoV-2 and the presence of acute respiratory failure, with lung infiltrations on the chest X-rays, requiring admission to the ICU.
- •
Bacterial co-infection: considered in patients with confirmed COVID-19 in which culture of the lower airway sample (bronchoaspirate [BAS] or bronchoalveolar lavage [BAL]) obtained within the first 24 hours of admission reveals the presence of a pathogenic microorganism at concentrations above the cut-off points defined for each technique.15
- •
Ventilator-associated pneumonia (VAP): defined according to the new ATS/IDSA guidelines,16 and corresponding to patients who develop a clinical condition characterized by increased radiological infiltration, changes in appearance of the secretions, and culture positivity (BAS or BAL sample) for pathogenic microorganisms at concentrations above the cut-off points defined for the technique, in a sample obtained 48 hours after the start of MV.
- •
Ventilator-associated pneumonia incidence rate per 1000 ventilator days, expressed as density (number of cases per day/person/exposure) and calculated using the formula: incidence rate = number of cases of VAP during the study period/total days/person/exposure to MV in the population during the study period * 1000.
- •
Failure of HFO: defined as the need for immediate intubation and subsequent mechanical ventilation. The need for OTI was based on clinical and blood gas criteria, and was left to the criterion of the physician in charge.
- •
Shock upon admission: defined as the need for any dose of noradrenalin within the first 6 hours of admission in order to maintain mean blood pressure, and once the required volume replacement measures have been adopted, based on dynamic parameters or echocardiography.
- •
Acute respiratory distress syndrome: classified according to the definition of Berlin17 into two groups: severe/moderate and mild.
- •
Acute renal failure was established based on the RIFLE classification,18 with three defined categories.
Previously published criteria were used in reference to the comorbidities and the rest of definitions.19
