Skeletal muscle wasting and weakness have proven to be important determinants of critically ill patients’ outcome. Reduced skeletal muscle mass on intensive care unit (ICU) admission has been associated with increased mortality and disability after discharge.1–3 Moreover, muscle wasting occurring during ICU stay has also been associated with adverse outcomes.4 However, the development of muscle atrophy in critically ill patients is highly heterogeneous among different muscle types.5,6 Pectoral muscle area (PMA) on ICU admission, determined by computed tomography (CT) scan, has been associated with mortality.1,7 On the contrary, tomographic evolution of PMA and its impact on patients’ outcomes has not been reported. Therefore, we aimed to determine if the evolutionary pattern of PMA after ICU admission was associated with patient survival. We hypothesized that PMA wasting would be greater in non-survivors.
Thirty mechanically ventilated patients admitted to the ICU of a University Hospital (Hospital de Clínicas, Montevideo) from February 2016 to April 2020 and requiring two chest CT scans were retrospectively included in the study. Median time from ICU admission to the first CT scan was 0 (0–1) days and 12 (9–15) days for the second one. PMA measurement was performed as previously described from a single axial slice of the CT scan.1 Muscles were manually shaded in the first axial slice above the superior aspect of the aortic branch using specific software (Weasis Medical Viewer) and PMA was computed in square centimeters as the aggregated area of right and left major and minor pectoral muscles (Supplementary Fig. 1). The study was approved by the institution’s Research Ethics Committee.
Categorical variables are reported as absolute numbers (percentage) and were compared using Chi-square test or Fisher exact test. Continuous variables are expressed as mean±standard deviation if normally distributed, or median (25th–75th percentile) if not. PMA evolution was analyzed through Wilcoxon signed-rank test. Student t-test or Mann–Whitney U test were performed to compare variables between groups. The Spearman correlation test was used to analyze bivariate correlations. Kaplan-Meier curves and the log-rank test were applied to compare ICU mortality in patient groups stratified by PMA. A P value < 0.05 was considered statistically significant.
