To compare the early administration of surfactant, before 12 h of life, versus late, in late preterm neonates (born between 34+0 and 36+6 weeks of gestation), with moderate-severe respiratory distress.
DesignRetrospective, observational, analytical, case-control study, with late preterm infants admitted between 2012–2021. It is divided into 2 groups: surfactant administered ≤ 12 h of life and >12 h and evolution is compared using univariate analysis.
SettingNeonatal Intensive Care Unit (NICU) level III of a Universitary Hospital.
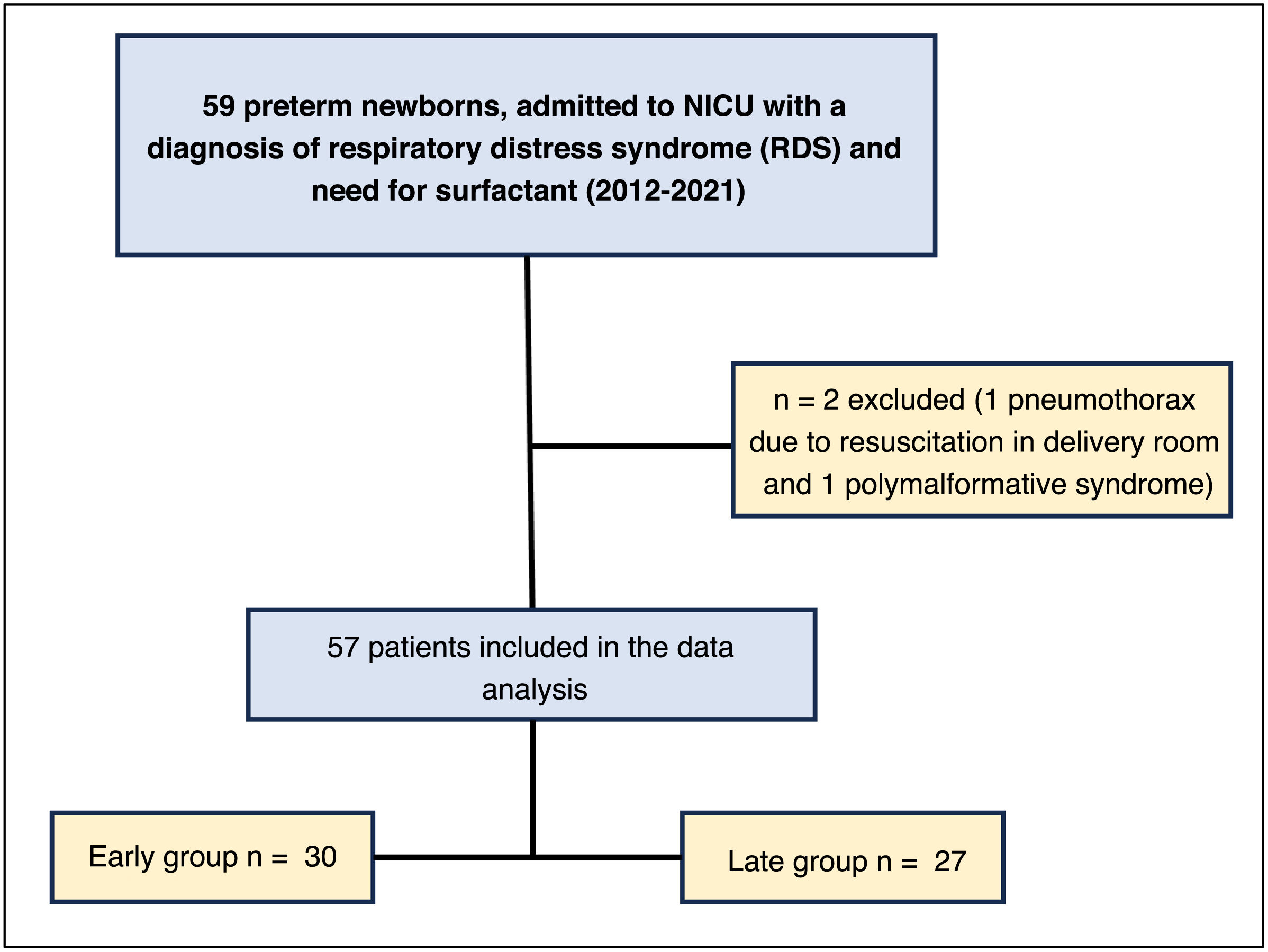
Patients or participants57 patients, 30 in the early group and 27 in the late group. Inclusion criteria: neonates from 34+0 to 36+6 weeks of gestation, with respiratory distress syndrome, in need of non-invasive ventilation and surfactant.
InterventionsNone.
Main variables of interestSociodemographic, clinical and evolutionary: redosing, duration of respiratory support, oxygen and time to stop requiring it after surfactant. Also, complications and length of hospitalization.
ResultsIn the early group there was less need for redosing (3.3% vs 48.1%, P < .001) and a decrease in duration, in days, of stay in the NICU (7 vs 10.5, P .002), invasive mechanical ventilation (2.4 vs 3.9, P .034), total respiratory support (4.6 vs 6.6, P .005) and oxygen therapy (0.4 vs 2.8, P < .001). Also, lower incidence of pneumothorax (0% vs 33.3%, P .001). Furthermore, 12 h after administration, 83.4% maintained FiO2 0.21, compared to 44.4% in the late administration.
