Immunosuppression in transplantation has experienced changes in recent years as a result of the introduction of new drugs that act upon the different pathways of the host immune response with the purpose of securing more individualized immune suppression, with fewer side effects.
Although following in the steps of other solid organ transplant modalities, lung transplantation, because of its special characteristics, has not yielded similar middle- and long-term results.
Improved understanding of the underlying rejection mechanisms, the pharmacodynamic control of drugs, new administration routes designed to reduce the side effects, and new drug substances or immune modulating processes will all contribute to improve the expectations associated to lung transplantation in the near future.
La inmunosupresión en el trasplante se ha modificado en los últimos años con el descubrimiento de nuevos fármacos que intentan atacar las distintas vías de la respuesta inmunológica, con la idea de conseguir una inmunosupresión más personalizada y con menores efectos secundarios.
A pesar de seguir los pasos de los otros trasplantes de órganos sólidos, el trasplante pulmonar, por sus especiales características no ha conseguido similares resultados a medio y largo plazo.
El mejor entendimiento de los mecanismos de rechazo, el control farmacodinámico de los fármacos, las nuevas vías de administración que disminuyan los efectos secundarios y los nuevos fármacos o procesos inmunomoduladores contribuyen a mejorar las expectativas de este trasplante en un próximo futuro.
Lung transplantation was the last solid organ transplant modality incorporated to the group of transplantation procedures known to afford good results. At present, it is an accepted treatment choice for a selected group of patients with end-stage lung disease.
While taking advantage of the experience gained with other types of organ transplants, lung transplantation, because of its special characteristics, has not yielded similar long-term results. In this context, survival rates of 80% in the first year and of 50% after 5 years of follow-up are regarded as adequate.
Regarding the immunological factors, the main problems posed by lung transplantation are:
- •
Direct graft contact with the exterior through the upper airway. Such direct communication not only facilitates exposure to germs and the development of infections, but also constitutes a vehicle for other harmful factors produced by the body itself (e.g., gastroesophageal reflux or nasal or oral cavity colonizations) or contained in the air we breathe. Such aggression in some cases triggers the host immune response, which can lead to rapid or progressive graft rejection if not adequately controlled.
- •
The impossibility of cross-matching prior to transplantation.
- •
The high antigen content of the donor lung.
These factors imply that lung transplantation requires important immunosuppression, particularly in the immediate postoperative period. Despite such measures, however, the acute rejection rate in this period remains high.
The introduction of new and more potent immunosuppressors that act upon the different pathways mediating the immune response allow us to provide more individualized immunosuppression. In the early days, the immunosuppressive therapy used was fundamentally supported by the experience gained in the transplantation of other organs, followed later on by the findings of retrospective studies often corresponding to a single center, and which documented the first specific experiences in lung transplantation. The lack of scientific evidence led to the conduction of randomized multicenter studies, which produced ideas but were unable to establish conclusive evidence reinforcing the use of such drugs in lung transplantation. For this reason, in the course of the present study most of the recommendations are based on publications with a low grade of evidence, and some of the recommended drugs have not been approved for use in lung transplantation.1
Classical immunosuppression has always included the utilization of three drugs, associated or not to induction, using mono- or polyclonal antibodies.
The inclusion of three drugs helps minimize their side effects and allows us to attack different pathways of the immune response. With this aim in mind, we usually combine a calcineurin inhibitor (cyclosporine or tacrolimus), an antiproliferative drug (azathioprine or mycophenolate mofetil) and corticosteroids.2
The present study reviews and offers an update on some of the most important aspects of immunosuppression in lung transplantation.
The current state of inductionThe main objective of induction treatment is to reduce acute rejection in the first moments of transplantation through inhibition of the proliferation or depletion of the T lymphocytes, which are regarded as the main effectors of the host cellular immune response.