Statistical analysisIn view of the characteristics of the study, no sample size calculation was made. The sample size therefore was the same as the number of patients admitted during the study period. Continuous variables were reported as the median and interquartile range (IQR), while categorical variables were reported as frequencies and percentages. Differences in distribution of the variables between groups of patients were explored using the chi-square test or the Fisher exact test (categorical variables). Differences between medians were evaluated using the nonparametric Wilcoxon test or Mann-Whitney U-test. Due to the limited number of cases, no multivariate analyses were made. Statistical significance was considered for p < 0.05. The SPSS® version 25.0 statistical package (IBM) was used throughout.
ResultsGeneral characteristicsFrom 1 February to 14 April 2020, a total of 380 cases of COVID-19 were diagnosed in our hospital. Of these, 43 (11.3%) required admission to the ICU due to acute respiratory failure, and constituted the subject of the present analysis. Seventeen patients (39.5%) were admitted from Internal Medicine, 15 (34.9%) were transferred from other hospitals, and 11 (25.6%) came from the Emergency Department of our hospital. Although the patients received conventional oxygen therapy and in some cases HFO before admission to our ICU, these data were not available for analysis. The median time from arrival in hospital to admission to the ICU was one day (IQR: 0.0-3.0).
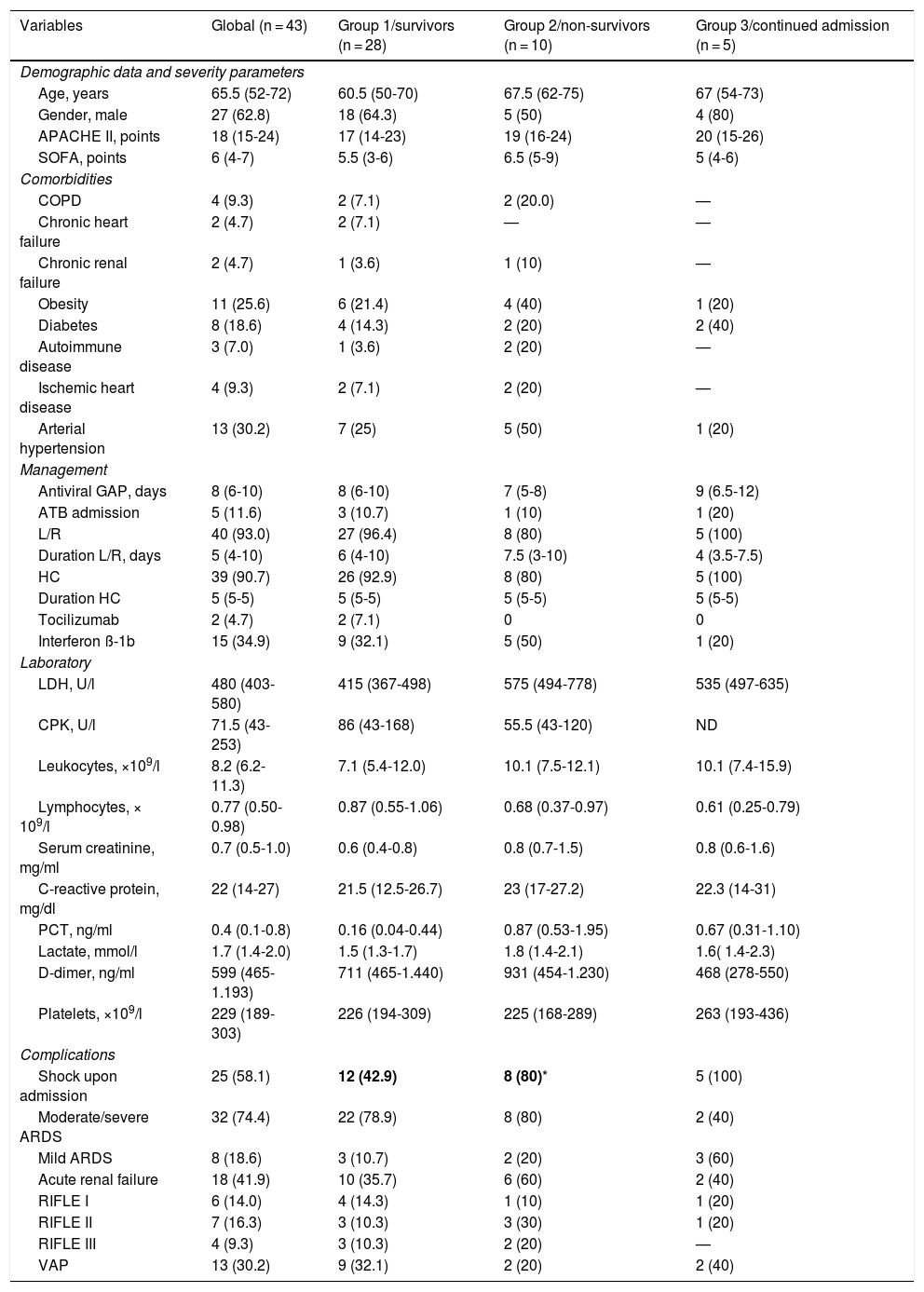
The baseline characteristics of the patients are shown in Table 1. At the time of the analysis, 28 patients were discharged live (group 1 = 65.1%), 10 patients died (group 2 = 23.3%) and 5 remained in admission (group 3 = 11.6%). The median number of days to death was 23 (IQR: 11-40), and only one patient (2.3%) died within the first 24 hours of admission. The mean duration of stay in the ICU for the patients in group 1 was 27 days (IQR: 13-34).
General characteristics of the 43 patients with severe COVID-19.