ConclusionsIn our study, early administration in late preterm infants provides benefits in terms of respiratory assistance and complications. We suggest expanding studies to establish recommendations in this group of patients.
Comparar la administración precoz de surfactante, antes de las 12 horas de vida, frente a tardía, en prematuros tardíos (nacidos entre 34+0 y 36+6 semanas de gestación) con distrés respiratorio moderado-grave.
DiseñoEstudio retrospectivo, observacional, analítico, caso-control, con prematuros tardíos ingresados entre 2012−2021. Se dividen en 2 grupos: surfactante administrado ≤ 12 horas de vida y >12 h y se compara evolución, mediante análisis univariante.
ÁmbitoUnidad de Cuidados Intensivos Neonatal (UCIN) nivel III de un Hospital Universitario.
Pacientes o participantesCincuenta y siete pacientes, 30 en el grupo precoz y 27 en el tardío. Criterios de inclusión: neonatos de 34+0 a 36+6 semanas de gestación, con síndrome de dificultad respiratoria y necesidad de ventilación no invasiva y surfactante.
IntervencionesNinguna.
Variables de interés principalesSociodemográficas, clínicas y evolutivas: redosificación, duración de soporte respiratorio, oxígeno y tiempo en dejar de precisarlo tras el surfactante. También complicaciones y duración de hospitalización.
ResultadosEn el grupo precoz hubo menor necesidad de redosificación (3.3% vs 48.1%, P < .001) y disminución de duración, en días, de estancia en UCIN (7 vs 10.5, P .002), ventilación mecánica invasiva (2.4 vs 3.9, P .034), soporte respiratorio total (4.6 vs 6.6, P .005) y oxigenoterapia (0.4 vs 2.8, P < .001). También, menor incidencia de neumotórax (0% vs 33.3%, P .001). Además, a las 12 h de la administración, mantenían FiO2 0.21 el 83.4%, frente a 44.4% en el tardío.
ConclusionesEn nuestro estudio, la administración precoz en prematuros tardíos proporciona beneficios en cuanto a asistencia respiratoria y complicaciones. Sugerimos la ampliación de estudios para poder establecer recomendaciones en este grupo de pacientes.
Late preterm infants, born between 34+0 and 36+6 weeks of gestation, account for 70%–74% of all preterm births. They exhibit characteristics that increase their risk of morbidity and mortality, up to 2–3 times greater than those born at term, and their health care costs are twice as high as those of full-term infants.1–3
The 2018 SEN34-36 group study,2 involving 9121 patients from 34 hospitals found that 58.6% of late preterm infants required admission to the Neonatology unit, with up to 15% requiring admission to the Neonatal Intensive Care Unit (NICU), with a mean length of stay of 11 and 12 days, respectively.
One of the main causes of hospital admission in this population is respiratory complications, with respiratory distress syndrome (RDS) developing in up to 10%. This leads to higher morbidity and mortality rates and longer lengths of stay, along with the additional disadvantage of mother-infant separation.1–3 Respiratory treatment, which is better established in more preterm neonates, generates controversy. For this reason, an expectant approach is sometimes taken regarding surfactant administration, deciding to use it later when the expected progression has not been observed.1–5
Current evidence in late preterm infants suggests a lower risk of mortality, air leaks, persistent pulmonary hypertension, and duration of respiratory support when they receive surfactant.6–8 As indicated in the latest European Consensus Guidelines, due to data heterogeneity, there is currently insufficient evidence to establish recommendations.3
Given the above, this study is presented. The primary endpoint was to describe the clinical progression after surfactant use in a group of late preterm infants. The secondary endpoint was to compare based on the timing of application to determine if early use is protective.
Materials and methodsStudy design and participantsWe conducted a retrospective, observational, and analytical study at a single center from January 2012 through December 2021, in a third-level neonatal intensive care unit (NICU). The project was approved by the Ethics Committee for Research with Medicines (CEIm) at the hospital (act 8/2024). Exemption from informed consent was accepted due to the retrospective design and data collection according to everyday practice.
Inclusion criteria: Newborns between 34+0 and 36+6 weeks of gestation with a diagnosis of RDS, requiring mechanical ventilation, either non-invasive (NIV), in the form of CPAP or intermittent positive pressure ventilation (IPPV); or invasive mechanical ventilation (IMV). They must have received surfactant.
Diagnostic criteria for RDS: Patients with typical radiological findings (reduced lung volume, reticular infiltrate) and requirements for oxygen and/or distress.
Exclusion criteria:
- •
Mild RDS, defined as RDS that resolves without receiving surfactant.
- •
Transient tachypnea of the newborn (TTN), defined as distress with rapid improvement (<12–24 h), FiO2 requirements < 0.3, and typical radiographic findings (hyperinflation, fluid in fissures).
- •
Air leaks due to resuscitation at birth.
- •
Major malformations, chromosomal abnormalities, and/or metabolic diseases.
Radiographs of each patient were analyzed by the researchers to differentiate between TTN and RDS.
Patients were categorized into 2 groups: those who received surfactants within 12 h of life, inclusive, and those who received surfactants later. The decision to use a 12-h cutoff value between early and late administration was arbitrary.
