Few studies have evaluated the impact in diagnosis and therapeutic management of basic transthoracic echocardiography in postoperated cardiac surgery. The aim of our study was to evaluate the impact of basic transthoracic echocardiography in the management of this kind of patients.
DesignOver an 18-month period, we prospectively studied all patients admitted to a university hospital Intensive Care Unit following heart surgery. We evaluated clinically all of them to establish a diagnosis and an initial treatment. We performed basic transthoracic echocardiography for a diagnosis evaluation that was compared with clinical diagnosis. If they differed, we assessed to change treatment and evaluate the therapeutic response. We performed a descriptive analysis.
ResultsWe included 136 patients and performed 203 echocardiographies. Transthoracic echocardiography differed of initial diagnosis in 101 (49.8%) echocardiographies. In 56 of these echocardiographies (55.44%), we could give an alternative diagnosis with a change in the treatment in 30 patients (53.6%). We found clinical improvement in 26 patients (86.76%) in the following 30−60 min.
ConclusionsBasic transthoracic echocardiography is useful in diagnostic and therapeutic management of postoperative cardiac surgery patients. We could not confirm the clinical diagnosis in half of the performed echocardiographies. In most patients in whom we observe a change in the diagnosis due to echocardiography, we observed a clinical improvement after changing the treatment.
Pocos estudios han evaluado el impacto en el diagnóstico y tratamiento de la ecocardiografía transtorácica básica en los pacientes postoperados de cirugía cardíaca. El objetivo de nuestro estudio fue valorar el impacto de la ecocardiografía transtorácica básica en el manejo diagnóstico y terapéutico de estos pacientes.
DiseñoDurante 18 meses se estudiaron prospectivamente todos los pacientes postoperados de cirugía cardíaca que ingresaron en el Servicio de Medicina Intensiva de un hospital universitario. Se realizó una valoración clínica a todos ellos para establecer un diagnóstico y tratamiento inicial. Se realizó una ecocardiografía transtorácica básica para valoración diagnóstica, que se comparó con la valoración clínica. En caso de discrepancia, se valoró cambiar el tratamiento en función a la ecocardiografía y se evaluó la respuesta terapéutica. Se realizó un análisis descriptivo de los hallazgos.
ResultadosSe incluyeron 136 pacientes y se realizaron 203 ecocardiografías. La ecocardiografía transtorácica difería del diagnóstico inicial en 101 (49,8%) ecocardiografías. En 56 de éstas (55,44%) se obtuvo un diagnóstico alternativo, lo que comportó un cambio en el tratamiento en 30 pacientes (53,6%). Encontramos mejoría clínica significativa en 26 de estos pacientes (86,76%) en los siguientes 30-60 minutos.
ConclusionesLa ecocardiografía transtorácica básica es útil en el manejo diagnóstico y terapéutico de los pacientes postoperados de cirugía cardíaca. En la mitad de las ecocardiografías realizadas no se pudo confirmar el diagnóstico clínico. En la mayoría de pacientes en los que observamos cambio en el diagnóstico debido a la ecocardiografía, se observó mejoría clínica tras el cambio de tratamiento.
Transthoracic echocardiography (TTE) is a noninvasive imaging technique that proves very useful in the critical care setting. It allows point-of-care (at the patient bedside), real-time dynamic imaging of the heart and also provides morphological and functional information. The data afforded by echocardiography often allow us to identify the causes of shock, and offer useful guidance for assessing treatment. Some studies have shown that echocardiography can provide information leading to significant modification of treatment in up to 52% of all critical patients. As a result, some groups have developed TTE protocols designed to optimize the treatment of these patients.1–20
Although studies have been made by intensivists on the usefulness of transesophageal echocardiography (TEE) in critical patients,21,22 very few publications have assessed the applicability and impact of specific goal-directed basic TTE upon the diagnostic and therapeutic management of patients in the postoperative period of heart surgery.1,23–25 Recently, our group has published a study showing that basic TTE can be performed in most such patients, with the generation of interpretable data.26
The present study was carried out to prospectively evaluate the impact of specific goal-directed basic TTE upon the diagnosis and treatment of patients following heart surgery.
