To describe the transfusion practice in the ICUs in Spain, according to national and international recommendations (guidelines).
DesignProspective, cross-sectional, multi-centre study.
ScopeData collection was carried out by means of a questionnaire sent electronically to the Heads of Service of 111 ICUs in Spain.
Participants1,448 patients were included, aged 61.8 (SD 15.7) years, 66.2% male, with an SOFA of 4.7 ± 3.8 and average stay of 10.62 ± 17.49 days.
VariablesDemographic and clinical variables of the patients were collected, as well as variables related to the transfusion act.
ResultsOf the 1,448 patients, 9.9% received al least one transfusion of any blood product, 3.7% fresh plasma, 3.9% platelets and 8.9% red blood cell concentrate, mainly by analytical criteria (36.2%). Hemoglobin had a mean of 7.8 g/dL (95% CI: 6-9-8.5) and 9.8 g/dL (95% CI: 8.5–11.2) before and after the transfusion, respectively, p < 0.001. The transfusion units had a mean of 2.5 ± 2.4 per patient. The most commonly used blood product was red blood cell concentrate (CH) (90.2%). Patients admitted for surgery had a higher transfusion rate (14.4%) than those admitted for medical pathology (8.9%) (p = 0.006). 5.4% (7/129) of patients who received CH died compared to 2.4% (31/1302) who did not (p = 0.04). Mortality of transfused patients was higher. The transfusion rate in most of hospitals was 5% to 20%, with 18 hospitals (16.21%) having transfusion rates between 20% and 50%. Hospitals with PBM programs and mass transfusion programs had a lower transfusion rate, although not statistically significant.
ConclusionsIn this multicenter cross-sectional study, a transfusion prevalence of 9.9% was observed in Spanish Critical Care Units. The most frequent blood product transfused was red blood cells and the main reasons for transfusion were acute anemia with hemodynamic impact and analytical criteria. Mortality of transfused patients was higher.
Describir la práctica transfusional en las UCIs de España, acorde con recomendaciones (guidelines) nacionales e internacionales.
DiseñoEstudio prospectivo, transversal y multicéntrico.
ÁmbitoLa recogida de datos se realizó mediante una encuesta enviada electrónicamente a los médicos intensivistas de 111 UCIs de España.
ParticipantesSe incluyeron 1.448 pacientes, de 61,8 (DE 15,7) años, el 66,2% varones, con un SOFA de 4,7 ± 3,8 y estancia media de 10,62 ± 17,49 días.
VariablesSe recogieron variables demográficas y clínicas de los pacientes, así como variables relacionadas con el propio acto transfusional.
ResultadosDe los 1.448 pacientes, el 9,9% recibieron al menos una transfusión de cualquier hemocomponente, 3,7% de plasma fresco, 3,9% de plaquetas y 8,9% de concentrado de hematíes, siendo la causa principal el umbral transfusional basado en la hemoglobina (36,2%). La hemoglobina tuvo una media de 7,8 g/dL (IC 95%: 6,9–8,5), y de 9.8 g/dl (IC95%: 8,5–11,2) antes y después de la transfusión respectivamente (p < 0,001). Las unidades transfundas tuvo una media por paciente de 2,5 ± 2,4 por paciente. El hemoderivado más utilizado fue el concentrado de hematíes (CH) (90,2%). Los pacientes ingresados por motivos quirúrgicos tuvieron una tasa de transfusión mayor (14,4%) respecto a los ingresados por patología médica (8,9%) (p = 0,006). El 5,4% (7/129) de los pacientes que recibieron CH fallecieron respecto el 2,4% (31/1302) que no lo recibieron (p = 0,04). La tasa de transfusión en la mayor parte de hospitales fue de 5% al 20%, habiendo 18 hospitales (16.21%) con tasas de transfusión entre el 20% y el 50%. Los hospitales con programas PBM y programas de transfusión masiva tuvieron una menor tasa de transfusión, aunque sin ser significativa.
ConclusionesEn este estudio multicéntrico de corte transversal se observó una prevalencia transfusional en las unidades de críticos españolas del 9,9%. El producto sanguíneo más frecuentemente trasfundido fueron los concentrados de hematíes, y los principales motivos de transfusión fueron la anemia aguda con repercusión hemodinámica y el umbral transfusional basado en la hemoglobina. La mortalidad de los pacientes transfundidos fue mayor.
The replacement of blood losses in the critical patient through the transfusion of blood products has changed substantially in recent decades.1 Those in charge of deciding transfusion must consider that the inappropriate use of these products increases patient morbidity-mortality, and that blood products are moreover valuable and rather scarce.