| Variables | Global (n = 43) | Group 1/survivors (n = 28) | Group 2/non-survivors (n = 10) | Group 3/continued admission (n = 5) |
|---|---|---|---|---|
| Demographic data and severity parameters | ||||
| Age, years | 65.5 (52-72) | 60.5 (50-70) | 67.5 (62-75) | 67 (54-73) |
| Gender, male | 27 (62.8) | 18 (64.3) | 5 (50) | 4 (80) |
| APACHE II, points | 18 (15-24) | 17 (14-23) | 19 (16-24) | 20 (15-26) |
| SOFA, points | 6 (4-7) | 5.5 (3-6) | 6.5 (5-9) | 5 (4-6) |
| Comorbidities | ||||
| COPD | 4 (9.3) | 2 (7.1) | 2 (20.0) | — |
| Chronic heart failure | 2 (4.7) | 2 (7.1) | — | — |
| Chronic renal failure | 2 (4.7) | 1 (3.6) | 1 (10) | — |
| Obesity | 11 (25.6) | 6 (21.4) | 4 (40) | 1 (20) |
| Diabetes | 8 (18.6) | 4 (14.3) | 2 (20) | 2 (40) |
| Autoimmune disease | 3 (7.0) | 1 (3.6) | 2 (20) | — |
| Ischemic heart disease | 4 (9.3) | 2 (7.1) | 2 (20) | — |
| Arterial hypertension | 13 (30.2) | 7 (25) | 5 (50) | 1 (20) |
| Management | ||||
| Antiviral GAP, days | 8 (6-10) | 8 (6-10) | 7 (5-8) | 9 (6.5-12) |
| ATB admission | 5 (11.6) | 3 (10.7) | 1 (10) | 1 (20) |
| L/R | 40 (93.0) | 27 (96.4) | 8 (80) | 5 (100) |
| Duration L/R, days | 5 (4-10) | 6 (4-10) | 7.5 (3-10) | 4 (3.5-7.5) |
| HC | 39 (90.7) | 26 (92.9) | 8 (80) | 5 (100) |
| Duration HC | 5 (5-5) | 5 (5-5) | 5 (5-5) | 5 (5-5) |
| Tocilizumab | 2 (4.7) | 2 (7.1) | 0 | 0 |
| Interferon ß-1b | 15 (34.9) | 9 (32.1) | 5 (50) | 1 (20) |
| Laboratory | ||||
| LDH, U/l | 480 (403-580) | 415 (367-498) | 575 (494-778) | 535 (497-635) |
| CPK, U/l | 71.5 (43-253) | 86 (43-168) | 55.5 (43-120) | ND |
| Leukocytes, ×109/l | 8.2 (6.2-11.3) | 7.1 (5.4-12.0) | 10.1 (7.5-12.1) | 10.1 (7.4-15.9) |
| Lymphocytes, × 109/l | 0.77 (0.50-0.98) | 0.87 (0.55-1.06) | 0.68 (0.37-0.97) | 0.61 (0.25-0.79) |
| Serum creatinine, mg/ml | 0.7 (0.5-1.0) | 0.6 (0.4-0.8) | 0.8 (0.7-1.5) | 0.8 (0.6-1.6) |
| C-reactive protein, mg/dl | 22 (14-27) | 21.5 (12.5-26.7) | 23 (17-27.2) | 22.3 (14-31) |
| PCT, ng/ml | 0.4 (0.1-0.8) | 0.16 (0.04-0.44) | 0.87 (0.53-1.95) | 0.67 (0.31-1.10) |
| Lactate, mmol/l | 1.7 (1.4-2.0) | 1.5 (1.3-1.7) | 1.8 (1.4-2.1) | 1.6( 1.4-2.3) |
| D-dimer, ng/ml | 599 (465-1.193) | 711 (465-1.440) | 931 (454-1.230) | 468 (278-550) |
| Platelets, ×109/l | 229 (189-303) | 226 (194-309) | 225 (168-289) | 263 (193-436) |
| Complications | ||||
| Shock upon admission | 25 (58.1) | 12 (42.9) | 8 (80)* | 5 (100) |
| Moderate/severe ARDS | 32 (74.4) | 22 (78.9) | 8 (80) | 2 (40) |
| Mild ARDS | 8 (18.6) | 3 (10.7) | 2 (20) | 3 (60) |
| Acute renal failure | 18 (41.9) | 10 (35.7) | 6 (60) | 2 (40) |
| RIFLE I | 6 (14.0) | 4 (14.3) | 1 (10) | 1 (20) |
| RIFLE II | 7 (16.3) | 3 (10.3) | 3 (30) | 1 (20) |
| RIFLE III | 4 (9.3) | 3 (10.3) | 2 (20) | — |
| VAP | 13 (30.2) | 9 (32.1) | 2 (20) | 2 (40) |
APACHE II: Acute Physiology and Chronic Health Evaluation; ATB: antibiotic; COPD: chronic obstructive pulmonary disease; antiviral GAP: days from symptoms onset to first dose of antiviral drug; HC: hydroxychloroquine; L/R: lopinavir/ritonavir; RIFLE: renal dysfunction scale; ARDS: acute respiratory distress syndrome; SOFA: Sequential Organ Failure Assessment; VAP: ventilator-associated pneumonia.
All comparisons taking group 1 as reference.
