Based on some of the recommendations of the SEMICYUC working groups, we developed a checklist and applied it in two periods, analyzing their behavior as a tool for improving safety.
DesignA comparative pre- and post-intervention longitudinal study was carried out.
SettingThe Intensive Care Unit (ICU) of a 400-bed university hospital.
PatientsRandom cases series in two periods separated by 6 months.
InterventionsWe developed a checklist with 24 selected indicators that were randomly applied to 50 patients. Verification was conducted by a professional not related to care (prompter). We analyzed the results and compliance index and carried out corrective measures with training. With 6 months of preparation, we again applied the random checklist to 50 patients (post-intervention period) and compared the compliance indexes between the two timepoints.
ResultsThere were no differences in demographic characteristics or evolution between the periods. The compliance index at baseline was 0.86±0.12 versus 0.91±0.52 in the post-intervention period (P=.023). An acceptable compliance index was obtained with the 24 indicators, though at baseline the compliance index was <0.85 for 5 recommendations. These detected non-compliances were worked upon through training in the second phase of the study. The post-intervention checklist evidenced improvement in compliance with the recommendations.
ConclusionsThe checklist used to assess compliance with a selection of recommendations of the SEMICYUC applied and moderated by a prompter was seen to be a useful instrument allowing us to identify points for improvement in the management of ICU patients, increasing the quality and safety of care.
Con algunas de las recomendaciones de los grupos de trabajo de la SEMICYUC, elaboramos un checklist y lo aplicamos en dos periodos. Analizamos su comportamiento como herramienta de mejora en la seguridad.
DiseñoEstudio longitudinal, comparativo pre y post-intervención.
ÁmbitoUnidad de Cuidados Intensivos (UCI) de un hospital universitario de 400 camas.
PacientesSerie de casos aleatorios en 2 periodos separados por 6 meses.
IntervencionesElaboramos un checklist con 24 indicadores seleccionados que aplicamos de forma aleatoria a 50 pacientes. La verificación fue conducida por un profesional no relacionado con el cuidado (prompter). Analizamos los resultados y el índice de cumplimiento y realizamos medidas correctoras con formación. Con 6 meses de preparación, aplicamos de nuevo el checklist aleatorio a 50 pacientes (periodo post-intervención) y comparamos el índice de cumplimiento entre ambos.
ResultadosNo observamos diferencias en demográficos ni en la evolución entre periodos. El índice de cumplimiento en el basal fue de 0,86±0,12 y en el periodo de post-intervención de 0,91±0,52; P=,023. Obtuvimos un índice de cumplimiento aceptable de los 24 indicadores, pero en el basal en 5 recomendaciones el índice de cumplimiento fue menor a 0,85. Estos incumplimientos detectados se trabajaron formativamente en la segunda fase. En el checklist post-intervención observamos una mejoría en el cumplimiento de las recomendaciones.
ConclusionesEl checklist utilizado para comprobar el cumplimiento de una selección de recomendaciones de la SEMICYUC aplicado y moderado por un prompter fue un instrumento útil que permitió establecer puntos de mejora en la atención de los pacientes de UCI aumentando la calidad y la seguridad.
The standardization in the implementation of clinical practice guidelines and protocols for the management of critically ill patients in the intensive care unit (ICU) has a positive impact on morbidity and mortality. In this sense, one of the most evident proofs on this regard has been the multimodal action of the Bacteriemia Zero campaign carried out in our setting at national and international level.1,2 On the other hand, errors of omission are difficult to identify, and they are a source of very important safety incidents in critically ill patients.3
We believe that the association between the greater compliance to the clinical practice guidelines and the fewer errors of omission regarding safety detected should be part of continuous improvement plans in intensive medicine services. Actually, it has been summarized in the «to do» and «not-to-do» recommendations of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) working groups. In an ongoing effort to improve the management of critically ill patients, SEMICYUC has been publishing different recommendations from its working groups that have been periodically reviewed using articles and proven scientific evidence until coming up with the actual recommendations of 2017.4,5
Our group conducted a collaborative commented and discussed selection during several multidisciplinary sessions of the recommendations established by SEMICYUC working groups.4,5 Finally, 24 indicators and/or recommendations were selected to elaborate a checklist that was passed on to a randomized sample of consecutive patients. The objective of our study was to design and verify a simple tool to carry out «to do», «to check», and «to act-and-plan» tasks through a checklist including «to do and not-to-do» recommendations from SEMICYUC working groups. This easy-to-use methodology will improve safety, minimize the errors of omission, and increase compliance to clinical practice guidelines regarding the processes of care during the management of our critically ill patients.