Previous studies have shown that blood products are often not used properly, contributing to place patients at risk by exposing them to the potential complications of transfusion, such as transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), transfusion-related immunomodulation (TRIM) and infections – especially if transfusion is unnecessary or is secondary to transfusion error.2,3 On the other hand, needless transfusion seriously reduces the availability of blood products for those patients who really do need them.4 The World Health Organization (WHO) considers that transfusion should be regarded as a current healthcare resource which when used correctly as a therapeutic intervention is able to save lives. Nevertheless, knowing the potential complications of the procedure, unnecessary transfusion must be avoided, and alternatives should be used whenever possible. The health authorities, care providers and physicians are crucial for the prevention, early diagnosis and management of those clinical situations in which blood transfusion may prove necessary.2
The above is encompassed within the concept of Patient Blood Management (PBM),5 which stresses the importance of multidisciplinary programs for the optimization of erythropoiesis.6 In Spain, article 40 of Royal Decree (RD) 1088/20051,7 regulates the existence and functions of hospital and regional transfusion committees and centers, with the primary aim of ensuring the correct use of blood and its different components. However, despite the existence of such legislation, there is a notorious variability of prescription practices among different centers, possibly due to differences in their degree of implementation of the PBM programs.
Aging of the population, combined with age-related comorbidities and the increased use of antiplatelet drugs and anticoagulants among the elderly, could be related to an increase in transfusion rates in Spain. In contraposition to this, some studies have reported that despite these conditioning factors, the transfusion rates have not increased as expected,8 and this could be due to the more or less intense work of the hospital transfusion committees, which seek to ensure a more rational use of transfusion practices.
The most widely used transfusional threshold-defining parameter remains the hemoglobin (Hb) concentration,9,10 despite the fact that it is also one of the main factors conditioning inappropriate transfusions. In many cases the values proposed in the clinical practice guides are not followed.11 This situation is more patent in elderly patients, since there is no scientific evidence warranting the idea that higher Hb levels improve the survival or quality of life of these patients, and hence more restrictive transfusion practices in this particular population group appear to be the safest option.12
It is not clear whether there are differences in the transfusion criteria and in the number and percentage of critical patients subjected to transfusion among the different Spanish Intensive Care Units (ICUs). In this regard, it is reported that the predominant activities in the ICU are of a medical nature, and if we consider the existence of a group of such Units specialized in polytraumatized patients – which are the Units characterized by a high transfusion demand – we effectively have a defined potential source of transfusional variability. Furthermore, this same article underscores the idea that our ICUs usually have no protocols, and few hospitals have transfusion committees. In turn, in those centers that do have such committees, the latter do not usually generate standards, or these are of little relevance. All this contributes to further accentuate the possible differences in relation to transfusion policies among different hospitals.
We postulate that there may be important variability in this regard. The aim of the present «Transfusion day» study was to determine the epidemiology of transfusion practices in Spanish ICUs, and to identify the factors that influence their possible variability.
Material and methodsStudy designA cross-sectional multicenter study was conducted in a sample of 111 Spanish ICUs (Annex I, see Supplementary material). The chosen cut-off date was 12 November 2018, as this was a commemorative day in the field of transfusion therapy. In effect, Dr. Norman Bethune, considered to be the first to develop a mobile blood transfusion service, during the Spanish Civil War, died on 12 November 1939. Pediatric ICUs were not included in the study.
PatientsWe included all the patients in the ICUs on the mentioned date of the study.
Study variablesPatient demographic and clinical data were collected, along with variables related to the transfusion act. Annex II (Supplementary material) contains the case report form (CRF) that was sent to the ICUs.
Hemodynamic repercussions were defined as the presence of clinical signs suggestive of hypoperfusion (sensory alterations, poor capillary filling, etc.) and particularly the presence of arterial hypotension. Likewise, acute anemia was defined based mainly on the criteria of the WHO (mild, moderate or severe anemia), though on relating it to bleeding, it was associated with dynamic terms; accordingly, the difference between acute and chronic was considered depending on the time of appearance.
Data collectionData monitoring and collection took place on 12 November 2018. Subsequent follow-up was limited to those patients who experienced some transfusion reaction.
Data collection was carried out using an electronic CRF (eCRF), and data analysis was performed using the STATA/SE® version 14.0 statistical package.
Statistical analysisQualitative variables were described as frequency distributions, while quantitative variables were expressed as the mean and standard deviation (SD) or confidence interval.