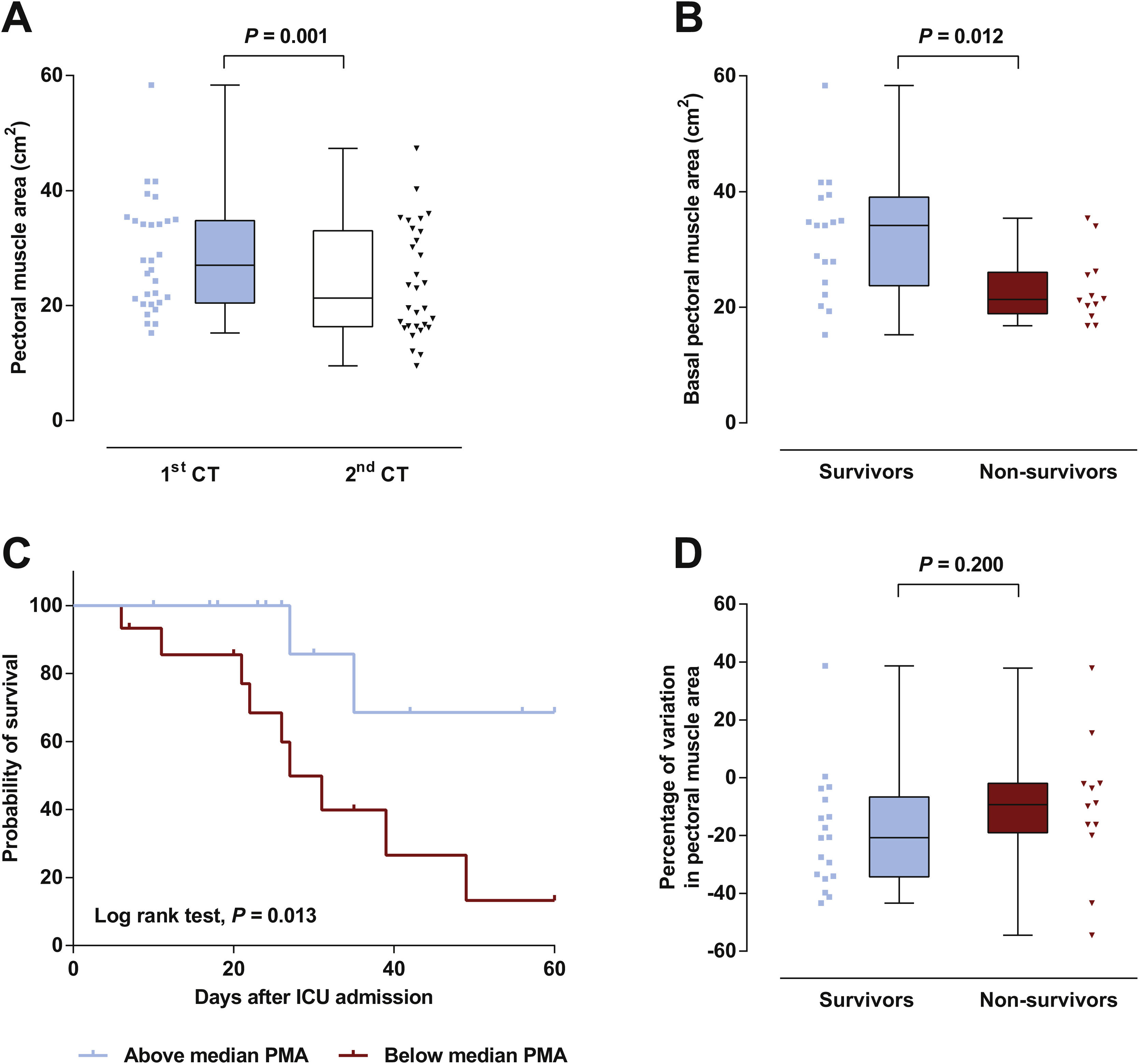
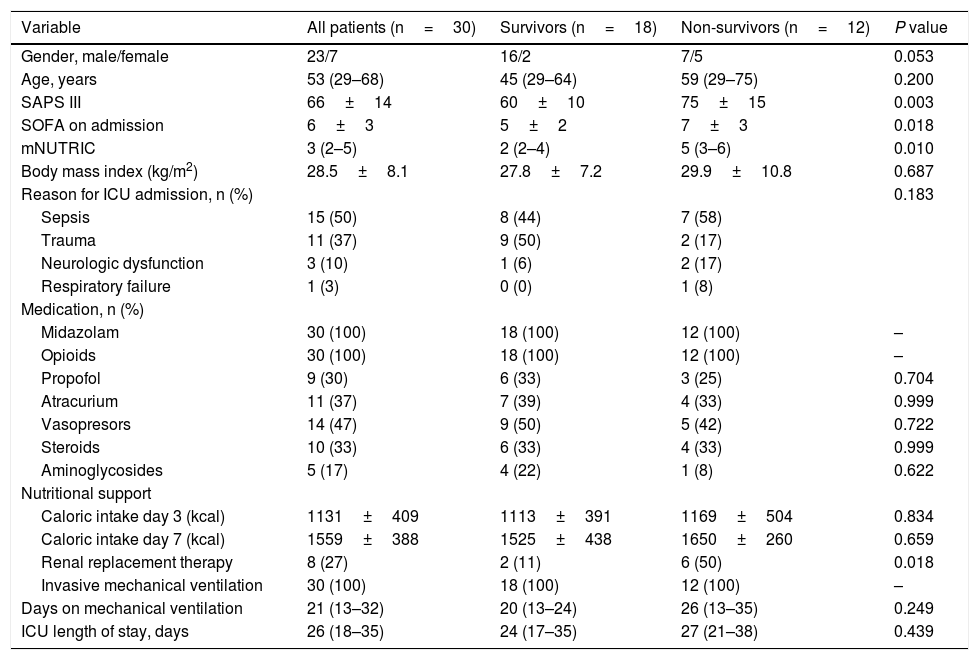
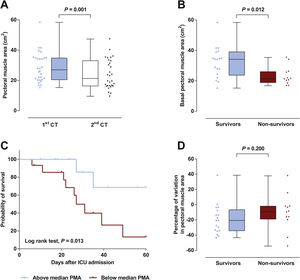
Patient’s characteristics are summarized in Table 1. Most patients were male (77%), median time on mechanical ventilation was 21 (13–32) days and ICU mortality was 40%. The vast majority (87%) presented a low nutritional risk as assessed by the modified NUTRIC score (mNUTRIC).8 Median PMA on admission CT scan (PMA1) was 27.0 (20.5–34.8)cm2, and was inversely correlated with patients’ age (rs=−0.506, P=0.004), mNUTRIC (rs=−0.717, P<0.001) and SAPS III (rs=−0.657, P< 0.001; Supplementary Figure 2). Males had a higher PMA on admission than females (34.1 (20.5–35.4)cm2versus 21.5 (19.3–24.3)cm2, P= 0.033). PMA measured on the second CT scan (PMA2) was significantly reduced from baseline to 21.3 (16.3–33.0) cm2 (P=0.001; Fig. 1A), with a median variation of −16.2 ([−33.6]–[−3.5])%. Neither PMA measurements (PMA1 and PMA2) nor its percentage of variation were correlated with the duration of mechanical ventilation or ICU stay. Baseline PMA was significantly higher in patients discharged alive from ICU than in non-survivors (34.1 (23.8–39.1) cm2versus 21.3 (18.9–26.0) cm2, P=0.012; Fig. 1B). A significant difference in ICU mortality was observed between patients whose admission muscle area was above or below the median PMA (13% versus 67%, respectively, P=0.013; Fig. 1C). The percentage of variation from PMA1 to PMA2 was not significantly different between survivors and non-survivors (P=0.200; Fig. 1D). However, a trend towards a more pronounced reduction in PMA was observed in survivors, which could be related to the higher muscle mass on admission in these patients. Of note, caloric intake on the third and seventh days was similar between survivors and non-survivors (P=0.834 and 0.659, respectively), and was not correlated with the percentage of variation from PMA1 to PMA2 (P=0.505 and 0.849, respectively).
Baseline characteristics of the cohort.
| Variable | All patients (n=30) | Survivors (n=18) | Non-survivors (n=12) | P value |
|---|---|---|---|---|
| Gender, male/female | 23/7 | 16/2 | 7/5 | 0.053 |
| Age, years | 53 (29–68) | 45 (29–64) | 59 (29–75) | 0.200 |
| SAPS III | 66±14 | 60±10 | 75±15 | 0.003 |
