To describe the main indications, clinical results and complications associated with fibrobronchoscopy in the Intensive Care Unit (ICU).
DesignA retrospective, single-center observational study was carried out.
SettingSeventeen beds in a medical/surgical ICU.
PatientsConsecutive patients undergoing fibrobronchoscopy during their stay in the ICU over a period of 5 years.
InterventionsFlexible bronchoscopy performed by an intensivist.
Main variables of interestFlexible bronchoscopy indications and complications derived from the procedure.
ResultsA total of 208 flexible bronchoscopies were carried out in 192 patients admitted to the ICU. Most of the procedures (193 [92.8%]) were performed in mechanically ventilated patients. The average patient age was 58±16 years, with an APACHE II score at admission of 19±7. The most frequent indication for flexible bronchoscopy was diagnostic confirmation of initially suspected pneumonia (148 procedures), with positive bronchoalveolar lavage findings in 46%. The most frequent therapeutic indication was the resolution of atelectasis (28 procedures). Other indications were the diagnosis and treatment of pulmonary hemorrhage, the aspiration of secretions, control of percutaneous tracheotomy, and difficult airway management. The complications described during the procedures were supraventricular tachycardia (3.8%), transient hypoxemia (6.7%), and slight bleeding of the bronchial mucosal membrane (2.4%).
ConclusionsA microbiological diagnosis of pneumonia and the resolution of atelectasis are the most frequent indications for flexible bronchoscopy in critically ill patients.
Flexible bronchoscopy performed by an intensivist in ICU is a safe procedure.
Describir las principales indicaciones, resultados clínicos y complicaciones de la fibrobroncoscopia en enfermos críticos.
DiseñoEstudio retrospectivo, observacional, de un solo centro.
ÁmbitoUnidad de Cuidados Intensivos (UCI) médico-quirúrgica de 17 camas.
PacientesPacientes consecutivos a los que se les realizó una fibrobroncoscopia durante un periodo de cinco años.
IntervencionesFibrobroncoscopia realizada por médicos especialistas en Medicina Intensiva con fines diagnósticos y/o terapéuticos.
Principales variables de interésIndicaciones y complicaciones derivadas de la fibrobroncoscopia.
ResultadosSe han realizado 208 fibrobroncoscopias en 192 pacientes; en el momento del procedimiento 193 (92,8%) recibían ventilación mecánica invasiva. La edad media de los pacientes incluidos fue de 58±16 años y el APACHE II al ingreso en UCI de 19±7. La mortalidad global fue del 31,3%. Las indicaciones más frecuente fueron en 148 (71,2%) casos por sospecha clínica de neumonía y en 28 (13,5%) para resolución de atelectasias. La fibrobroncospia fue eficaz en 120 (57,7%) casos, con resolución de la atelectasia en 20 casos, 71,4% y obteniendo resultados positivos del LBA en 68 (46%) de los casos con sospecha de neumonía. Se han detectado 27 complicaciones menores en 208 (13%) pacientes. Las complicaciones más frecuentes han sido: taquicardia supraventricular (3,8%), hipoxemia transitoria (6,7%) y hemorragia leve de la mucosa bronquial (2,4%).
ConclusionesEl diagnóstico microbiológico de neumonías y la resolución de atelectasias fueron las indicaciones más frecuentes. La fibrobroncoscopia realizada por especialistas de Medicina Intensiva es un procedimiento eficaz y seguro.
Fibrobronchoscopy has become the procedure of choice in most airway explorations, and is an important tool in the diagnosis and treatment of a number of pulmonary disorders in critically ill patients.1–4 The expansion of its use in Intensive Care Units (ICUs) is attributable to the fact that the technique is relatively easy to perform at the patient bedside,2 avoids the need for potentially dangerous patient transfer outside the ICU, and poses few complications.5 As a result, fibrobronchoscopy is presently considered to be an essential element in critical care.5,6
Although in principle critical patients are more prone to develop complications during fibrobronchoscopy, individuals subjected to mechanical ventilation, thanks to their secured airway, are paradoxically at lesser risk than when fibrobronchoscopy is performed in spontaneously breathing patients. The present study describes the main indications, clinical outcomes, effectiveness of bronchoalveolar lavage (BAL), and complications of fibrobronchoscopy in critical patients.