ProceduresPorcine surfactant—poractant alfa—was used at an initial dose of 200 mg/kg and, if needed, redosed at 100 mg/kg. The criteria for administration were those established during the years of data collection: FiO2 ≥ 0.3–0.4 despite CPAP ≥ 6 cm H2O, or moderate-to-severe persistent distress with radiological findings consistent with RDS. Redosing was decided if oxygen requirements persisted or if invasive mechanical ventilation was required, after 6−12 h from the first dose, with a maximum of 3 doses.
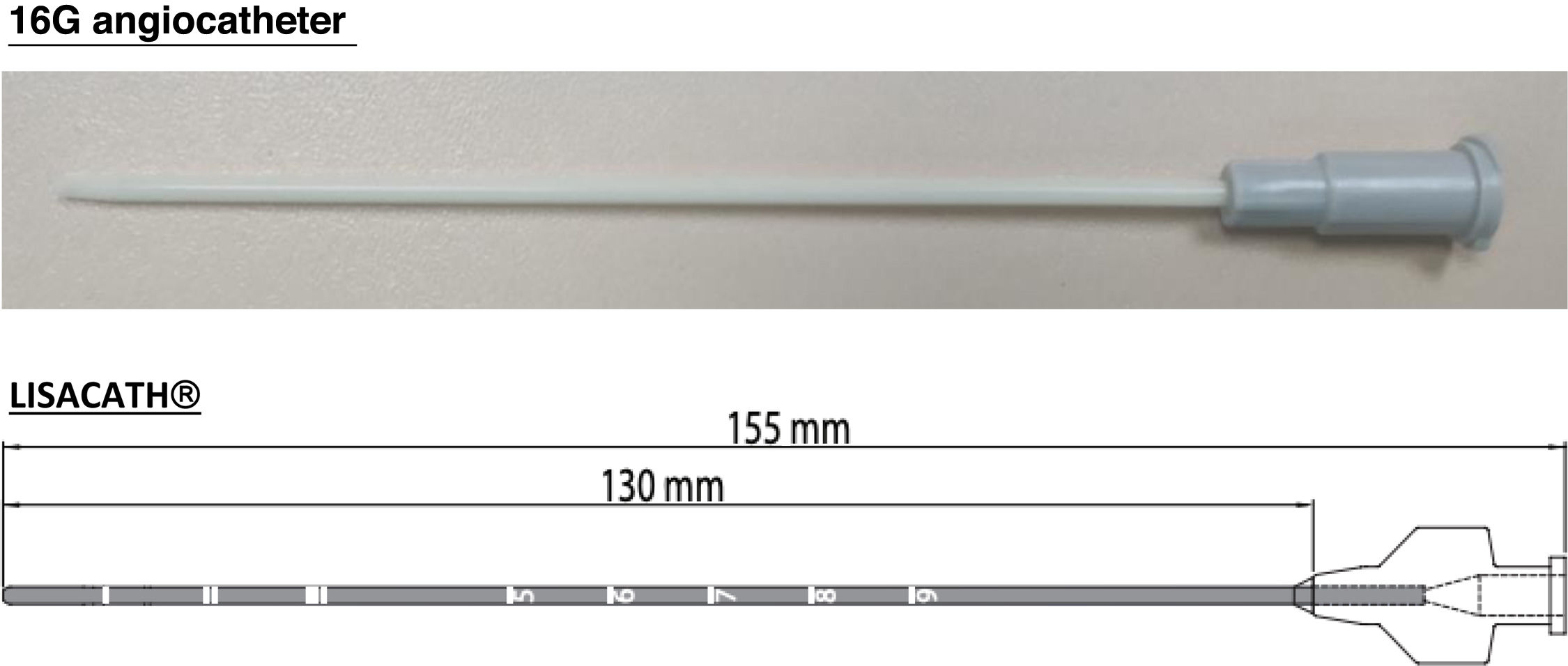
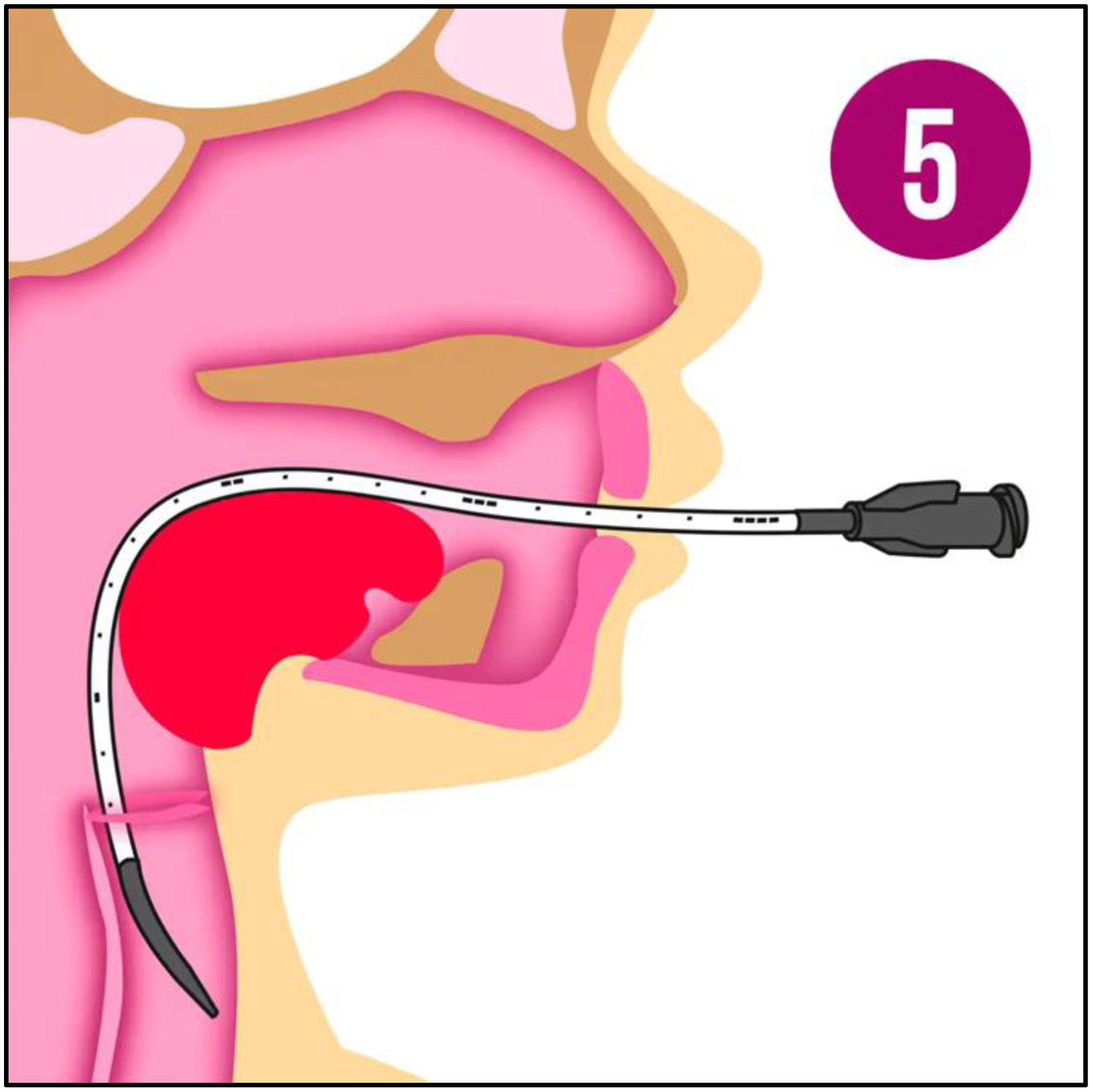
Surfactant was administered via endotracheal tube, as intubation was required at the physician's discretion, or by a minimally invasive method (LISA: less invasive surfactant administration). Initially, a 16G angiocatheter was used based on the study by Dargaville et al.9–11 Later, starting in 2017, the LISAcath® catheter from Chiesi, built with polyurethane with a 5-Fr diameter, was used (Fig. 1). The technique consists of instilling surfactant through the catheter, placed via direct laryngoscopy beyond the vocal cords while the patient remains on NIV (Fig. 2). During and after the procedure, aspiration through an orogastric tube is performed to ensure correct administration.
Catheters used in the LISA technique: 16G angiocatheter (image obtained by the author) and LisaCath®.19,20
Performing the LISA technique with Surfcath®21 catheter, currently used in our unit.