Material and methodsThe study design starts by selecting a set of recommendations from SEMICYUC working groups extracted from the ones published in 2017 and the «not-to-do» recommendations from 2018.4,5
The project was approved by the hospital clinical research and ethics committee and a consent form was prepared for every patient. The written informed consent was signed by all the patients or their families if they remained unconscious.
The selection of the indicators (eg, bed positioned at 30°) and/or the recommendations (eg, removing unnecessary catheters) was based on a brainstorming session conducted in the unit with the participation of 4 intensivists, the attending physician, and the nurse supervisor. All of them with specific training on patient safety issues. The complex extraction of the indicators and/or recommendations to be reviewed never happened. Instead, only those that were easier to measure and useful to the cases in our unit were reviewed based on the subjective criteria of the evaluation team.
A checklist with 24 indicators and/or recommendations was elaborated that was included in the final checklist and eventually completed at the emergency ward at the bedside. The health professionals responsible for the well-being of the patients attended this round (doctor and nurse) plus an intensivist without any direct responsibility in the patient’s clinical care who acted as conductor, auditor or prompter. The latter also moderated the selection of the indicators and was the clinician in charge at the unit. See checklist with the indicators and/or recommendations selected on Table 1.
Complete checklist that was randomly run at the bedside.
| Recommendation | C | I | NA | |
|---|---|---|---|---|
| Bioethics | ||||
| 1. | Are there indications for LLST or not to perform CPR maneuvers in the patient’s PMH? | |||
| 2. | If LLST has been suggested has POD or CAD been suggested too? | |||
| Cardiology | ||||
| 3. | In the presence of hemodynamic alterations, US of the vena cava or the heart-lung? | |||
| Nephrology | ||||
| 4. | In AKI, are the RIFLE or AKIN scores registered in the patient’s PMH? | |||
| 5. | In AKI with RIFLE o AKIN scores=3, has CRRT been started? | |||
| Infections | ||||
| 6. | Does the patient carry unnecessary catheters? | |||
| 7. | If MV, is the bed positioned at>30°? | |||
| 8. | In sepsis, have antibiotics been administered within the first 3h? | |||
| Technology | ||||
| 9. | Have the alarm limits been individually adjusted for every patient? | |||
| 10. | Have the general treatment forms been updated in the patient’s PMH? | |||
| Respiratory | ||||
| 11. | In MV, is TV adjusted to the predicted <8mL/kg? | |||
| 12. | In MV, is plateau pressure adjusted to <30mmHg? | |||
| 13. | If ARDS, MV, and PaO2/FiO2 ratio <150, has the patient been placed in the prone position? | |||
| Metabolism | ||||
| 14. | Have the patient’s nutritional needs (EN/PN) been estimated-taken into consideration? | |||
| 15. | Is glucose level under control and <180mg/dL? | |||
| Neurology | ||||
| 16. | If TBI and Glasgow <8 and lesions on the CT scan, does the patient carry an ICP catheter? | |||
| 17. | If TBI and Glasgow <8 and lesions on the CT scan, pCO2>35mmHg? | |||
| Planning | ||||
| 18. | Has the treatment-plan been discussed between doctor and nurse? | |||
| Sedation | ||||
| 19. | Are the VAS, BPS, and RASS scales being measured? | |||
| 20. | Has sedoanalgesia been modified based on the scores? | |||
| Toxicology | ||||
| 21. | In the presence of poisoning and if indicated, has CRRT been initiated? | |||
| Transfusion | ||||
| 22. | In a stable hemodynamic condition, no transfusion has been given with Hb levels>7.5g/dL? | |||
| Donation | ||||
| 23. | Has donation been discussed if the patient has evolved to a brain death condition? | |||
| 24. | Are there any AD or similar documents included in the patient’s PMH? | |||
| [0.2–5] Total score Compliance indicator: [Total C/24-total NA] | ||||
AD, advanced directives; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ARDS, acute respiratory distress syndrome; BPS, Behavioral Pain Scale; C, correct recommendation; CAD, controlled asystole donation; CPR, cardiopulmonary resuscitation; CRRT, continuous renal replacement therapy; CT scan, computed tomography scan; EN, enteral nutrition; Hb, hemoglobin; I, incorrect recommendation; ICP, intracranial pressure; LLST, limitation of life-sustaining treatments; MV, mechanical ventilation; NA, recommendation non-applicable to such patient; PMH, past medical history; PN, parenteral nutrition; POD, potential organ donation; RASS, Richmond Agitation-Sedation Scale; TBI, traumatic brain injury; TV, tidal volume; US, ultrasound; VAS, visual analogue scale.