Patients and methodsA retrospective, observational, single-center study was made in a clinical-surgical ICU with a polyvalent unit of 10 beds and a coronary unit with 7 beds. Over a period of 5 years, the study included consecutive patients subjected to fibrobronchoscopy while admitted to the ICU. The following clinical characteristics were analyzed: patient age, APACHE II score upon admission, mortality, indications of fibrobronchoscopy, and complications of the technique. Exploration was not performed in cases where the clinical and/or hemodynamic condition of the patient was unable to guarantee safe fibrobronchoscopy. The following exclusion criteria were established: endotracheal tube under 8mm in diameter, pneumothorax evidenced on the chest X-rays prior to fibrobronchoscopy, oxygen saturation <90% as determined by pulsioxymetry, with FiO2 1, severe acidosis (pH<7.20), and hemodynamic instability defined by systolic blood pressure <90mmHg despite the administration of vasoactive drugs.
During the procedure, continuous electrocardiographic monitorization was carried out using Datex-Ohmeda S/5 bedside monitors. Blood pressure was noninvasively measured using the M-NE12STPR module, and in those cases where the value was found to be under 90mmHg, the pressure was checked using a manual cuff and sphygmomanometer. Depending on the reason for admission to the ICU, the patients were classified as surgical patients (admitted to the ICU from the operating room or from surgical areas after a recent operation), neurological patients (admitted to the ICU with central nervous system disease), trauma patients (admitted to the ICU due to severe trauma), immune depressed patients (admitted to the ICU with immune system disorders) and clinical patients (admitted to the ICU from the emergency care area or medical specialty hospitalization wards).
Following verbal and written informed consent, the technique was carried out using an Olympus type 40/240 bronchoscope connected to an Olympus CLK-4 halogen light source. In the patients subjected to mechanical ventilation, the technique was performed through the orotracheal tube by means of a special adaptor valve (Mallinckrodt model 331/5661). The ventilation mode during the procedure was: control pressure with FiO2 1 and without changes in previous positive end-expiratory pressure (PEEP). Following the procedure, FiO2 was adjusted to the clinical condition of the patient. Regarding the sedation used, in the case of patents on mechanical ventilation we did not modify the continuous infusion they were already receiving—adding further midazolam doses (intravenous boluses of 1–3mg) if needed—while spontaneously breathing patients received local nasal and oropharyngeal lidocaine, together with intratracheal administration of the drug via inter-cricothyroid membrane puncture. In the patients with clinically suspected pneumonia, bronchoalveolar lavage (BAL) was performed with 150ml of physiological saline solution in three 50-ml aliquots instilled in the location suggested by the chest X-rays or at middle lobe level in the case of bilateral infiltration. We did not administer local anesthetics through the bronchoscopy canal, and aspiration was avoided until lodging in the study zone was secured. On performing BAL, the first 20ml recovered were discarded, and a sample of the remaining fluid was processed for microbiological study involving direct staining (gram stain), staining for mycobacteria and fungal species, bacterial culture in the usual media (blood agar, chocolate agar, MacConkey, Brucella agar and BHI), and culture in specific media for Mycobacteria (liquid medium: Bactec MGIT-960-BD), fungi (Sabouraud agar) and Legionella (BCYE). In immune deficient patients, specific staining for Pneumocystis jiroveci was used. We also applied immunofluorescent techniques for Legionella. No epithelial cell and neutrophil quantifications were made as sample quality control. BAL was considered positive7 in the presence of the growth of over 104 colony forming units/ml (cfu/ml). A chest X-ray study was made after the procedure to evaluate the presence of pneumothorax. The complications were classified as major or minor according to the need to apply treatment, adopting the British Thoracic Society guidelines referred to diagnostic bronchoscopy6—defining tachycardia as a heart rate of >100bpm, hypoxemia as oxygen saturation under 90% with FiO2 1, and bleeding based on direct visualization through the bronchoscope during the procedure. All the interventions were carried out by intensivists previously trained in the technique in the Department of Pneumology.
The data obtained were analyzed using the SPSS version 18 statistical package for MS Windows. Qualitative variables were reported with the percentage distribution of each of the categories. Quantitative variables in turn were reported as the mean and standard deviation in the case of a normal distribution, and as the median in the case of a non-normal distribution.
ResultsDuring the study period the mean annual admission rate was 1182 patients, with an average of 357 (30.2%) subjects on mechanical ventilation a year. We analyzed a total of 208 fibrobronchoscopic procedures in 192 patients, representing 3.51% of the total patients and 11.65% of the subjects requiring mechanical ventilation.