Sociodemographic variables related to the prenatal and perinatal situation of the patients were collected, as well as the clinical situation before and after surfactant administration. Data on the timing and type of administration, as well as the subsequent progression and possible complications, were also gathered. All these variables are included in Appendix 1.
Statistical analysisDescriptive data analysis was performed using R software version 4.2.3. Categorical variables are expressed as absolute numbers (n) and percentages (%), and analyzed using the chi-squared test with Yates' correction if necessary. Continuous variables that follow a normal distribution are compared using the mean ± standard deviation (SD) and analyzed with the Student's t-test. Otherwise, variables are expressed as median and range and analyzed using the Mann-Whitney U test. Statistical significance is considered if P < .05.
ResultsSigns of respiratory distressBetween 2012 and 2021, a total of 966 late preterm infants were born in our hospital area. Among them, 140 patients (14.5%) required admission to the NICU with a diagnosis of respiratory distress. Fifty-seven of these (40.7%) were considered to have TTN; 24 (17.1%), RDS without the need for surfactant; and 59 (42.1%), RDS requiring surfactant. A total of 135 out of the 140 patients (96.4%) required respiratory support at some point during their admission.
Epidemiological characteristicsA total of 57 out of the 59 patients who required surfactant were included in the study, with 30 newborns (52.6%) in the early surfactant group and 27 (47.4%) in the late group (Fig. 3).
The characteristics of the study groups are shown in Table 1, and distribution by year of data collection in Fig. 4.