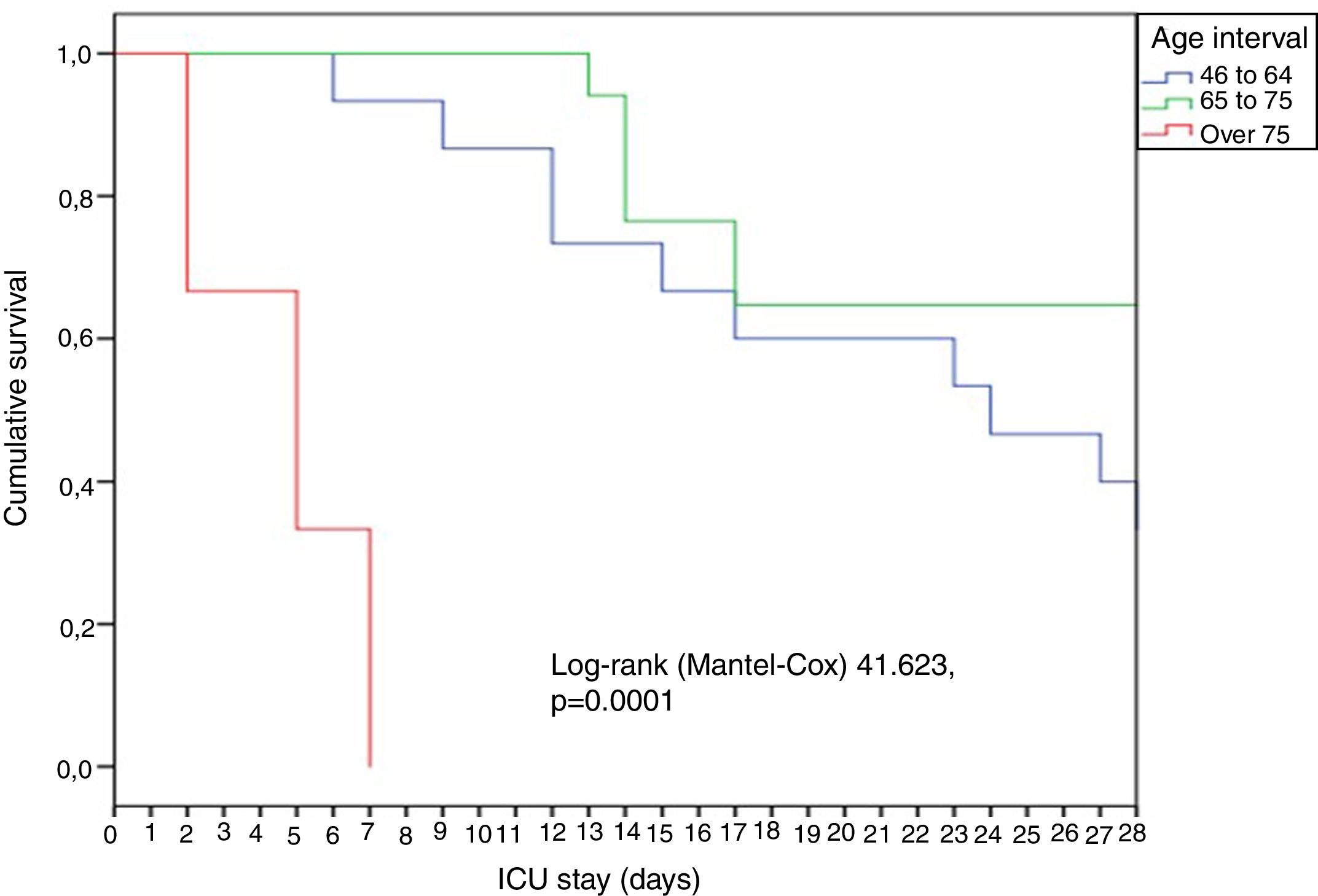
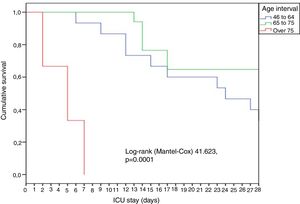
In general, the patients were young (65 years), predominantly males, and with great disease severity as evidenced by the median APACHE (18) and SOFA scores (6). A significant rise (p = 0.02) in mortality was observed over the increasing age intervals: 18-45 years (0%), 46-64 years (13.3%), 65-75 years (35.3%) and > 75 years (66.7%). The patients over 75 years of age died early, within the first week (Fig. 1).