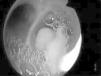
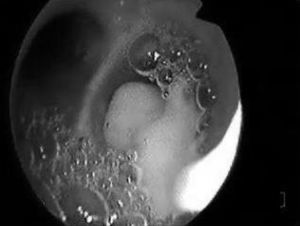
In 15 cases (7.2%), bronchoscopy was carried out in patients under spontaneous breathing conditions or subjected to noninvasive mechanical ventilation in BIPAP mode, and in 193 cases (92.8%) it was carried out in patients subjected to mechanical ventilation. The clinical characteristics of the patients are shown in Table 1, distributed according to whether bronchoscopy was performed under conditions of mechanical ventilation or not. The main indications of fibrobronchoscopy are indicated in Table 2. The predominant indication was diagnostic evaluation, particularly suspected pneumonia, where positive microbiological cultures were obtained in 70 cases (46.4%), followed by therapeutic indications—fundamentally the resolution of atelectasis (percentage resolution rate 71.4%), particularly those caused by central mucus plugging. Fig. 1 shows the endoscopic view of a patient with complete lung atelectasis in which a central mucus plug was aspirated from the right main bronchus.
Characteristics of the study series.
| Total | IMV | Non-IMV |
| n=208 | n=193 | n=15 |
| Age, years | 58.2±16.5 | 53.6±17.6 |
| APACHE II upon admission to ICU | 19.6±6.8 | 11.6±5 |
| Diagnosis upon admission (%) | ||
| Clinical | 57.5 | 66.7 |
| Surgical | 10.9 | 0 |
| Trauma | 8.8 | 6.7 |
| Neurological | 8.8 | 0 |
| Immune depression | 14 | 26.7 |
| Mortality | 32.1 | 20 |
Data expressed as mean±standard deviation.
Non-IMV: noninvasive mechanical ventilation; IMV: invasive mechanical ventilation.
Indications of fibrobronchoscopy in the patients studied.
| Total | IMV | Non-IMV |
| n=208 | n=193 | n=15 |
| Clinically suspected pneumonia | 140 (72.5%) | 8 (53.3%) |
| Atelectasis | 28 (14.5%) | 0 |
| Pulmonary hemorrhage | 8 (4.1%) | 0 |
| Aspiration of secretions (bronchorrhea) | 6 (3.1%) | 0 |
| Tracheostomy control | 5 (2.6%) | 0 |
| Pathological study | 3 (1.6%) | 2 (13.3%) |
| Difficult airway | 0 (0%) | 2 (13.3) |
| Others | 3 (1.6%) | 3 (20.1%) |
Data presented as absolute and relative frequencies (percentages).
Non-IMV: noninvasive mechanical ventilation; IMV: invasive mechanical ventilation.
Minor complications were recorded in 27 patients (13%). The complications described during bronchoscopy were supraventricular tachycardia (3.8%), transient hypoxemia (6.7%) and mild bleeding of the bronchial mucosa as a result of friction with the bronchoscope (2.4%). There were no cases of pneumothorax related to the procedure, or need for orotracheal intubation in the patients not subjected to mechanical ventilation at the time of bronchoscopy.
DiscussionThe present study describes the diagnostic and therapeutic usefulness of fibrobronchoscopy in the critical patient. Among other indications, the technique has been shown to be effective in diagnosing pneumonia and in resolving atelectasis. It is safe, and the associated complications are not serious and are observed in a small percentage of cases.
Pneumonia is the most common nosocomial infection in the ICU,8–10 causing important mortality,11,12 prolonging mechanical ventilation and generating important costs due to the lengthening of hospital stay.13,14 In our ICU we apply the “invasive” strategy,15–18 based on the use of fibrobronchoscopy for microbiological diagnosis and quantitative culturing of samples selectively obtained from the purportedly affected zone. This is the strategy recommended by the main scientific societies12,19 for diagnosing ventilation associated pneumonia (VAP). In our series, almost one-half of the patients subjected to BAL yielded a positive microbiological result—the technique being indicated when clinically suspecting the existence of ventilation associated pneumonia or in immune depressed patients, where BAL has been shown to offer increased performance.20 The resolution of atelectasis was the most frequent therapeutic indication. The efficacy of bronchoscopy in this context is subject to controversy. Kreider et al.,21 in a systematic literature review, observed important variations in the efficacy of fibrobronchoscopy, between 19 and 89%22,23—the best results being obtained in application to lobar atelectasis produced by a central mucus plug, while the poorest performance corresponded to subsegmental atelectasis. Marini et al.24 compared fibrobronchoscopy versus intense respiratory physiotherapy and found no differences between the two groups. In this study, the presence of an air bronchogram on the chest X-rays was associated to an increased delay in resolution.