The comparative analysis of the qualitative variables was based on the chi-squared test, while the comparison of quantitative variables was carried out using the Student t-test or Mann–Whitney U-test, depending on whether or not the data exhibited a normal distribution as confirmed by the Shapiro–Wilks test. The nonparametric Wilcoxon test was used to contrast hemoglobin before and after transfusion.
The multivariate analysis was based on binary logistic regression models with the forward conditional method, entering the transfusion of at least one blood component of any kind as dependent variable, while those variables showing statistical significance in the bivariate analysis were entered as independent variables. Model calibration analysis was made using the Hosmer–Lemeshow statistic. The discriminatory capacity of the model was assessed by means of the area under the receiver-operating characteristic (ROC) curve. The adjusted results of the multivariate model were reported as the odds ratio (OR) and 95% confidence interval (95%CI), and were graphically displayed by means of a nomogram assigning a score to each factor related to transfusion, and allowing calculation of the probability that a patient will undergo transfusion. A type I error of under 5% was regarded as significant.
Ethical aspectsThe study was carried out in abidance with the ethical principles of the Declaration of Helsinki, version of Fortaleza (Brazil) 2013 (available on the website of the World Medical Association – https://www.wma.net/wp-content/uploads/2016/11/wmj22.pdf) regarding human research, the good epidemiological practice principles of the ICH (International Conference on Harmonization), and applicable Spanish legislation (http://www.ieaweb.org/GEP07.htm).
The study was approved by the Clinical Research Ethics Committee of Hospital Universitario La Paz (Madrid, Spain).
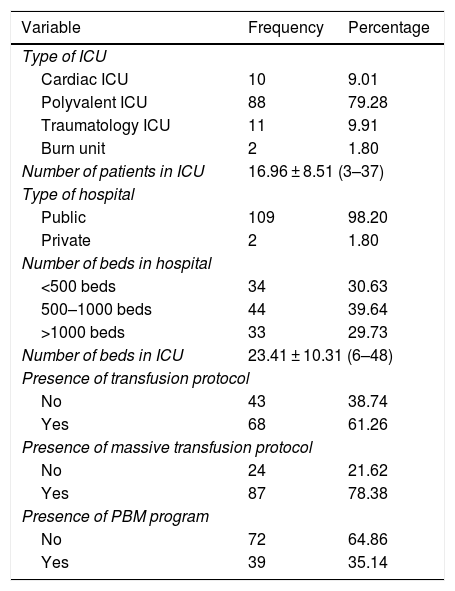