Over a first period of 2 months, the checklist was randomly passed on to 50 consecutive patients on 3 different days of the week. Patients admitted to the ICU during the first 3 days of disease progression were considered eligible for the checklist; after this time, the checklist was passed on to the next patient. The answers to the questionnaire were recorded on a tablet at the bedside in a form specifically designed for this purpose. The answers were saved in an independent file stored inside the unit server mainframe. These servers run on the hospital internal security protocols. When the checklist was completed and when all health professionals agreed, data were stored in the specific file with the action «saved» as the electronic signature of the answers given. Results were collected and the recommendations with correct answers, incorrect answers, and non-applicable answers were estimated in each patient. The compliance indicator was estimated per patient using the following formula: total number of correct answers / (24-total number of non-applicable answers) estimated immediately and independently after running the checklist in every patient. Similarly, when completing data mining from the first group of patients, the correct, incorrect, and non-applicable answers were grouped. Also, the compliance index of every indicator and/or recommendation was estimated with the following formula: total number of correct answers / (50-total number of non-applicable answers).
Within the next 6 months the baseline results were used to conduct clinical training sessions on the recommendations at stake with compliance indicators <0.85 in order to reinforce the aspects that still had room for improvement. These 40minute-clinical sessions were designed based on the indicators and/or recommendations with low compliance levels during the first period and directed by an expert intensivist in the issue at stake. Consequently, 2 sessions were conducted on an estimate of the nutritional needs and on the need to keep the levels of glycemia <180mg/dL. One of the sessions discussed the management of cranial traumas, another how to approach patients with limitation of life-sustaining treatments and the possibility of potential organ donation. Finally, one doctor-patient session discussed the need to remove unnecessary catheters and keep good communication going among all health professionals.
During the second period conducted 6 months later the same checklist was randomly run among other 50 patients for another 2 months and the compliance indicators were established per patient and per indicator or parameter and then compared to the results obtained during the baselines period.
The statistical analysis was descriptive, and the qualitative variables were expressed as number with the characteristic and its percentage, and the quantitative variables as mean and standard deviation. A comparative study was conducted after the intervention with respect to the early or baseline period using the chi-square test for the qualitative variables and the Student t test for the quantitative ones. Since the study was just exploratory and we did not have similar data from other studies or did not know the behavioral pattern in our setting, we did not conduct any estimates on the size of the sample. Variables with P values <.05 were considered statistically significant.