Material and methodsSelection of patientsThe study was carried out in the Department of Intensive Care Medicine of a University general hospital with 16 polyvalent beds that attends 100 post-heart surgery patients a year.
We included all patients over 18 years of age subjected to heart surgery and consecutively admitted to the Intensive Care Unit (ICU) between November 2014 and April 2016, with the physical presence of an intensivist with certified training in basic echocardiography.
The study protocol was approved by the local Clinical Research Ethics Committee. All patients gave written informed consent to participation in the study.
Clinical characteristicsAt the time of patient admission, we recorded the general demographic characteristics, the reason for admission, main comorbidities, severity scores (APACHE II, EuroSCORE) and hemodynamic and echocardiographic data prior to surgery, if available.
A clinical and hemodynamic evaluation of the patient was made by one of the 6 intensivists of the Department to establish an initial diagnosis, in accordance with routine clinical practice in post-heart surgery patients: hypovolemia, left ventricular dysfunction, right ventricular dysfunction, biventricular dysfunction, vasoplegia, massive mitral valve insufficiency or tamponade, and treatment was prescribed based on the diagnosis.
Basic echocardiographyFollowing the initial clinical evaluation and within the first 8 h of admission, another intensivist trained in basic echocardiography performed basic TTE. At the time of the exploration, the use of mechanical ventilation was documented, as well as the administration of vasoactive medication, and the hemodynamic parameters (heart rate, blood pressure, central venous oxygen saturation, lactate concentration).
The echocardiographic study was repeated daily in the presence of signs of tissue hypoperfusion (arterial hypotension, evidence of poor peripheral perfusion, elevated lactate levels, oliguria). The professional performing the echocardiographic evaluation was blinded to the initial tentative diagnosis.
The echocardiographic analysis comprised systematic assessment of the long and short axes parasternal projections and four-chambers apical and four-chambers subcostal projections with visualization of the inferior vena cava. For each acoustic window, imaging difficulty was rated as either high or low. All explorations were made using the 1.5–4.6 MHz M5S-D echocardiographic probe of the Vivid-e (General Electrics) system, available 24 h in the ICU of our center.
The analyzed echocardiographic parameters were:
- 1)
Left ventricle: visual size defined as normal or dilated, visual systolic function classified as normal (≥ 50%) or depressed (< 50%), and contraction pattern defined as either homogeneous or heterogeneous.
- 2)
Right ventricle: visual size defined as normal or dilated, and visual systolic function (normal or depressed).
- 3)
Valves: presence of massive mitral valve insufficiency.
- 4)
Inferior vena cava: classified as normal, dilated or not visualized.
- 5)
Pericardial fluid with or without cardiac tamponade, classified as either present or absent.
The echocardiographic patterns were defined as5,6,27:
- •
Severe hypovolemia: small hyperdynamic ventricles, with obliteration of the left ventricular cavity (kissing walls), and small inferior vena cava showing great variation with respiratory movements (under mechanical ventilation, inferior vena cava < 1.5 cm with collapse > 50%; under spontaneous ventilation, inferior vena cava < 1 cm with collapse > 50%).
- •
Left ventricular failure: reduction of global left ventricular motility, heterogeneous contractility pattern suggesting myocardial ischemia, or dilatation of the left ventricular cavity suggesting chronic heart disease.
- •
Right ventricular failure: dilatation of the right ventricle and paradoxical motion of the interventricular septum - which in the critical patient is basically associated to acute respiratory distress syndrome (ARDS) or severe pulmonary thromboembolism. Isolated right ventricular dilatation is suggestive of right ventricular infarction. Other associated findings may be a dilated inferior vena cava without collapse.
- •
Tamponade: presence of pericardial effusion with diastolic collapse of the right atrium and ventricle, and dilated inferior vena cava without respiratory collapse under spontaneous breathing conditions.
- •
Acute massive mitral valve insufficiency: left ventricle of normal size (indicative of acute valve disease), left ventricle with normal or hyperdynamic systolic function secondary to volume overload of the left ventricle and massive reflow in the color Doppler exploration.