ResultsDescription of the ICUsTable 1 summarizes the main characteristics of the ICUs that participated in the study. The great majority of the hospitals (98.20%) were public centers, and the distribution of the total number of beds was very heterogeneous, with the compilation of data on hospitals of all sizes. Of note is the fact that 78.38% had a massive transfusion protocol, 61.26% had a transfusion protocol, and only 35.14% had a PBM program.
Description of the ICUs participating in the study.
| Variable | Frequency | Percentage |
|---|---|---|
| Type of ICU | ||
| Cardiac ICU | 10 | 9.01 |
| Polyvalent ICU | 88 | 79.28 |
| Traumatology ICU | 11 | 9.91 |
| Burn unit | 2 | 1.80 |
| Number of patients in ICU | 16.96 ± 8.51 (3–37) | |
| Type of hospital | ||
| Public | 109 | 98.20 |
| Private | 2 | 1.80 |
| Number of beds in hospital | ||
| <500 beds | 34 | 30.63 |
| 500–1000 beds | 44 | 39.64 |
| >1000 beds | 33 | 29.73 |
| Number of beds in ICU | 23.41 ± 10.31 (6–48) | |
| Presence of transfusion protocol | ||
| No | 43 | 38.74 |
| Yes | 68 | 61.26 |
| Presence of massive transfusion protocol | ||
| No | 24 | 21.62 |
| Yes | 87 | 78.38 |
| Presence of PBM program | ||
| No | 72 | 64.86 |
| Yes | 39 | 35.14 |
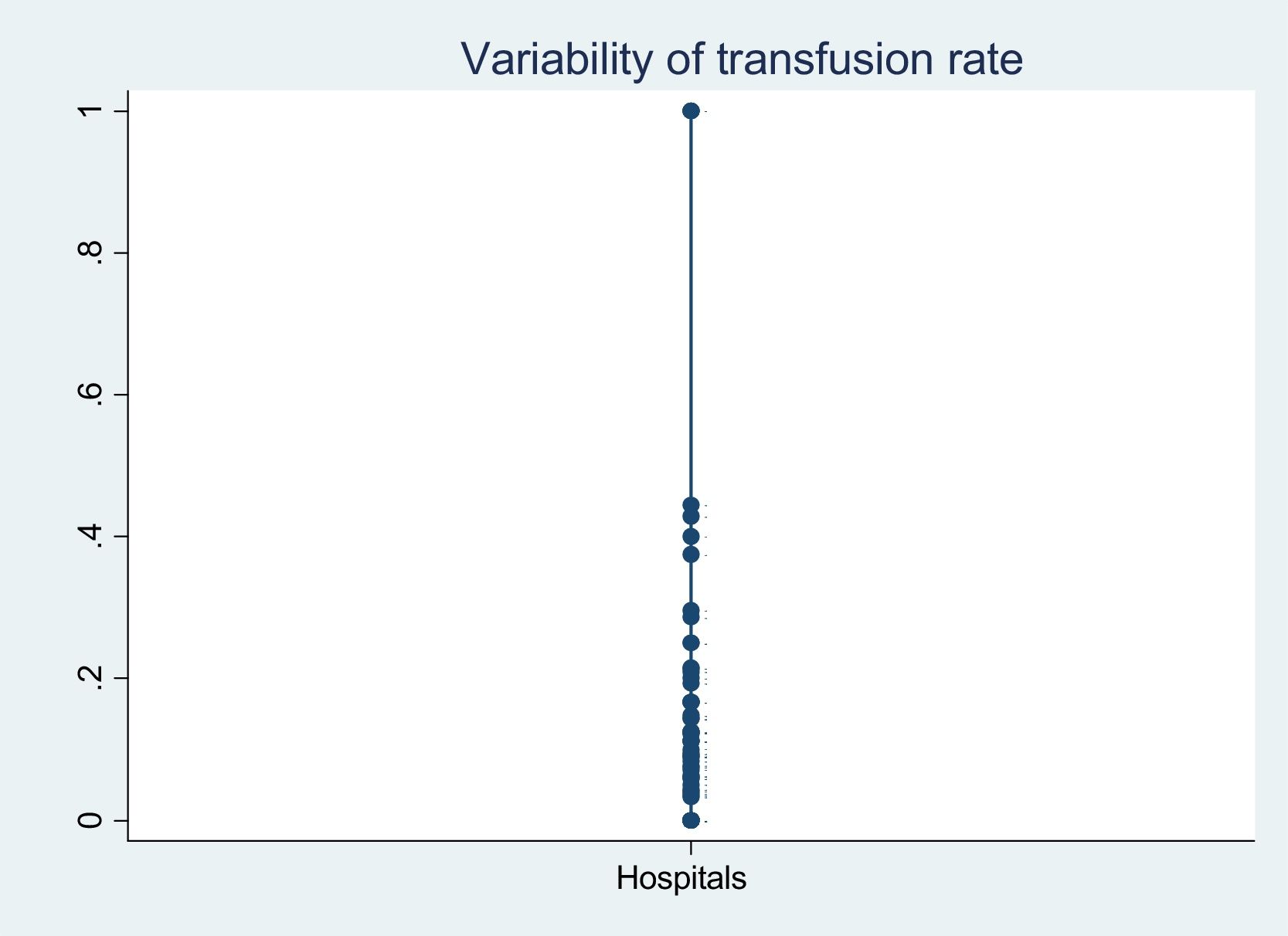
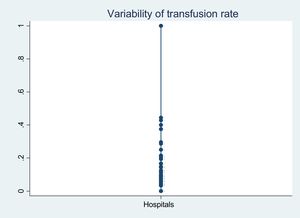
With regard to variability of the transfusion rates (Fig. 1), we found that only one hospital transfused 100% of the patients admitted to the ICU at that time. Most of the hospitals showed a rate of 5–20%, with 18 hospitals (16.21%) presenting transfusion rates of 20–50%. On the other hand, the hospitals with PBM programs transfused 12.24% of the patients – this figure being somewhat lower than in the hospitals with no such programs (16.30%) (p = 0.264). Similar results were obtained in relation to the transfusion and massive transfusion protocols, where the transfusion rates were similar, regardless of whether or not the protocol was established in the hospital (p = 0.883 and p = 0.197, respectively).