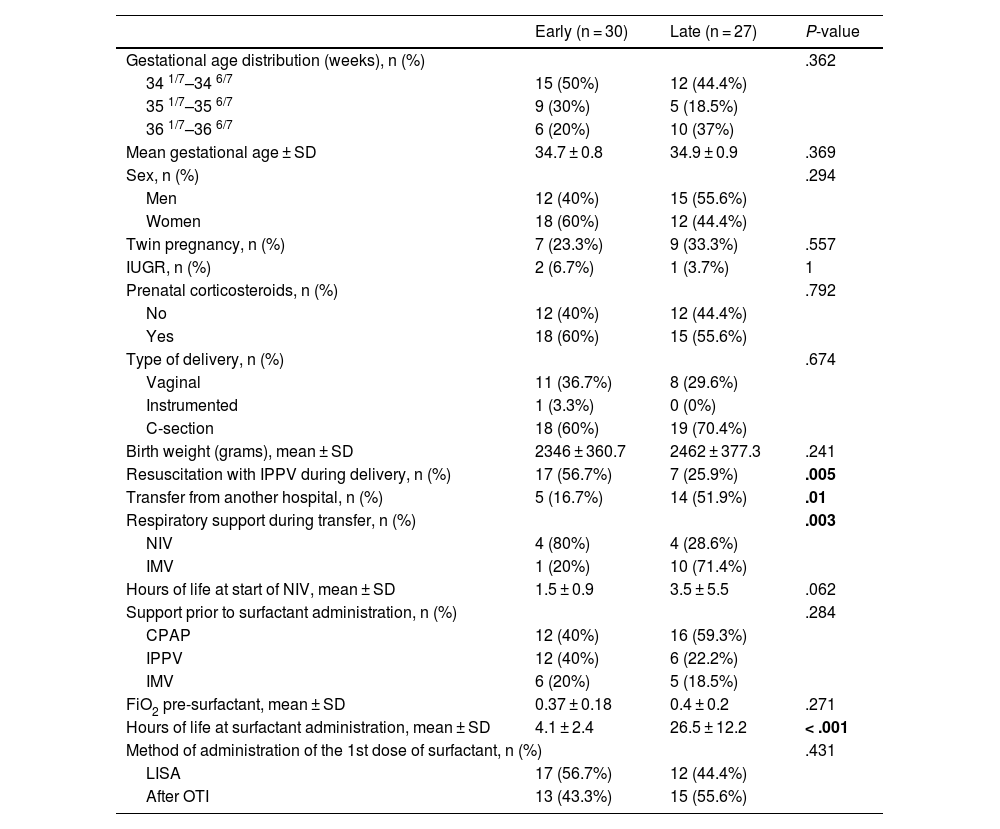
Demographic and clinical characteristics of the included patients, as well as the timing and method of surfactant administration in each group.
| Early (n = 30) | Late (n = 27) | P-value | |
|---|---|---|---|
| Gestational age distribution (weeks), n (%) | .362 | ||
| 34 1/7–34 6/7 | 15 (50%) | 12 (44.4%) | |
| 35 1/7–35 6/7 | 9 (30%) | 5 (18.5%) | |
| 36 1/7–36 6/7 | 6 (20%) | 10 (37%) | |
| Mean gestational age ± SD | 34.7 ± 0.8 | 34.9 ± 0.9 | .369 |
| Sex, n (%) | .294 | ||
| Men | 12 (40%) | 15 (55.6%) | |
| Women | 18 (60%) | 12 (44.4%) | |
| Twin pregnancy, n (%) | 7 (23.3%) | 9 (33.3%) | .557 |
| IUGR, n (%) | 2 (6.7%) | 1 (3.7%) | 1 |
| Prenatal corticosteroids, n (%) | .792 | ||
| No | 12 (40%) | 12 (44.4%) | |
| Yes | 18 (60%) | 15 (55.6%) | |
| Type of delivery, n (%) | .674 | ||
| Vaginal | 11 (36.7%) | 8 (29.6%) | |
| Instrumented | 1 (3.3%) | 0 (0%) | |
| C-section | 18 (60%) | 19 (70.4%) | |
| Birth weight (grams), mean ± SD | 2346 ± 360.7 | 2462 ± 377.3 | .241 |
| Resuscitation with IPPV during delivery, n (%) | 17 (56.7%) | 7 (25.9%) | .005 |
| Transfer from another hospital, n (%) | 5 (16.7%) | 14 (51.9%) | .01 |
| Respiratory support during transfer, n (%) | .003 | ||
| NIV | 4 (80%) | 4 (28.6%) | |
| IMV | 1 (20%) | 10 (71.4%) | |
| Hours of life at start of NIV, mean ± SD | 1.5 ± 0.9 | 3.5 ± 5.5 | .062 |
| Support prior to surfactant administration, n (%) | .284 | ||
| CPAP | 12 (40%) | 16 (59.3%) | |
| IPPV | 12 (40%) | 6 (22.2%) | |
| IMV | 6 (20%) | 5 (18.5%) | |
| FiO2 pre-surfactant, mean ± SD | 0.37 ± 0.18 | 0.4 ± 0.2 | .271 |
| Hours of life at surfactant administration, mean ± SD | 4.1 ± 2.4 | 26.5 ± 12.2 | < .001 |
| Method of administration of the 1st dose of surfactant, n (%) | .431 | ||
| LISA | 17 (56.7%) | 12 (44.4%) | |
| After OTI | 13 (43.3%) | 15 (55.6%) | |
SD: Standard deviation; IQR: interquartile range; IUGR: Intrauterine growth restriction; NIV: Non-invasive ventilation; IMV: Invasive mechanical ventilation; IPPV: Intermittent positive pressure ventilation; CPAP: Continuous positive airway pressure; OTI: Orotracheal intubation; LISA: Less invasive surfactant administration.
The statistically significant p-values have been highlighted in bold.