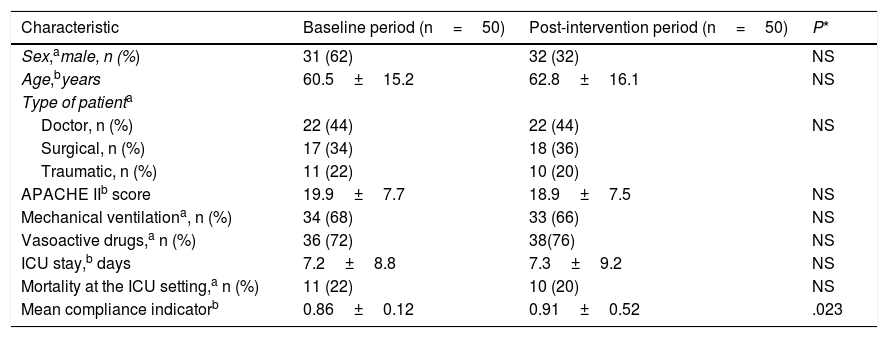
ResultsThe patients’ clinical characteristics at baseline period and after the intervention are shown on Table 2. No significant differences were seen between both groups regarding demographic data and severity. No differences were seen either between both periods in the percentage of patients on mechanical ventilation, in the percentage of patients treated with vasoactive drugs, in the days spent at the ICU or in the mortality rate reported at the ICU setting. This lack of significant differences allowed group comparison.
Comparison of the patients’ clinical and evolutionary characteristics and compliance indicator between both study periods.
| Characteristic | Baseline period (n=50) | Post-intervention period (n=50) | P* |
|---|---|---|---|
| Sex,amale, n (%) | 31 (62) | 32 (32) | NS |
| Age,byears | 60.5±15.2 | 62.8±16.1 | NS |
| Type of patienta | |||
| Doctor, n (%) | 22 (44) | 22 (44) | NS |
| Surgical, n (%) | 17 (34) | 18 (36) | |
| Traumatic, n (%) | 11 (22) | 10 (20) | |
| APACHE IIb score | 19.9±7.7 | 18.9±7.5 | NS |
| Mechanical ventilationa, n (%) | 34 (68) | 33 (66) | NS |
| Vasoactive drugs,a n (%) | 36 (72) | 38(76) | NS |
| ICU stay,b days | 7.2±8.8 | 7.3±9.2 | NS |
| Mortality at the ICU setting,a n (%) | 11 (22) | 10 (20) | NS |
| Mean compliance indicatorb | 0.86±0.12 | 0.91±0.52 | .023 |
In the baseline group, the compliance indicator went from 0.64 to 1.0 being the average compliance indicator=0.86±0.12. In the post-intervention group, the compliance indicator went from 0.75 to 1.0 being the average compliance indicator as high as 0.91±0.52. P values=.023 were considered statistically significant.
In order to find the indictors and/or recommendations with low compliance indicators, all indicators with compliance indices <0.85 were studied to find the weakest points and also to know which ones need better training between the first and the second period.
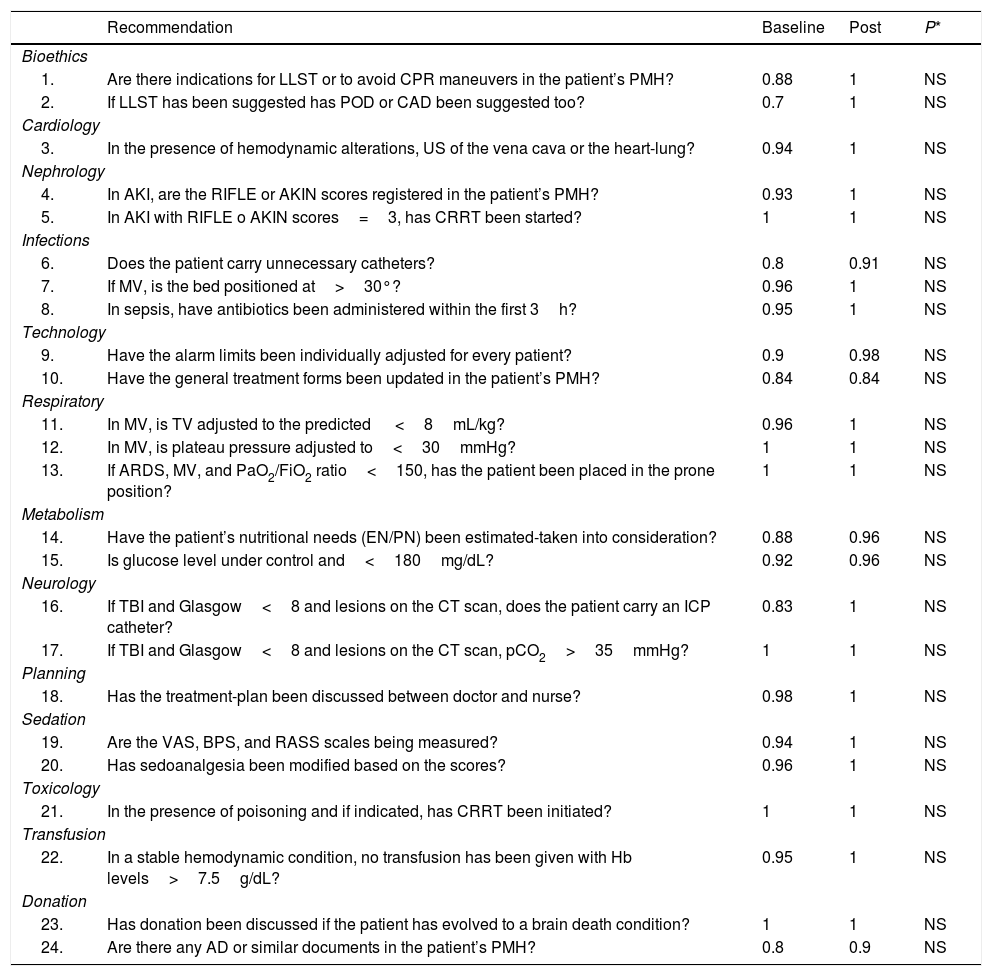
The comparison of correct results question by question based on the compliance indicator and its statistical significance between the baseline and post-intervention periods is shown on Table 3. No significant differences were seen between both periods. However, the compliance indicator of the recommendations of the second period was higher compared to baseline. Actually, these were the study most remarkable results. Since we introduced targeted thematic training sessions between both periods, we can think that these sessions would have prompted higher compliance indicators although other unassessed factors may have impacted these indicators too.
Comparison of the compliance indicator of every recommendation between the baseline checklist and the post-intervention (Post) checklist and statistical significance.
| Recommendation | Baseline | Post | P* | |
|---|---|---|---|---|
| Bioethics | ||||
| 1. | Are there indications for LLST or to avoid CPR maneuvers in the patient’s PMH? | 0.88 | 1 | NS |