All the explorations were digitally recorded and later evaluated by an intensivist with advanced training in TTE (A.O.), rating the quality of the images as optimal (complete identification of the endocardial margins of the left ventricle), suboptimal (identification of > 50% of the endocardial margins of the left ventricle) or poor (identification of < 50% of the endocardial margins of the left ventricle, or visualization not possible). The analysis was based only on the optimal and suboptimal echocardiographic images. We determined the percentage of cases in which echocardiography confirmed the initial tentative or suspected diagnosis. If this diagnosis was not confirmed, we determined the percentage of cases in which echocardiography afforded an alternative diagnosis and the percentage of changes in treatment. Lastly, clinical improvement associated to the different therapeutic changes was analyzed.
In the event of doubt regarding the echocardiographic data, a second echocardiographic assessment was made by a cardiologist with expertise in advanced echocardiography.
Statistical analysisA descriptive study was made, analyzing percentage clinical improvement secondary to treatment optimization. Continuous variables were reported as the median and standard deviation (SD), or as percentages.
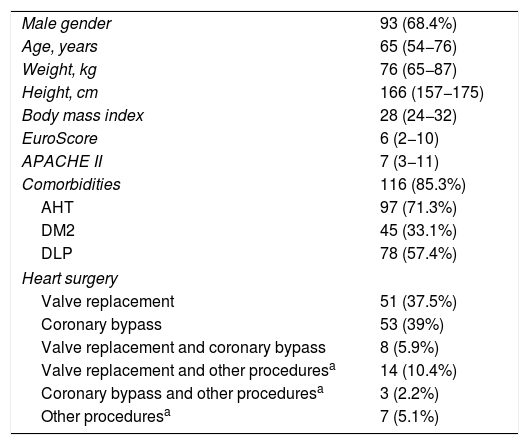
ResultsPatient characteristicsThe study sample consisted of 136 post-heart surgery patients consecutively enrolled in the period between November 2014 and April 2016, and representing a total of 203 echocardiographic studies. No patients were excluded during the study period. The demographic and clinical data of the sample are reported in Table 1.
Demographic and clinical data, and severity predictors.
| Male gender | 93 (68.4%) |
| Age, years | 65 (54−76) |
| Weight, kg | 76 (65−87) |
| Height, cm | 166 (157−175) |
| Body mass index | 28 (24−32) |
| EuroScore | 6 (2−10) |
| APACHE II | 7 (3−11) |
| Comorbidities | 116 (85.3%) |
| AHT | 97 (71.3%) |
| DM2 | 45 (33.1%) |
| DLP | 78 (57.4%) |
| Heart surgery | |
| Valve replacement | 51 (37.5%) |
| Coronary bypass | 53 (39%) |
| Valve replacement and coronary bypass | 8 (5.9%) |
| Valve replacement and other proceduresa | 14 (10.4%) |
| Coronary bypass and other proceduresa | 3 (2.2%) |
| Other proceduresa | 7 (5.1%) |
DLP: dyslipidemia; DM2: type 2 diabetes mellitus; AHT: arterial hypertension.
The figures express the mean (standard deviation) or frequency (percentage).
With regard to the type of heart surgery, most patients underwent valve replacement either isolatedly (51 patients; 37.5%) or associated to other procedures (22 patients; 16.3%). A total of 64 coronary revascularization surgeries were performed (47.1%), either isolatedly or associated to other procedures. Seven patients underwent other types of surgery (5.1%).
With regard to the valve replacements, 49 (67.12%) comprised biological valves, 21 (28.77%) mechanical valves, and three (4.11%) corresponded to annuloplasties. There were 54 aortic valve replacements (39.7%), 17 mitral valve replacements (12.5%), four mitral annuloplasties (2.94%) and 15 tricuspid annuloplasties (11.03%).
All patients were subjected to preoperative TTE performed by a cardiologist during admission or on an ambulatory basis a few days before admission. This echocardiographic study showed that most patients had a left ventricle with no hypertrophy (72 patients; 52.94%) or dilatation (115 patients; 84.56%), and with a normal ejection fraction (Simpson’s rule 4C) (127 patients; 93.38%). The right ventricle was not dilated in most patients (131 patients; 96.32%), and exhibited normal function in all cases. Most of the patients had a non-dilated inferior vena cava (128 patients; 94.11%).