In the early surfactant group, a higher need for resuscitation with IPPV at birth was observed, with a statistically significant difference (Table 1). The median APGAR score at 1 min of life was 8 in the early group and 9 in the late group, and at 5 min, it was 9 and 10, respectively.
Before surfactant administration, most patients received NIV (Table 1), with a positive end-expiratory pressure (PEEP) of 5−6 cm H2O.
Surfactant administrationThe situation prior to the administration of the first dose of surfactant and the subsequent progression are shown in Tables 1 and 2.
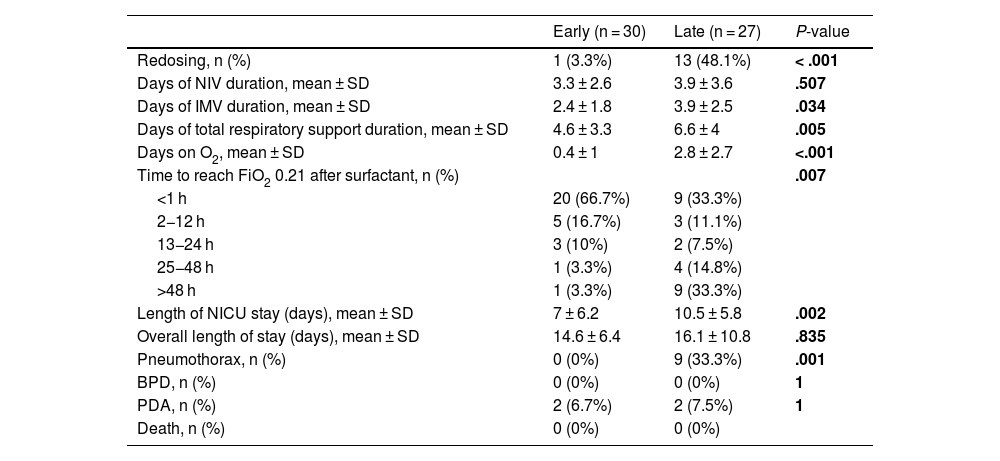
Results and comparison of the outcomes in each group after surfactant administration.
| Early (n = 30) | Late (n = 27) | P-value | |
|---|---|---|---|
| Redosing, n (%) | 1 (3.3%) | 13 (48.1%) | < .001 |
| Days of NIV duration, mean ± SD | 3.3 ± 2.6 | 3.9 ± 3.6 | .507 |
| Days of IMV duration, mean ± SD | 2.4 ± 1.8 | 3.9 ± 2.5 | .034 |
| Days of total respiratory support duration, mean ± SD | 4.6 ± 3.3 | 6.6 ± 4 | .005 |
| Days on O2, mean ± SD | 0.4 ± 1 | 2.8 ± 2.7 | <.001 |
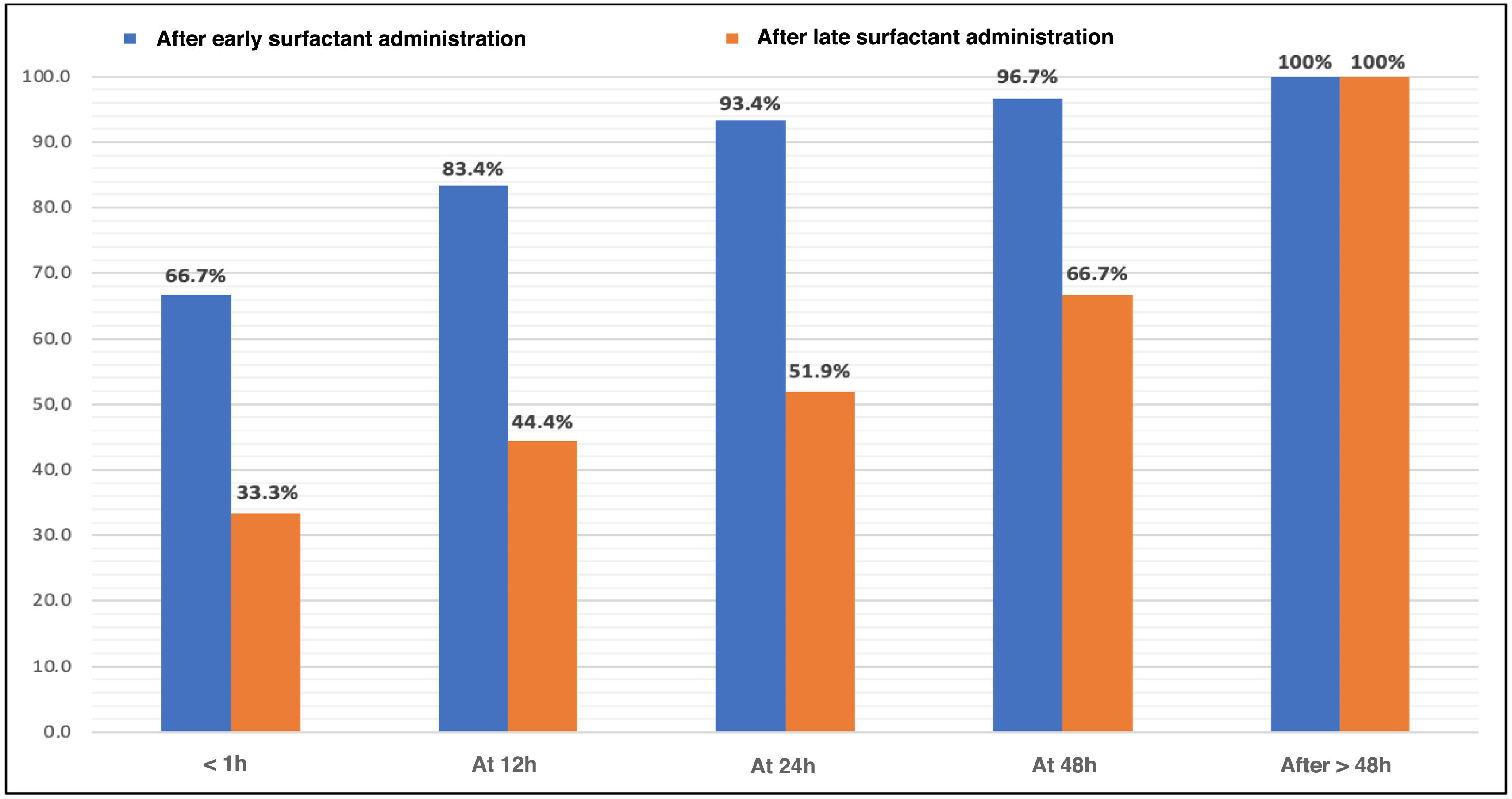
| Time to reach FiO2 0.21 after surfactant, n (%) | .007 | ||
| <1 h | 20 (66.7%) | 9 (33.3%) | |
| 2−12 h | 5 (16.7%) | 3 (11.1%) | |
| 13−24 h | 3 (10%) | 2 (7.5%) | |
| 25−48 h | 1 (3.3%) | 4 (14.8%) | |
| >48 h | 1 (3.3%) | 9 (33.3%) | |
| Length of NICU stay (days), mean ± SD | 7 ± 6.2 | 10.5 ± 5.8 | .002 |
| Overall length of stay (days), mean ± SD | 14.6 ± 6.4 | 16.1 ± 10.8 | .835 |
| Pneumothorax, n (%) | 0 (0%) | 9 (33.3%) | .001 |
| BPD, n (%) | 0 (0%) | 0 (0%) | 1 |
| PDA, n (%) | 2 (6.7%) | 2 (7.5%) | 1 |
| Death, n (%) | 0 (0%) | 0 (0%) | |
SD: Standard deviation; NIV: Non-invasive ventilation; IMV: Invasive mechanical ventilation; NICU: Neonatal intensive care unit; BPD: Bronchopulmonary dysplasia; PDA: Patent ductus arteriosus.
The statistically significant p-values have been highlighted in bold.
Redosing was found to be up to 12 times higher when surfactant was administered later, with a statistically significant difference (P < .001).
In the late group, the percentage of patients transferred to our unit from other hospitals was up to 3 times higher (Table 1). Eleven of those 14 patients (78.6%) met the criteria for surfactant administration before 12 h of life. However, only 6 of the 11 (54.5%) received it at the referring hospital, and it was administered later.
Inter-group comparisonPatients in the early group required fewer days of IMV, total respiratory support, additional oxygen, and shorter NICU stays, with statistically significant differences (Table 2).