| 2. | If LLST has been suggested has POD or CAD been suggested too? | 0.7 | 1 | NS |
| Cardiology | ||||
| 3. | In the presence of hemodynamic alterations, US of the vena cava or the heart-lung? | 0.94 | 1 | NS |
| Nephrology | ||||
| 4. | In AKI, are the RIFLE or AKIN scores registered in the patient’s PMH? | 0.93 | 1 | NS |
| 5. | In AKI with RIFLE o AKIN scores=3, has CRRT been started? | 1 | 1 | NS |
| Infections | ||||
| 6. | Does the patient carry unnecessary catheters? | 0.8 | 0.91 | NS |
| 7. | If MV, is the bed positioned at>30°? | 0.96 | 1 | NS |
| 8. | In sepsis, have antibiotics been administered within the first 3h? | 0.95 | 1 | NS |
| Technology | ||||
| 9. | Have the alarm limits been individually adjusted for every patient? | 0.9 | 0.98 | NS |
| 10. | Have the general treatment forms been updated in the patient’s PMH? | 0.84 | 0.84 | NS |
| Respiratory | ||||
| 11. | In MV, is TV adjusted to the predicted <8mL/kg? | 0.96 | 1 | NS |
| 12. | In MV, is plateau pressure adjusted to<30mmHg? | 1 | 1 | NS |
| 13. | If ARDS, MV, and PaO2/FiO2 ratio<150, has the patient been placed in the prone position? | 1 | 1 | NS |
| Metabolism | ||||
| 14. | Have the patient’s nutritional needs (EN/PN) been estimated-taken into consideration? | 0.88 | 0.96 | NS |
| 15. | Is glucose level under control and<180mg/dL? | 0.92 | 0.96 | NS |
| Neurology | ||||
| 16. | If TBI and Glasgow<8 and lesions on the CT scan, does the patient carry an ICP catheter? | 0.83 | 1 | NS |
| 17. | If TBI and Glasgow<8 and lesions on the CT scan, pCO2>35mmHg? | 1 | 1 | NS |
| Planning | ||||
| 18. | Has the treatment-plan been discussed between doctor and nurse? | 0.98 | 1 | NS |
| Sedation | ||||
| 19. | Are the VAS, BPS, and RASS scales being measured? | 0.94 | 1 | NS |
| 20. | Has sedoanalgesia been modified based on the scores? | 0.96 | 1 | NS |
| Toxicology | ||||
| 21. | In the presence of poisoning and if indicated, has CRRT been initiated? | 1 | 1 | NS |
| Transfusion | ||||
| 22. | In a stable hemodynamic condition, no transfusion has been given with Hb levels>7.5g/dL? | 0.95 | 1 | NS |
| Donation | ||||
| 23. | Has donation been discussed if the patient has evolved to a brain death condition? | 1 | 1 | NS |
| 24. | Are there any AD or similar documents in the patient’s PMH? | 0.8 | 0.9 | NS |
AD, advanced directives; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ARDS, acute respiratory distress syndrome; BPS, Behavioral Pain Scale; C, correct recommendation; CAD, controlled asystole donation; CPR, cardiopulmonary resuscitation; CRRT, continuous renal replacement therapy; CT scan, computed tomography scan; EN, enteral nutrition; Hb, hemoglobin; I, incorrect recommendation; ICP, intracranial pressure; LLST, limitation of life-sustaining treatments; MV, mechanical ventilation; NA, recommendation non-applicable to such patient; PMH, past medical history; PN, parenteral nutrition; POD, potential organ donation; RASS, Richmond Agitation-Sedation Scale; TBI, traumatic brain injury; TV, tidal volume; US, ultrasound; VAS, visual analogue scale.