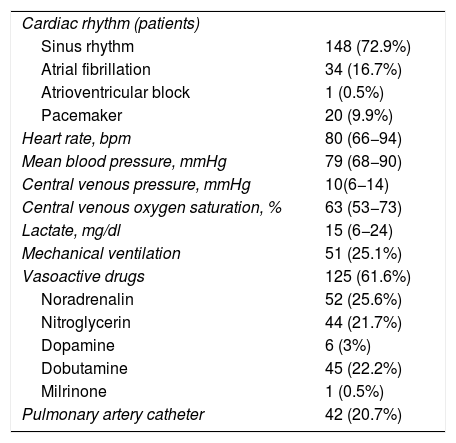
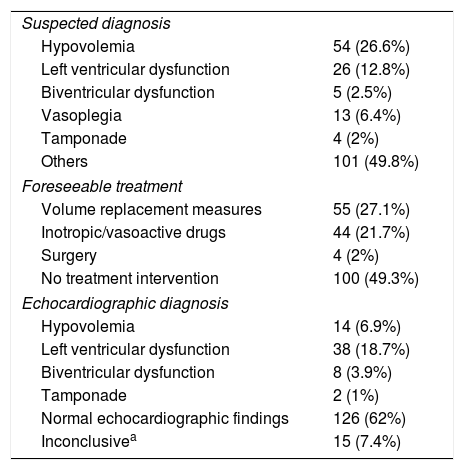
Postoperative echocardiographyA total of 203 TTEs were performed, distributed as follows: 97 patients (71.3%) underwent a single echocardiographic study; 19 (14%) underwent two echocardiographic studies; 14 (10.3%) underwent three echocardiographic studies; four (2.9%) underwent four echocardiographic studies; and two patients (1.5%) underwent 5 echocardiographic studies. Table 2 shows the clinical data at the time of echocardiography. The main indication of the first echocardiographic study was hemodynamic instability (74 cases; 54.4%), and the main initially suspected diagnosis was hypovolemia (54 cases; 26.6%). The other 62 echocardiographic studies (45.6%) were performed with some other suspected diagnosis, or as controls. The echocardiographic studies performed on the following days were due to hemodynamic instability. With regard to the contemplated initial management based on the clinical data, in most cases (100 cases; 49.3%) no surgery was foreseeable. Table 3 reports the suspected diagnoses, the contemplated management before echocardiography and the echocardiographic diagnosis.
Clinical data at the time of echocardiography.
| Cardiac rhythm (patients) | |
| Sinus rhythm | 148 (72.9%) |
| Atrial fibrillation | 34 (16.7%) |
| Atrioventricular block | 1 (0.5%) |
| Pacemaker | 20 (9.9%) |
| Heart rate, bpm | 80 (66−94) |
| Mean blood pressure, mmHg | 79 (68−90) |
| Central venous pressure, mmHg | 10(6−14) |
| Central venous oxygen saturation, % | 63 (53−73) |
| Lactate, mg/dl | 15 (6−24) |
| Mechanical ventilation | 51 (25.1%) |
| Vasoactive drugs | 125 (61.6%) |
| Noradrenalin | 52 (25.6%) |
| Nitroglycerin | 44 (21.7%) |
| Dopamine | 6 (3%) |
| Dobutamine | 45 (22.2%) |
| Milrinone | 1 (0.5%) |
| Pulmonary artery catheter | 42 (20.7%) |
The figures express the mean (standard deviation) or frequency (percentage).
Suspected diagnosis and foreseeable treatment before echocardiography, and echocardiographic diagnosis.
| Suspected diagnosis | |
| Hypovolemia | 54 (26.6%) |
| Left ventricular dysfunction | 26 (12.8%) |
| Biventricular dysfunction | 5 (2.5%) |
| Vasoplegia | 13 (6.4%) |
| Tamponade | 4 (2%) |
| Others | 101 (49.8%) |
| Foreseeable treatment | |
| Volume replacement measures | 55 (27.1%) |
| Inotropic/vasoactive drugs | 44 (21.7%) |
| Surgery | 4 (2%) |
| No treatment intervention | 100 (49.3%) |
| Echocardiographic diagnosis | |
| Hypovolemia | 14 (6.9%) |
| Left ventricular dysfunction | 38 (18.7%) |
| Biventricular dysfunction | 8 (3.9%) |
| Tamponade | 2 (1%) |
| Normal echocardiographic findings | 126 (62%) |
| Inconclusivea | 15 (7.4%) |
The figures express the mean (standard deviation) or frequency (percentage).