Induction with OKT3 was used in the first cardiopulmonary transplants, and posteriorly the polyclonal antibodies played an important role in the beginning of lung transplantation–though the high infection rate involved encouraged the avoidance of induction except in selected cases. In 2001, a comparative study of OKT3, ATG and daclizumab showed an increased bacterial infection rate among the patients treated with OKT3, in comparison with the other two induction regimens; as a result, OKT3 was abandoned as an induction agent in lung transplantation. None of the induction agents delayed the development of chronic rejection or improved patient survival.3
Polyclonal antibodies and IL-2 antagonists usually have been found to be effective in reducing the number of acute rejections in the immediate postoperative period. On the other hand, these drugs allow us to postpone the start of immunosuppression in cases of postsurgical renal failure.4
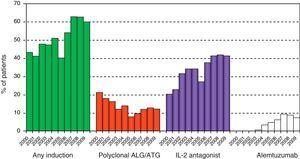
Although the main randomized, prospective multicenter trial (LUNAS) only reported a lesser number of acute rejections among the patients treated with Basiliximab®, and showed no significant differences with respect to the development of bronchiolitis obliterans syndrome (BOS) or survival (personal communication), the absence of side effects recorded in this trial and in other clinical studies made with other chimeral monoclonal antibodies4,5 is possibly the main reason why in recent years a larger number of transplant groups have again started to use induction in the initial management of lung transplantation, as it can be seen in the figures of the ISHLT registry (Fig. 1).6
Use of induction in recent years in lung transplantation. ISHLT registry. Analysis limited to patients receiving prednisone and alive at hospital discharge. ALG, anti-lymphocytic globulin; ATG, anti-thymocytic globulin; and IL-2R, interleukin-2 receptor. (Adapted from Christie et al.6)
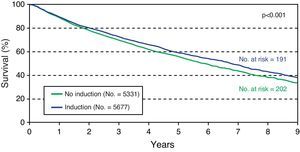
The data of this international registry, which show improved survival among patients with induction (Fig. 2),6 the possibility of reducing the number of acute rejections and of avoiding renal damage, with a reduction in the start or a lowering of the levels required for correct immunosuppression, are all sufficiently important factors that likewise support the use of induction therapy.5
Evolution of lung transplant survival between 2000 and 2009, stratified according to the use of induction therapy. ISHLT registry. (Adapted from Christie et al.6)
A relatively new development is the use of alemtuzumab for induction in a limited number of hospitals. This is a humanized monoclonal antibody targeted not only to antigen CD52, present on the surface of the B and T cells, but also in macrophages, monocytes, NK cells and thymocytes. Alemtuzumab produces important leukocyte depletion, with recovery of the different cell populations in different post-transplantation periods,7 resulting in less severe acute rejection episodes and a decrease in cytomegalovirus (CMV) rates compared with induction using thymoglobulin. However, a current publication has found no differences in survival or acute rejection in patients treated with and without alemtuzumab.8 A recent retrospective study has analyzed the data collected on a prospective basis in a single center corresponding to 336 lung graft recipients classified according to the type of induction used: thymoglobulin, alemtuzumab, daclizumab, or no induction. An analysis was made of patient and graft survival, the acute cellular rejection rate, lymphocytic bronchiolitis and bronchiolitis obliterans, and lymphoproliferative disorders following transplantation. Alemtuzumab offered better results in comparison with the other options, except as regards the lymphoproliferative syndromes, where no differences were observed.9
New developments in maintenance immunosuppressionAnticalcineurinic drugs remain the basic option in immune suppression among lung transplant patients. Tacrolimus and Neoral® cyclosporine have been shown to be excellent immunosuppressors. Monitorization, which is exclusively pharmacokinetic, is one of the main problems for achieving the most effective and individualized immunosuppression, and the side effects of these drugs represent an important cause of long-term morbidity–mortality in these patients.
A number of clinical trials have associated tacrolimus with a lesser incidence of acute rejection and BOS.10,11 It has also been seen that switching from cyclosporine A (CsA) to tacrolimus stabilizes the evolution of BOS,12 and recently a multicenter study has shown that after three years, tacrolimus reduces the risk of developing BOS with respect to CsA.13 Thus, the latest data of the international registry show that the use of tacrolimus is greater both in immunosuppression at discharge and in maintenance.6
The main novelty in this area has been the introduction of Advagraf®, a new tacrolimus formulation affording prolonged release of the drug. This new medication allows the utilization of tacrolimus in a single dose, thereby contributing to improve adherence to therapy. To date, the experience gained with this formulation in lung transplantation has been limited to single-center series involving a limited number of stable patients converted to Advagraf®, and in which the change was found to be safe14. A pharmacokinetic study has also been published involving 20 stable patients subjected to mg: mg conversion from Prograf® to Advagraf®–the resulting data referred to AUC and Cmin being comparable, with a good AUC/Cmin correlation for both formulations.15 In the transplantation of other organs, a number of studies point to the safety of Advagraf®, particularly in relation to kidney, liver and heart transplantation.16
Another novelty, of special interest in the immediate postoperative period, refers to the administration of tacrolimus via the sublingual route, this being particularly indicated in cases of gastroparesis in the immediate postoperative interval. Although the mechanism of absorption of the product has not been established, this route helps achieve correct blood drug concentrations and avoids the need for intravenous dosing and its corresponding neurotoxicity.17
Regarding the anti-lymphocytic agents, in recent years the mTOR (mammalian target of rapamycin) drugs have been introduced. These are semisynthetic derivatives of the natural immunosuppressive macrolide rapamycin, with purported immunosuppressor, antineoplastic and antifibrotic actions.
In the cells, these drugs form a complex that suppresses cytokine mediated T cell proliferation, inhibiting progression of the G phase to the S phase of the cell cycle.
Sirolimus and everolimus are the two main drugs of this family, with a common mechanism of action, and sharing much of the toxicity and side effects.
The main complication of this group of drugs is that their antiproliferative effect interferes with healing. They therefore cannot be used in the immediate postoperative period. Some studies have related the use of these agents to an increased incidence of bronchial suture dehiscences.18
Although there is little supporting evidence for the use of mTOR drugs in the usual management of lung transplant patients,19 the published studies and personal experience has caused the Spanish groups to recommend their use in renal failure20 and BOS, in patients with more than three months and less than 5 years of follow-up, provided the change in medication is made in the early stages of both complications. Likewise, it appears advisable to use their antiproliferative effect in selected patients with certain malignant tumors after transplantation.21
Inhalatory immunosuppressorsOne of the advantages in lung transplantation is the administration of treatments via the inhalatory route, thus allowing direct access of the medication to the transplanted organ. On the other hand, this administration route avoids systemic side effects, since the drug is absorbed in only small amounts.