Forty-four percent of the patients registered no comorbidities. None of the healthcare staff members of our hospital were admitted to the ICU due to COVID-19 during the observation period. The main comorbidities were arterial hypertension (30.2%) and obesity (25.6%), followed by diabetes mellitus (18.6%), chronic obstructive pulmonary disease (COPD)(9.3%) and ischemic heart disease (9.3%). Of the 13 patients with arterial hypertension, 7 (53.8%) had a history of drug treatment in the form of angiotensin converting enzyme inhibitors (ACEIs) (n = 2) or angiotensin receptor antagonists (ARAs)(n = 5). Only the presence of shock upon admission was more frequent among the non-survivors (80%) than among the survivors (group 1 = 42.9%) (Table 1). The other variables considered showed no statistically significant differences between the groups.
TreatmentThe time from symptoms onset to the first antiviral drug dose was considerable, with a median of 8 days (IQR: 6-10) – no differences being observed between the groups. Lopinavir/ritonavir was administered in 40 cases (93%), with a median duration of only 5 days (IQR: 3.5-10) due to complications related to important transaminase elevation requiring treatment suspension. Hydroxychloroquine was used in 39 patients (90.7%), with a median duration of 5 days. Interferon ß-1b was only administered to 15 patients (34.9%), due to drug availability problems, and only two patients (4.7%) received tocilizumab in other centers, before transfer to our ICU (Table 1). The administration of corticosteroids was not considered among the treatments for COVID-19. Such medication was only used upon admission to the ICU in a single patient (2.3%) as continuation of pre-existing chronic treatment. Five patients (11.6%) received methylprednisolone as rescue therapy due to persistent lung infiltrations after the second week of stay, and four patients (9.3%) received the medication due to other indications such as thrombocytopenia (n = 1), hemolytic anemia (n = 1) and skin rash (n = 2).
Ventilatory supportAll the patients required some type of ventilatory support during the first hours of admission to the ICU. High-flow oxygen was used as initial treatment for respiratory failure due to COVID-19 in 27 patients (62.7%). However, after 24 hours, only four patients responded favorably – this representing a failure rate of 85.2% (4/27). On day 7, only two patients maintained HFO, and these were discharged live, with no need for any other type of ventilatory support. The median time to HFO failure was 8 hours (IQR: 6-20). All the patients in which HFO failed were subsequently intubated and ventilated. Invasive mechanical ventilation was used as first treatment option for acute respiratory failure in 16 patients (37.2%). However, during the first 24 hours, 37 (86%) of the 43 patients were ventilated, and finally after 7 days, 41 (95%) required IMV. Of the patients subjected to IMV, 34 (82%) required at least one ventilation episode in prone decubitus, with a median of three maneuvers (IQR: 1-5) per patient – though some underwent over 10 ventilation periods in prone decubitus. Only one patient (2.3%) was referred to the reference hospital for ECMO, and this individual finally died. The median number of days of IMV was 27 (IQR: 15-38), and proved similar in the survivors (23 days [IQR: 12-30]) and non-survivors (27 days [IQR: 13-38]; p = 0.37). Of the 37 patients that required IMV, 32 (86.5%) had completed their course in the ICU at the time of the analysis, with a crude mortality rate of 28.1% (9/32), which was similar to that recorded among the patients who did not require IMV in the first 24 hours (16.6%; p = 0.55).
A total of 93% of the patients (40/43) met criteria of ARDS, which proved moderate/severe in 32 cases (74.4%) and mild in 8 (18.6%). Of the 32 patients with moderate/severe ARDS, 27 (72.9%) received IMV after 24 hours, but four (14.8%) remained with HFO, and one patient with a face mask and reservoir. All of the patients with mild ARDS required IMV after 24 hours.