Most of the echocardiographic studies were made with the patient in supine decubitus (197 patients; 97%), and the mean duration of the exploration was 14.46 ± 2.16 min. Evaluation was made of imaging difficulty and imaging quality in each of the planes. In most cases, difficulty was low in all planes, and imaging quality was found to be optimal or suboptimal in most patients. The poorest imaging quality corresponded to the subcostal plane for visualizing the inferior vena cava. On analyzing imaging quality in each plane, we found the long axis parasternal plane to yield 85 echocardiographic studies (41.9%) of optimal quality, 81 (39.9%) of suboptimal quality and 37 (18.2%) of poor quality. The short axis parasternal plane in turn yielded 80 echocardiographic studies (39.4%) of optimal quality, 82 (40.4%) of suboptimal quality, and 41 (20.2%) of poor quality. The four-chambers apical plane yielded 135 echocardiographic studies (66.5%) of optimal quality, 42 (20.7%) of suboptimal quality, and 26 (12.8%) of poor quality. The subcostal plane yielded 122 echocardiographic studies (60.1%) of optimal quality, 29 (14.3%) of suboptimal quality, and 52 (25.6%) of poor quality. Lastly, with regard to visualization of the inferior vena cava, there were 116 echocardiographic studies (57.1%) of optimal quality, 19 (9.4%) of suboptimal quality, and 68 (33.5%) of poor quality. All the echocardiographic studies had some plane of optimal or suboptimal imaging quality, thus allowing echocardiographic assessment in all cases.
On analyzing the different echocardiographic parameters, we found the left ventricle to be of normal size (175 cases; 86.2%) and with normal ventricular function in most cases (151 cases; 74.4%), but with heterogeneous contractility in most patients (111 cases; 54.7%). Only 12 patients (5.9%) presented kissing walls. The right ventricle in turn was of normal size and function in most of the cases (184 cases; 90.6%). Sixty patients (29.6%) showed the presence of pericardial fluid, but only two (1.5%) presented signs of tamponade, and thus underwent surgery again.
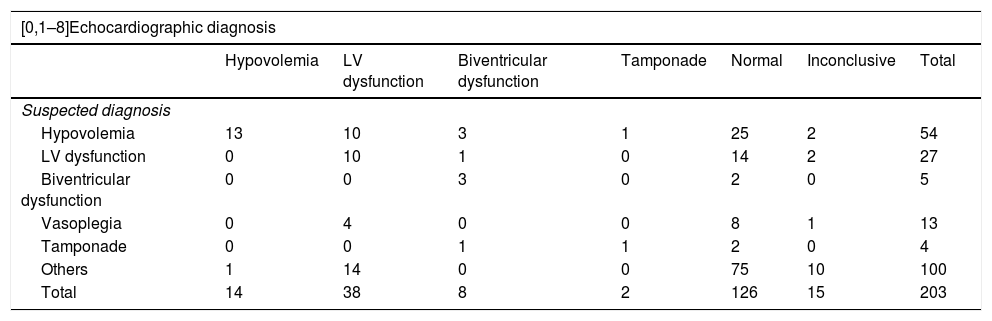
Table 4 compares the suspected diagnoses based on the clinical findings versus the echocardiographic diagnosis. Overall, the echocardiographic diagnosis was consistent with the suspected clinical diagnosis in 102 cases (50.2%). In one-half of the cases (101 cases, 49.8%), echocardiography differed from the clinical diagnosis. In these 101 cases, echocardiography proved normal in 45 (44.6%); no alternative diagnosis therefore could be established. In 56 cases (55.4%), echocardiography offered a diagnosis different from the suspected clinical diagnosis, and treatment changes were decided in 30 of these cases (53.6%). The changes predominantly involved the start of inotropic treatment (14 cases, 25%). In relation to whether the changes were made on occasion of the first echocardiographic study or as a result of the subsequent echocardiographic explorations, we found that in a large percentage of cases in which a change in diagnosis occurred (34 cases, 60.71%), the change took place on occasion of the first echocardiographic study and implied a modification of the patient management approach in 22 cases (64.71%). In the other 22 cases with discrepancies between the echocardiographic diagnosis and the clinical diagnosis, the treatment changes occurred on occasion of the subsequent echocardiographic studies. All the changes made can be seen in Table 5.