| SOFA on admission | 6±3 | 5±2 | 7±3 | 0.018 |
| mNUTRIC | 3 (2–5) | 2 (2–4) | 5 (3–6) | 0.010 |
| Body mass index (kg/m2) | 28.5±8.1 | 27.8±7.2 | 29.9±10.8 | 0.687 |
| Reason for ICU admission, n (%) | 0.183 | |||
| Sepsis | 15 (50) | 8 (44) | 7 (58) | |
| Trauma | 11 (37) | 9 (50) | 2 (17) | |
| Neurologic dysfunction | 3 (10) | 1 (6) | 2 (17) | |
| Respiratory failure | 1 (3) | 0 (0) | 1 (8) | |
| Medication, n (%) | ||||
| Midazolam | 30 (100) | 18 (100) | 12 (100) | – |
| Opioids | 30 (100) | 18 (100) | 12 (100) | – |
| Propofol | 9 (30) | 6 (33) | 3 (25) | 0.704 |
| Atracurium | 11 (37) | 7 (39) | 4 (33) | 0.999 |
| Vasopresors | 14 (47) | 9 (50) | 5 (42) | 0.722 |
| Steroids | 10 (33) | 6 (33) | 4 (33) | 0.999 |
| Aminoglycosides | 5 (17) | 4 (22) | 1 (8) | 0.622 |
| Nutritional support | ||||
| Caloric intake day 3 (kcal) | 1131±409 | 1113±391 | 1169±504 | 0.834 |
| Caloric intake day 7 (kcal) | 1559±388 | 1525±438 | 1650±260 | 0.659 |
| Renal replacement therapy | 8 (27) | 2 (11) | 6 (50) | 0.018 |
| Invasive mechanical ventilation | 30 (100) | 18 (100) | 12 (100) | – |
| Days on mechanical ventilation | 21 (13–32) | 20 (13–24) | 26 (13–35) | 0.249 |
| ICU length of stay, days | 26 (18–35) | 24 (17–35) | 27 (21–38) | 0.439 |
Categorical variables are presented as n (%) and continuous variables as median (25th–75th percentile) or mean±standard deviation. P value represents significance between survivors and non-survivors.
(A) A significant decrease in pectoral muscle area (PMA) was observed between first (1st) and second (2nd) CT scans. (B) Survivors presented a higher PMA on admission than non-survivors. (C) Survival curves for patients with admission PMA below or above the median value of 27.0cm2. (D) No significant difference was observed in the variation of PMA between 1st and 2nd CT scans.
CT-determined cross-sectional area of different skeletal muscles at the time of admission to the ICU has been associated with prognosis. In concordance with our results, Jaitovich et al. reported higher survival in patients with larger admission PMA.1 Furthermore, reduced baseline erector spinae,2 psoas,9 and total abdominal muscles’ cross-sectional area (evaluated at the third lumbar vertebra)3 have been associated with negative outcomes in critically ill patients. While the relevance of different