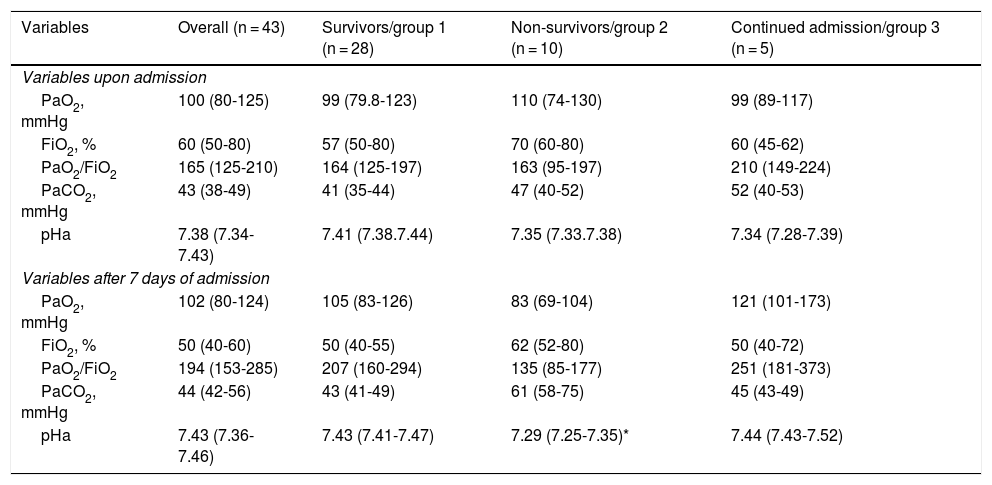
Independently of the ventilatory support received, the median PaO2/FiO2 upon admission was 165 (IQR: 125-210), and the ratio increased significantly up until day 7, reaching 194 (IQR: 153-285; p = 0.05). A similar PaO2/FiO2 behavior was recorded in the survivors, though without reaching statistical significance (p = 0.07), while the non-survivors showed a discrete decrease in PaO2/FiO2 after 7 days despite the treatment provided (Table 2).
Variables related to oxygenation of the 43 patients, stratified according to outcome.
| Variables | Overall (n = 43) | Survivors/group 1 (n = 28) | Non-survivors/group 2 (n = 10) | Continued admission/group 3 (n = 5) |
|---|---|---|---|---|
| Variables upon admission | ||||
| PaO2, mmHg | 100 (80-125) | 99 (79.8-123) | 110 (74-130) | 99 (89-117) |
| FiO2, % | 60 (50-80) | 57 (50-80) | 70 (60-80) | 60 (45-62) |
| PaO2/FiO2 | 165 (125-210) | 164 (125-197) | 163 (95-197) | 210 (149-224) |
| PaCO2, mmHg | 43 (38-49) | 41 (35-44) | 47 (40-52) | 52 (40-53) |
| pHa | 7.38 (7.34-7.43) | 7.41 (7.38.7.44) | 7.35 (7.33.7.38) | 7.34 (7.28-7.39) |
| Variables after 7 days of admission | ||||
| PaO2, mmHg | 102 (80-124) | 105 (83-126) | 83 (69-104) | 121 (101-173) |
| FiO2, % | 50 (40-60) | 50 (40-55) | 62 (52-80) | 50 (40-72) |
| PaO2/FiO2 | 194 (153-285) | 207 (160-294) | 135 (85-177) | 251 (181-373) |
| PaCO2, mmHg | 44 (42-56) | 43 (41-49) | 61 (58-75) | 45 (43-49) |
| pHa | 7.43 (7.36-7.46) | 7.43 (7.41-7.47) | 7.29 (7.25-7.35)* | 7.44 (7.43-7.52) |
FiO2: fractional inspired oxygen; PaCO2: arterial carbon dioxide pressure; PaO2: arterial oxygen pressure.
All comparisons taking group 1 as reference.
*p < 0.05 Data reported as median and interquartile range (IQR).
**p < 0.001.
A total of 11.6% of the patients (n = 5) received antimicrobial treatment (ATB) upon admission to the ICU due to clinically suspected bacterial co-infection. Four patients received ceftriaxone plus a macrolide, and one patient was treated with piperacillin / tazobactam plus a macrolide. No pathogenic microorganisms were isolated from the lower airway samples (BAS = 5) of these patients. In all subjects, antigen testing in urine for S. pneumoniae and Legionella spp. proved negative, in the same way as the bronchoaspirate and blood cultures performed upon admission to the ICU. No case of bacterial co-infection was recorded in our series. The median PCT concentration upon admission was 0.45 ng/ml (IQR: 0.04-2.45), while the median C-reactive protein concentration was 23 mg/dl (IQR: 10-28). On the other hand, 13 of the 43 patients (30.2%) developed VAP, representing an incidence of 13.3 cases/1000 days of IMV. The microorganisms isolated were S. anginosus (n = 3), P. aeruginosa (n = 3), methicillin-sensitive S. aureus (MSSA)(n = 2), E. coli (n = 1), S. oralis (n = 1), K. pneumoniae (n = 1), E. faecalis (n = 1) and Corynebacterium spp. (n = 1). None of the respiratory sample analyzed proved positive for Aspergillus spp.