This study shows that the use of a checklist with a selection of different «to do and not-to-do» recommendations established by SEMICYUC can be used as a dynamic, fast, and easy-to-use tool to assess our daily work and draw lines for improvement in the management of critically ill patients. In most patients, this checklist was run at the bedside in less than 3min.
Based on the improved compliance indicator of certain parameters, changes can be made in the checklist—that can be repeated periodically—and even established as a routine for all ICU patients. Also, it takes proven scientific evidence to the critically ill patient’s bedside. The checklist can also play an auditor role in our daily routine by analyzing process and safety indicators and standards.
Landmark studies have established that the use of a 19-item checklist in surgical patients reduces mortality among these patients as described in the study conducted by Haynes et al.6 Nonetheless, this depends on the setting where these items are applied to, on the implication of health professionals, and on the capacity of the leader as seen in the results without decreased mortality or fewer perioperative complications of another similar study conducted in Canada.7
We believe that the relevance of our study is that it shows that to change and improve the work of health professionals, we need to overcome resistance to change8 and appreciate the work done by multidisciplinary teams.9,10 Also, it is very important that the different processes become standardized and protocolized so that everybody can work the same way. We reviewed the study conducted by Gómez Tello et al.11 that describes the standards that all clinical information systems should meet to protocolize procedures and actions regarding quality and safety during the management of critically ill patients. In our setting, in their different studies conducted on audits in the intensive care setting, Bodí et al.12,13 showed us real-time randomized analyses as a useful tool to assess the structural safety, process, and results of critically ill patients.
Previous experiences with the implementation of checklists in the intensive care setting are encouraging if we look at the study conducted by Weiss et al.14 It is a pre- and post-intervention study that confirmed that both mortality and the average ICU stay decreased. This checklist was being run by a facilitator at the ICU, the so-called «prompter». However, in another recent multicenter study, these positive results could not be confirmed.15 Nonetheless, in this study, the checklist was not run by the prompter at the bedside. This means that results are very different depending on the group, setup, and implementation of the checklist. Our study proposes a simple checklist run by an intensivist at the bedside (with a prompter) with scientifically proven indicators by the scientific community in our specialty.