The time required to stop needing supplemental oxygen after the first surfactant dose was also analyzed, as shown Fig. 5 as cumulative percentages. In the early group, 20 patients (66.7%) reached FiO2 values of 0.21 within 1 h, while in the late group, that percentage required, at least, 24 h (P = .007).
DiscussionIn this retrospective study, we analyze the possible benefits that may arise from the early administration of surfactant. Administration before 12 h of life in our study has been associated with less respiratory support and fewer days at the NICU. Additionally, the number of days requiring oxygen has decreased, as well as the time needed to stop requiring it after the first dose. On the other hand, a lower incidence of pneumothorax was also observed in this group.
When comparing the epidemiological characteristics of both groups, no statistically significant differences were found. A higher number of patients who required administration of IPPV during resuscitation in the delivery room was observed in the early surfactant group. However, when analyzing the APGAR score, a median > 8 was obtained at both 1 and 5 min of life in both groups. Therefore, this difference was not considered clinically relevant, as these values suggest that progression was favorable. Most late preterm infants who required IPPV likely received it only within the first few moments of support in the delivery room, with a good response, not needing further resuscitation. In the late group, a higher transfer rate was observed, which may have contributed to a more unfavorable clinical course, both due to the transfer stress and possibility of delayed treatment.
The mean FiO2 before surfactant administration was >0.3 in both groups. In most patients from the late group, radiographic and clinical criteria were met within the first 12 h of life. However, in 12 (44.4%) cases, it was administered non-invasively at a later time, while in the other 15 (55.6%), it was administered only when intubation was needed, at the physician's discretion. This could be due to the tendency to optimize respiratory support and take a watchful waiting approach in late preterms. These infants typically present adequate respiratory effort, and the administration of surfactant involves an invasive maneuver.12 On the other hand, the later implementation of the LISA technique in hospitals without NICUs may have contributed to the delay in reaching the intubation criteria, which were probably later than the surfactant administration criteria.