DiscussionOur study describes the course of 43 seriously ill patients with COVID-19 during the first four weeks of stay in the ICU of a tertiary hospital. Despite the limited number of patients, the results obtained are of great interest due to the existing lack of knowledge of the evolution of this new disease and the differences in the characteristics of the patients. One of the main findings of our study was that one of every two admitted patients had no major comorbidities. This observation is consistent with the data reported by other studies8–11 that describe the absence of comorbidities in over 60% of the patients. In a way similar to what was seen in the influenza A (H1N1)pdm09 pandemic,20 obesity was very common in our patients. In contrast, obesity was not mentioned in the studies carried out in China9 or Italia,3 though it is indeed cited in the published experience in Vitoria (Basque Country)7 and in a recent study in the United States21 – where the incidence of obesity was even higher (41.7%). This situation could complicate direct extrapolation of the international data,3,8–11,21,22 since different populations are involved.
Another relevant finding was that the mortality rate (23.2%) in our series was lower than that in the study from Vitoria7 (31%), despite the fact that the patients were of similar age and severity, and presented a similar frequency of IMV (94%). Different publications also report a higher mortality rate. In the study published by Yang et al.,9 the overall mortality rate was 61.5%, though on considering the patients subjected to IMV, the figure reached 81% (30/37) – which is far higher than the rate obtained in our study (28.1%). It should be noted that in the mentioned study, of the 52 critical patients, only 22 (42%) received IMV. Although the authors did not report the time from failure of other oxygenation techniques to the start of IMV, the high mortality among the patients subjected to IMV suggests an important delay in orotracheal intubation. On the other hand, Wu et al.10 reported an overall mortality rate of 21.9%. In their study, of the 210 included patients, 165 (78.5%) received some type of ventilatory support, though there was a notoriously low incidence of IMV (3%), while almost half of the patients (48.8%) received nasal cannulas and 30% were subjected to NIV. In turn, Grasselli et al.,4 in Italy, reported a mortality rate of 26%. Although this study made no mention of severity scales, the included population appears to have been more similar to our own series, since of the 1591 subjects, 1287 (80%) were admitted to the ICU, and of these, 89% required IMV. Although the authors associated mortality to the age of the patients, the median age was no different from that of our own series (63 versus 65 years). In New York,21 of 5700 patients with COVID-19, only 6.5% (n = 373) were admitted to the ICU, and 320 of these individuals (85.8%) required IMV. The mortality rate in this subgroup was very high (88.1%). Similar mortality was recorded by Arentz et al.22 in a small population of 21 patients. The high mortality in this series could be related to the fact that the median age was older than in most communications (70 years, IQR: 40-92). In contrast to the above, our mortality rate was markedly higher than in the study published by Guan et al.8 (only 1.4%). In the latter study, most of the 1099 patients were not considered to be in serious condition (none required ventilation). In the cases considered to be serious (15.7%; 173/1.099), the mortality rate was 8.1%, despite the fact that of these individuals only 25 (2.2%) received IMV. It is clear that the international studies involve other types of populations and particularly other types of ventilatory support, and this makes it very difficult to extrapolate such experiences to our own setting. The data obtained therefore need to be interpreted with caution.
Another observation of interest is the fact that despite the contraindications to the use of HFO,12 the latter was started in over 60% of the patients as first line treatment. Nevertheless, the technique failed in over 85% of the cases. Although the available data do not allow us to evaluate the impact of a delay in intubation upon the patient course, a gap of 8 hours from HFO failure to intubation possibly may have no strong influence upon the clinical course, considering the observed mortality rate.
A matter of concern was the high VAP rate observed (30% or 13 cases/1000 days of IMV), which more than doubled the usual VAP rate in our ICU. This incidence was higher than that reported by Xang et al.9 (13.5%), though the latter authors did not specify the days of risk exposure – thereby making it difficult to establish comparisons. The urgency of care during the pandemic, the use of personal protection equipment (PPE), the rotation of scantly trained staff, and a decrease in the VAP preventive measures may help explain this increase. Nevertheless, such a high incidence must be confirmed by other studies.