Comparison of the clinical diagnoses versus the echocardiographic diagnoses.
| [0,1–8]Echocardiographic diagnosis | |||||||
|---|---|---|---|---|---|---|---|
| Hypovolemia | LV dysfunction | Biventricular dysfunction | Tamponade | Normal | Inconclusive | Total | |
| Suspected diagnosis | |||||||
| Hypovolemia | 13 | 10 | 3 | 1 | 25 | 2 | 54 |
| LV dysfunction | 0 | 10 | 1 | 0 | 14 | 2 | 27 |
| Biventricular dysfunction | 0 | 0 | 3 | 0 | 2 | 0 | 5 |
| Vasoplegia | 0 | 4 | 0 | 0 | 8 | 1 | 13 |
| Tamponade | 0 | 0 | 1 | 1 | 2 | 0 | 4 |
| Others | 1 | 14 | 0 | 0 | 75 | 10 | 100 |
| Total | 14 | 38 | 8 | 2 | 126 | 15 | 203 |
LV: left ventricle.
Changes in treatment due to the echocardiographic findings.
| Volume replacement measures | 1 (3.33%) |
| Start of inotropic/vasoactive drugs | 14 (46.67%) |
| Surgery | 1 (3.33%) |
| Othersa | 14 (46.67%) |
The figures express frequency (percentage).
In one case presenting diagnostic uncertainty, we contacted the Cardiology Department, which performed a new echocardiographic study that confirmed our own echocardiographic suspicion (severe pericardial effusion with signs of tamponade). We therefore consulted Heart Surgery, which decided repeat surgery, followed by clinical improvement.
Of the 30 cases in which treatment was modified on the basis of the echocardiographic diagnosis, 26 (86.67%) showed significant clinical improvement, with amelioration of the hemodynamic parameters (blood pressure, central venous oxygen saturation, increased diuresis, with a lesser need for inotropic and vasoactive drugs) over the following 30-60 min. On examining the treatment changes, we found that 14 patients (46.7%) started inotropic or vasoactive drug treatment, while another 14 patients (46.7%) underwent some other intervention such as the suspension of volume replacement measures or of inotropic medication. One patient (3.3%) received volume replacement measures, and another (3.3%) required repeat surgery because of tamponade. Four patients (13.33%) experienced no clear improvement as a result of the treatment adjustments. Two of them had started inotropic or vasoactive drug treatment (one with poor tolerance of dobutamine due to tachycardia, and another with scant hemodynamic improvement after dobutamine dosing). In another case, due to the presence of bradycardia, we decided to increase the heart rate by means of a pacemaker, though the tissue hypoperfusion parameters did not improve as a result. Lastly, in the fourth case, the clinical suspicion was hypovolemia with normal echocardiographic findings; we therefore decided not to administer volume replacement measures - though no significant clinical changes were observed.
DiscussionFollowing demonstration of the feasibility of basic echocardiography in the most extreme situations after heart surgery,26 it was important to study the impact of basic echocardiography on the management of post-heart surgery patients. In the present study, echocardiography did not confirm the clinically suspected diagnosis in almost one-half of the cases. On the other hand, echocardiography offered an alternative diagnosis in over 50% of these cases. In turn, the clinicians decided a change in treatment based on the result of the echocardiographic exploration in 30 cases (14.8% of the total patients and 53.6% with a change in diagnosis). This percentage corresponds to the lower range of the data reported in other studies, with treatment modification rates of up to 52%.1,21,24,28 This may be because patients with different clinical characteristics were involved. The previous studies included medical-surgical patients in which cardiac disorders not foreseeable on the basis of the initial clinical condition could be involved. In contrast, all of our patients were in the postoperative period of heart surgery, and consequently the fundamental causes of shock were easier to suspect on the basis of the clinical data. In some cases the clinical suspicion was hypovolemia, though with basic echocardiography we are only able to detect the presence of severe hypovolemia. Other studies in heart surgery patients offer similar data referred to the impact of transesophageal echocardiography,29–31 though these studies were made during the actual operation, resulting changes in management approach during surgery. In our case we used basic transthoracic echocardiography, which is totally noninvasive, during the immediate postoperative period.