A randomized, prospective study of inhalatory cyclosporine versus placebo showed significant improvements in terms of survival and BOS-free interval, with no differences in adverse effects or infections.22 These findings corresponding to a single center have stimulated the conduction of multicenter trials which are presently in progress.
Inhalatory corticosteroids have been used in many respiratory diseases, though the studies conducted in lung transplantation have produced no significant evidence supporting their use. In any case, some authors suggest their utilization in lymphocytic bronchiolitis, based on the possible reduction of airway inflammatory markers.23
Immune modulating drugsSome drugs used for prophylactic purposes in transplantation have revealed immune modulating activities that advise their utilization with this new function in lung transplantation.
In a heart transplant study, pravastatin was found not only to lower blood cholesterol but also to reduce the acute rejection rate and coronary disease, and to improve survival in the first year after transplantation. In lung transplantation, a retrospective study compared a group of 39 patients administered statins versus a control group of 161 patients not administered such drugs–with the observation of significant improvements in survival and lesser acute and chronic rejection rates. On the basis of these results, and since many studies have shown the statins to possess antiinflammatory and immune modulating properties, some groups have considered adding such treatment on a systematic basis in patients with suspected or confirmed BOS.24
Azithromycin and other macrolides have demonstrated immune modulating effects in cystic fibrosis, in addition to action upon bacterial adhesion and biofilm formation in the airway. This indicates that azithromycin exerts a protective effect against infection and subsequent inflammation in these patients. On the other hand, the drug increases gastric motility, thereby offering the possibility of improving gastroesophageal reflux. It has been shown to benefit patients who are beginning to develop BOS. The individuals who responded best to treatment showed >15% neutrophils and high levels of IL-8 in the bronchoalveolar lavage. As a result, azithromycin is presently regarded as a long-term treatment in this group of patients. Although the different mechanisms of action could stimulate a more global use of the drug, it must be remembered that resistances can develop, particularly in patients susceptible to developing infections produced by atypical mycobacteria.25
Humoral rejectionAcute rejection classically has been regarded as a predominantly cellular phenomenon. However, in recent years it has been shown to generally coexist with humoral rejection mediated by anti-HLA antibodies that may be present in the recipient or which may be formed de novo in the post-transplantation period. The underlying mechanism of action is still not fully understood, though humoral rejection makes an important contribution to graft dysfunction over the middle and long term.26 Although acute humoral rejection is very infrequent, there appears to be growing evidence of the participation of humoral immunity in the development of many cases of chronic graft dysfunction.27 The detection of these antibodies in the course of routine patient follow-up, and subsequent treatment with immunoglobulins and rituximab to secure the depletion of B lymphocytes, are regarded as a still non-standardized but acceptable treatment option.28
PhotopheresisWith the purpose of lowering the potent immunosuppression needed to avoid acute and chronic rejection, along with its side effects, some programs started to use extracorporeal photopheresis in lung transplantation. This technique separates part of the blood enriched with mononuclear cells and incubates it with a photosensitizing agent, subjected to ultraviolet irradiation, followed by reinfusion into the patient. It has been shown that these lymphocytes, monocytes and dendritic cells, sequestrated in the recipient spleen and liver, possess immune modulating activity through different mechanisms.29 Although the indications of this technique have not been fully defined, the results of the existing retrospective studies suggest that it may prove useful in bronchiolitis obliterans, provided the treatment is started in the early stages of the disease, and in patients with repeated acute rejection episodes.30,31
Generic drugsAlthough the current economical situation and existing legislation regarding the expiry of patent rights to different immunosuppressors favor the use of generic drugs, transplantation–and particularly lung transplantation–requires special considerations for a number of reasons.
The necessary immune suppression implies the observation of very narrow blood concentration margins. It is therefore essential for the corresponding bioequivalence studies to be made under the conditions of these patients, and not in healthy volunteers. It must be remembered that the treatment of transplant patients includes drugs that exhibit interactions or toxicity, in which the bioavailability of the immunosuppressor drug and the corresponding blood levels achieved are essential in order to maintain the function of the grafted organ. Allowing broad variability above or below the required levels can give rise to a loss of the rejection-infection balance, thereby facilitating deterioration of the transplanted organ.32
ConclusionImmune suppression in lung transplantation has undergone changes in recent years thanks to the development of new drugs that intervene in different pathways of the host immune response, and allow a tendency towards more individualized immunosuppression protocols. The marked differences between lung transplantation and the transplantation of other solid organs point to the need for prospective studies designed to establish evidence and provide orientations regarding the use of these new drugs. Very close follow-up of these patients, allowing early detection and management of the problems that affect the rejection-infection balance and the side effects of immunosuppression, remain the principal factor which we can act upon with a view to secure good middle and long term results.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Borro JM. Avances en la inmunosupresión del trasplante pulmonar. Med Intensiva. 2013;37:44–9.