Lastly, our data show that among the non-survivors, PaO2/FiO2 upon admission did not improve after 7 days despite the treatment provided. In the survivors, however, PaO2/FiO2 was seen to increase after 7 days. It therefore may be postulated that a lack of improvement in PaO2/FiO2 after one week of treatment could be a prognostic factor to be considered in future studies.
Our study clearly has important limitations that need to be mentioned. The first and possibly most important limitation is the small number of patients involved, which may preclude the identification of differences between groups due to type I error. Nevertheless, given the novel characteristics of this pandemic, our results do contribute to existing knowledge – though the findings must be confirmed by studies involving larger patient samples. On the other hand, our results describe the evolution in a special type of ICU, and might not be extendable to other areas or ICUs. It is clear that both the indication of admission to the ICU, and the complexity of care of these patients, vary greatly among different centers and countries. Such information therefore needs to be analyzed carefully in each study.
In conclusion, although our data describe a not particularly old patient population with a low prevalence of comorbidities, COVID-19 is seen to often require IMV due to ARDS, and is characterized by a high incidence of HFO failure and important mortality. A lack of improvement of PaO2/FiO2 after one week of active treatment could be regarded as a variable associated to early mortality – though these data require confirmation in future studies.
AuthorshipStudy conception and design: AR, MB, LC, GM, RC, and ST.
Data acquisition and analysis: AR, MB, JG, GM, RC, EPP, ST, CBB, RSP, LC and XT.
Data interpretation: AR, MB, LC, GM, RC, ESP, LC, ESP and EPP.
Important intellectual contribution to the contents of the study: AR, MB, GM, RC, ESP, LC, and LC.
Drafting of the manuscript: AR, MB, GM, and RC.
Critical review of the contents of the article: JG, EPP, CBB, RSP, ST and XT.
All the authors approved the final manuscript submitted for evaluation and possible publication.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to the present article.
AR has received a research grant from Gilead Science for the study of nebulized antibiotics, and has received fees for teaching conferences from Biomerieux, Astellas, Pfizer, Thermo Fisher, MSD, Gilead, Shionogi and BRHAMS. There are no conflicts of interest in relation to the present study, however. The rest of the authors have no conflicts of interest.
The HJ23-COVD-19 working group thanks all the healthcare staff of HJ23 related to the care of patients with COVID-19 for their unwavering dedication and effort during the period of this study. This article could not have been produced without them.
The findings and the conclusions of the present manuscript are the responsibility of the authors, and do not necessarily represent the official position of the Catalan Health Institute.
Clinical laboratory
Natalia Bastón-Paz, Carolina Sarvisé-Buil, Frederic Gómez-Bertomeu, Gemma Recio-Comi, Carla Martin-Grau, Silvia Montolio-Breva, Victoria Rivera-Moreno, Modest Sabaté-Piñol, Carmen Molina-Clavero, Nuria Serrat-Orús, Maria Teresa Sans-Mateu and Cristina Gutiérrez-Fornes.
Epidemiology and prevention of nosocomial infections
M. Montserrat Olona-Cabases.
ICU Nursing Department
Xavier Teixidó, Diana Gil-Castillejos and Nuria Burló-Arévalo.
Clinical Pharmacy
Laura Canadell and Erika Esteve-Pitarch.
ICU physicians
María Bodi, Alejandro Rodríguez, Gerard Moreno, Christian Villavicencio, Mari Carmen Gilavert, Sara Rosich, Ángel Pobo, Mónica Magret, Gonzalo Sirgo, Vanessa Blázquez, Federico Esteban, Iulen Leache, Paula Perello, Iban Oliva, Manuel Samper, Oriol Plans, Marc Cartanyá, Sandra Canelles, Raquel Carbonell, Neus Guasch, Cristina Ferré, Sara Manrique, Xavier Daniel, Silvia Urgeles, Ivan David, Marina Roure, Natalia Murillo, Marina Sánchez and Melina Salgado.
ICU Data-Analyses
Josep Gómez, Manuel Ruiz-Botella, Jordi Albiol and Eduard Mayol.
Please cite this article as: Rodríguez A, Moreno G, Gómez J, Carbonell R, Picó-Plana E, Benavent Bofill C, et al. Infección grave por coronavirus SARS-CoV-2: experiencia en un hospital de tercer nivel con pacientes afectados por COVID-19 durante la pandemia 2020. Med Intensiva. 2020. https://doi.org/10.1016/j.medin.2020.05.018