Heiberg et al.25 conducted a meta-analysis of 15 studies evaluating the use of transthoracic and transesophageal echocardiography in the postoperative period of heart surgery. The authors concluded that echocardiography is increasingly used in the postoperative period of heart surgery, and that its use results in significant changes in the diagnosis and management of these patients – though the mentioned studies were not designed to assess the patient course over time. Of the studies included in the meta-analysis, 7 analyzed the use of transthoracic echocardiography,2,32–37 and documented changes in diagnosis and management in 10-52% of the cases. Our own findings fall within this range. Of note is the observation that the treatment introduced after echocardiography resulted in clinical improvement in 86.67% of our patients – thus confirming the clinical usefulness of echocardiography in patients following heart surgery.
An interesting observation in our series is the fact that 74% of the echocardiographic explorations showed a normal left ventricular ejection fraction (LVEF), and that 90% recorded a right ventricular ejection fraction (RVEF) within normal ranges. This could be because in most of our patients the preoperative LVEF and RVEF values were respectively normal in 125 patients (92%) and in all patients – thus explaining that these parameters were preserved in the postoperative period. Nevertheless, we found the introduction of vasoactive medication due to the echocardiographic diagnosis to be the most frequent change in treatment, resulting in clinical improvement of the patient.
Thus, our results show that the echocardiographic study results in a change in diagnosis in up to 50% of all heart surgery patients. Furthermore, in over one-half of these cases changes in patient management are decided as a consequence of the echocardiographic findings - and this in turn is associated to clinical improvement in the great majority of cases. Nevertheless, in the event of doubts, advanced echocardiography should be performed by an adequately trained intensivist or a cardiologist, as required.
Our study has some limitations. In effect, it is a single-center investigation involving one same surgical team; our findings therefore cannot be extrapolated to other centers. All surgeries were elective and were performed in clinically stable patients; the risk of postoperative complications was therefore lower. Some patients already presented ventricular dysfunction at preoperative echocardiography; as a result, in the postoperative period, such dysfunction persisted in the echocardiographic study – without this having clinical repercussions. Likewise, basic echocardiography is only able to detect severe hypovolemia, and thus some patients could have presented hypovolemia that was not evidenced in the echocardiographic study. These circumstances may have influenced the observation of a low percentage change in the echocardiographic diagnosis versus the clinical diagnosis.
ConclusionsBasic transthoracic echocardiography is useful for the diagnostic and therapeutic management of patients following heart surgery. In most cases in which a change in diagnosis was made based on the echocardiographic findings, clinical improvement was noted as a result of the modification of treatment.
Financial supportNone.
Author contributionsStudy conception and design: Dr. Olga Moreno, Dr. Ana Ochagavía, Dr. Antonio Artigas, Dr. Francisco Baigorri.
Data acquisition, analysis and interpretation: Dr. Olga Moreno, Dr. Ana Ochagavía, Dr. Sandra Barbadillo, Dr. Roser Tomás, Dr. Maria Dolores Bosque, Dr. Cristina Fortià.
Drafting of the manuscript: Dr. Olga Moreno, Dr. Ana Ochagavía, Dr. Antonio Artigas, Dr. Francisco Baigorri.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors thank Cristina Mora, Cristina Espinal, María Luisa Martínez, Susana Millan and Eva Torrents (Hospital Universitari General de Catalunya) for their contribution to data collection.
Please cite this article as: Moreno O, Ochagavía A, Artigas A, Barbadillo S, Tomás R, Bosque MD, et al. Impacto en el manejo diagnóstico/terapéutico de la ecocardiografía básica dirigida a objetivos concretos en una UCI de cirugía cardíaca. Med Intensiva. 2020;155:534–541.