The value of checklists as default safety verification tools in the intensive care setting should be discussed. Still, they have not always been regarded as a valid methodology to correct safety defects.16 However, in our setting, real-time random safety audits (AASTRE) have been used including structural, procedural, and result indicators in the same randomly applied questionnaire. The data collected allow us to introduce elements to improve the quality of critical care. These elements are easy to implement as the pilot study conducted on this group clearly showed.17 Other studies conducted by the same group have confirmed the utility of checklists as well.18
Our methodology would be a checklist for the default verification of safety measures including structural, procedural, and result indicators. Still, it should be easier compared to the AASTRE that includes twice as many indicators.17,18 These safety and quality indicators and/or recommendations we chose are consistent with the treatment recommendations established by SEMICYUC. Actually, this is the added value of our methodology. As it occurred in other studies,15 ours did not show any differences in the progression of mortality since it included a low number of patients. Actually, in order to see an impact of such caliber a larger number of patients should be recruited.
In this sense, we should mention the study conducted by Byrnes et al.19 that analyzed a mandatory checklist run at the bedside including a wide range of goals. The authors believe this checklist was a useful tool to implement better clinical practices for a better management of the patients. These authors concluded that implementing a checklist is easy, cost-effective, and prevents the errors of omission occurred during the basic management of critically ill patients. Dubose et al.20 studied «Quality Rounds Checklist» and concluded that this method facilitates a sustained improvement of clinically significant prophylactic measures in the management of trauma. Also, he added that its routine daily use just takes a few minutes per patient, is cost-effective, and improves results. Both studies are consistent with our study. However, we included proven and consistently recommended indicators backed by scientific evidence and elaborated by SEMICYUC working groups.
Another important issue here is whether the checklist should be run on a piece of paper or through an electronic registry included the patient’s past medical history. There is a study21 that compared both methods and saw no differences between the two. Still, with the print format more mistakes are made. A study recently published22 discussed the creation of a dynamic checklist using a computer with the methodology of the metamodel that combines the experts’ clinical experience and the computer specialists’ expertise. However, we believe that such a checklist is very difficult to implement in the routine clinical practice.
Our study has several limitations: 1) It was conducted only in a single center and included very few patients in an exploratory case series study that did not consider the size of the sample. 2) The checklist included 24 indicators and/or recommendations established by SEMICYUC based on subjective criteria from health professionals who work at the ICU setting from clinical discussion sessions held. 3) Between the baseline period and the post-intervention period improvement sessions targeted at the recommendations with lower initial compliance were conducted. This means that the approach can be very different from the methodological point of view, and the impact this has on improvement as well.
For all these reasons, we believe that the added value of our work is the use of «to do and not-to do» recommendations from SEMICYUC working groups. These recommendations were carefully chosen based on modern publications and scientific evidence available. Also, electronic forms that run on the clinical information system can be created. These forms should be updated daily with corrections of the deviations seen in the compliance index of all indicators. These indicators can be changed in time when a proper predetermined compliance indicator has been achieved. It seems evident that work at the intensive care setting needs to be systematic, multidisciplinary, and with an orderly arranged transmission of clinical information among the different healthcare providers to avoid critical safety omissions.23
In conclusion, our study confirms that a methodology of implementation, verification, and correction based on a checklist that included certain «to do and not-to-do» recommendations established by SEMICYUC working groups isa valid tool to correct and improve the deviations seen when trying to comply with safety and quality indicators and parameters during the management of patients at the intensive care setting.
Contribution from the authorsJosep-Maria Sirvent: Project and final writing of the manuscript.
Carles Cordon: Project and field study.
Silvia Cuenca: Design of the checklist.
Cristina Fuster: Training sessions.
Carol Lorencio: Training sessions.
Patricia Ortiz: Reviews of safety standards.
Conflicts of interestNone reported.
Please cite this article as: Sirvent J-M, Cordon C, Cuenca S, Fuster C, Lorencio C, Ortiz P. Aplicación, comprobación y corrección a partir de un checklist elaborado con algunas de las recomendaciones (“hacer y no hacer”) de los grupos de trabajo de la SEMICYUC. Med Intensiva. 2021;45:88–95.