In terms of respiratory support, NIV was initiated shortly after the onset of distress in both groups, although it was delayed by up to 2 h in the late vs the early group, which could again be due to the difference in the number of transfers. In NICU-free hospitals and if clinical situation allows it, high- or low-flow oxygen therapy may be initiated with a watchful waiting approach, expecting improvement without escalating to NIV, as its initiation would require intensive care and the transfer of the patient to a different center with such a unit. However, literature indicates that delaying the initiation of NIV could also be a factor leading to worse progression.13 It would be important to unify criteria for the initiation of respiratory support and administration of surfactants.
After the early administration of surfactants, most patients no longer required additional FiO2 within the first 24 h, with a high percentage not requiring oxygen just 1 h after receiving it, maintaining this benefit throughout the length of stay. The patient who required a 2nd dose in the early group was the only one who received the first dose at exactly 12 h of life, taking more than 48 h to reach FiO2 values of 0.21. In contrast, in the late group, it took up to 48 h after administration to reach the same percentage of patients without oxygen, while redosing was required in 13 newborns (48.1%). In fact, 8 of the 9 patients who took more than 48 h to stop requiring oxygen received a 2nd dose. The patient who did not receive a 2nd dose had been intubated for developing a pneumothorax, the main complication of RDS.14 In our study, no air leak was observed when surfactant was administered early, while a significantly higher number was seen in those who received it later. Air leaks typically occur in RDS patients with a slow disease progression, so,15 as observed in our study and reflected in the literature, administering treatment within the first hours of life may help reduce this complication.
Current evidence shows that in more immature preterm infants, the administration of surfactants within the first hours of life has beneficial effects.16 However, in newborns closer to term, there is no consensus on the optimal timing for administration.3,15,17 The optimal timeframe should be the subject of further study. The review of the current literature includes a study published by the Cochrane Neonatal Group in 2012 that analyzed the effects of surfactant administration within the first 2 h in intubated newborns with RDS.18 Currently, the SURFON1 study is underway, comparing the effects of surfactants vs a watchful waiting approach in late preterm and early term infants. This study considers the optimal administration time to be within the first 2−8 h of life but accepts administration up to 24 h.
The limitations of this work should be taken into consideration. It is a retrospective study with a small sample size, which leads to potential data loss and allows for only hypothesis generation. These limitations inherent to the study design have been addressed by emphasizing recruitment and minimizing losses. Additionally, the study was conducted in a single center, analyzing years when the LISA technique was being implemented, with its corresponding learning curve. Of note, the potential bias from the higher number of transfers in the late group, which may have impacted the later administration of surfactants. Although the method of administration was not used as a variable in the analysis, the percentage of each method was similar in both groups with no significant differences. The severity of distress was also not considered, as the Silverman score was not recorded in most health records. Moreover, the initiation of feeding and whether it was fully established before surfactant administration or the prior gasometric status were not taken into account.
In the analyzed series, the patients who received surfactant before 12 h of life showed a better progression. Further prospective, multicenter studies with larger sample sizes are needed to establish the true utility of these results and address all the above-mentioned limitations.
ConclusionsIn our sample of late preterm infants with RDS, early administration of surfactant has been associated with benefits such as a quick and sustained withdrawal from supplemental oxygen, less need for respiratory support, and shorter NICU stays. Additionally, it has reduced the development of complications.
Therefore, further structured studies are needed to compare the benefits of early surfactant administration with other treatment options in this gestational age range.
CRediT authorship contribution statementDr. Bartual is the principal investigator of this project and, therefore, responsible for the study design, data collection, data analysis, and drafting of this manuscript. Dr. Vizcaíno has been actively involved in the study design and data analysis, as well as in the drafting of the manuscript. Dr. Ferrández contributed to the study design and drafting of the manuscript.
FundingNone declared.
*Sociodemographic:
- •
Total number of late preterm infants born between 2012–2020 in our area
- •
Number of late preterm infants diagnosed with TTN
- •
Number of late preterm infants diagnosed with RDS, with and without surfactant requirement
- •
Year of collection
- •
Distribution by gestational age: 341/7–346/7/351/7–356/7/361/7–366/7
- •
Sex: male/female
- •
Multiple pregnancy: yes/no
- •
Intrauterine growth restriction: yes/no
- •
Prenatal corticosteroids: yes/no
- •
Type of delivery: vaginal/assisted/C-section
- •
Birth weight
- •
Transfer from another hospital: yes/no
*Respiratory suport:
- •
Resuscitation with IPPV during delivery: yes/no
- •
Type of respiratory support during transfer: NIV/IMV
- •
Hours of life at initiation of NIV
- •
Type of mechanical ventilation before surfactant administration: CPAP/IPPV/IMV
- •
PEEP received in case of NIV
*Surfactant administration:
- •
FiO2 required before surfactant
- •
Hours of life at the time of surfactant administration
- •
Mode of administration of the 1st dose of surfactant: LISA/after OTI
*Progression:
- •
Redosing: yes/no
- •
Days on NIV after surfactant
- •
Days on IMV after surfactant
- •
Total days of respiratory support after surfactant (NIV, IMV, high and low flow oxygen therapy)
- •
Days with FiO2 > 0.21 after the 1st dose of surfactant
- •
Time to reach FiO2 values of 0.21 after the 1st dose of surfactant: <1 h/2−12 h/13−24 h/25−48 h/>48 h
- •
Length of NICU stay (days)
- •
Overall length of stay (days)
*Complications:
- •
Pneumothorax: yes/no
- •
BPD: yes/no
- •
PDA: yes/no
- •
Mortality: yes/no