muscles’ mass on admission has been consistently demonstrated, their evolution during critical illness and its impact on patients’ prognosis seems more heterogeneous. Upper and lower limb muscle atrophy after ICU admission has been described by numerous authors,5,10 and its development is associated with increased mortality.4 On the contrary, abdominal muscle area was not reduced in patients with acute pancreatitis requiring ICU admission.6 Previous work by Vivier et al. studied pectoral muscle thickness using ultrasound in 34 critically ill patients.11 Overall, no significant difference was found in pectoral muscle thickness between day 1 and day 5 of ICU stay. Moreover, when the authors analyzed the subgroup of patients that developed pectoral muscle atrophy (i.e., thickness reduction ≥ 10% between days 1 and 5) found no difference in mortality compared to the other patients. In contrast to Vivier et al., we found a significant decrease in PMA after ICU admission. This might be explained by a longer period between measurements (9 versus 5 days) and different methods used to evaluate pectoral muscle mass (CT scan versus ultrasound). However, neither of the studies found an association between the reduction in pectoral muscle mass and mortality. The different relationship between limb or pectoral muscle atrophy and patients’ outcome could be related to the distinct functional domains involved (i.e., locomotor and non-locomotor).
Our study has certain limitations. First, it was a retrospective study conducted in a single center, including a small number of patients with heterogeneous characteristics. Therefore, the independent impact of PMA on patients’ outcomes after adjusting for other variables (e.g., age, SAPS III, etc.) could not be determined. Second, data regarding the catabolic state and fluid balance of the patients could not be obtained. Finally, muscle strength and functional status after ICU discharge was evaluated.
In conclusion, development of pectoral muscle atrophy after ICU admission was demonstrated by CT scan for the first time. Admission PMA was associated with patients’ survival, although further studies are required in order to confirm these results and evaluate the effect of pectoral muscle atrophy developed during ICU stay.
FundingNone.
Conflict of interestsNone.