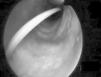
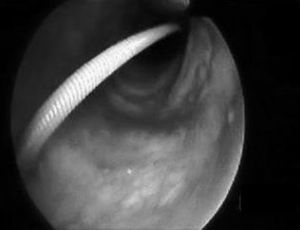
Percutaneous tracheotomy25,26 is a common practice in the ICU. It produces few complications, though these are potentially serious, including false access routes, pneumothorax, subcutaneous emphysema, transesophageal fistulas, etc. Such problems are mainly due to a lack of visualization of the airway while performing the technique. Bronchoscopic control minimizes the complications of the procedure,27 particularly in patients with difficult anatomical characteristics at neck level. Its use in such cases increases the clinical safety of procedure that is routinely used in the ICU. Fig. 2 shows guide insertion in the tracheal lumen during tracheotomy performed in one of the patients included in the study. The availability of fibrobronchoscopy in the ICU is essential for dealing with the so-called “difficult airway”,28,29 though in practice this is an infrequently applied indication,5,22 since experience in performing the technique is required. The third indication in order of frequency in our series was the management of pulmonary hemorrhage. In such cases bronchoscopy proved useful not only in diagnostic terms but also for treatment purposes in the presence of active bleeding.30,31 There have been descriptions of the plugging of bleeding points by means of a Fogarty catheter32 positioned through the endoscope aspiration canal. The same technique has also been reported using a Swan-Ganz catheter.3 We in turn contribute our experience with the intrapulmonary administration of activated coagulation factor VII instilled through the bronchoscope canal in two life-threatening emergencies involving alveolar hemorrhage refractory to conventional treatment, and which responded favorably to this technique. Nevertheless, this experience must be viewed with caution, since few cases have been described in the literature, and further studies are needed to evaluate such treatment and its efficacy.33,34 Other indications comprised a case of alveolar proteinosis, vocal cord examination in a case of hanging, a case of drowning in sea water in which abundant algae and sand were aspirated through the bronchoscopy canal, a case of chest trauma in which bronchoscopy was used to explore bronchial tree injuries under direct visualization,35,36 and a case of direct intubation guided by the bronchoscope through a tracheal prosthesis, in which intubation initially did not prove possible.
The safety of fibrobronchoscopy has been warranted by a number of authors.1–5 Complications have been documented in 13% of the cases, but lacked clinically significant repercussions; the technique therefore can be regarded as safe in the critical patient, provided it is performed under certain conditions that ensure increased clinical safety. Under patients and methods we defined those situations in which we consider that the procedure is not advisable, since the risks appear to outweigh the benefits. In this context, we stress the importance of the diameter of the orotracheal tube, which must be 2mm greater than that of the bronchoscope; the need for continuous monitorization during the procedure; and the ventilation mode. As a novelty, our experience contradicts the classical recommendation in most critical medical texts to suspend PEEP during the procedure.37 In none of our patients subjected to mechanical ventilation did we suspend PEEP that was applied prior to performing the technique, on the grounds that suspending PEEP could worsen the already impaired respiratory function of the patient. It should be noted that there were no cases of pneumothorax in pressure controlled ventilation mode with close monitorization of inspiratory pressure, in accordance with the recommendations of Lawson et al., who reported improved respiratory volumes in pressure control versus volume control mode.38
The present study is not without limitations, considering its retrospective design. Fibrobronchoscopy was performed upon indication by the supervising physician with no prior protocol defining the indication; this circumstance therefore influenced sample selection. Nevertheless, the indications of the technique are discussed and agreed by the medical team in daily clinical sessions, with the aim of minimizing variability in clinical practice. Another limitation is the fact that this is a single-center study. We therefore consider it necessary to conduct further studies to assess our observations before they can be generalized. On the other hand, the study also has its strengths: its main strong point is that this is the largest series in critical patients to have been published in recent years—contributing novel aspects such as the safety of the procedure in pressure controlled ventilation mode, without the need to suspend prior PEEP. In consistency with the above, and on the basis of our own experience, we consider that the availability of fibrobronchoscopy and of personnel adequately trained in the use of the technique is highly recommendable in dealing with lung disease in critical patients.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Estella Á. Análisis de 208 fibrobroncoscopias realizadas en una unidad de cuidados intensivos. Med Intensiva. 2012;36:396–401.