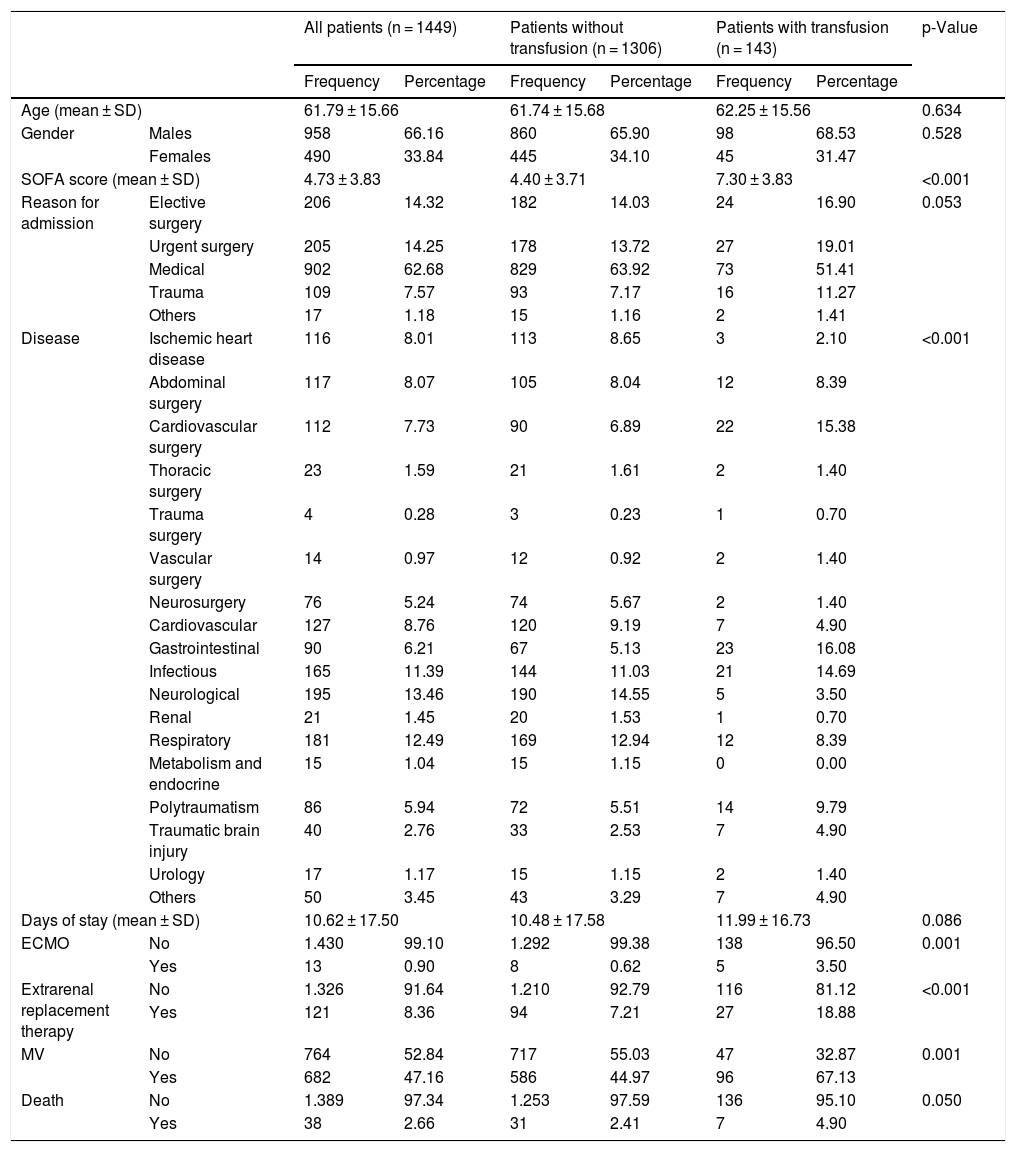
Patient characteristicsThe study included a total of 1448 patients, with a mean age of 62 ± 16 years and a predominance of males (66.2%). No significant differences in age or gender distribution were observed between the patients who were transfused and those who were not (Table 2).
Demographic and clinical characteristics of the patients admitted to the ICU.
| All patients (n = 1449) | Patients without transfusion (n = 1306) | Patients with transfusion (n = 143) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | Frequency | Percentage | |||
| Age (mean ± SD) | 61.79 ± 15.66 | 61.74 ± 15.68 | 62.25 ± 15.56 | 0.634 | ||||
| Gender | Males | 958 | 66.16 | 860 | 65.90 | 98 | 68.53 | 0.528 |
| Females | 490 | 33.84 | 445 | 34.10 | 45 | 31.47 | ||
| SOFA score (mean ± SD) | 4.73 ± 3.83 | 4.40 ± 3.71 | 7.30 ± 3.83 | <0.001 | ||||
| Reason for admission | Elective surgery | 206 | 14.32 | 182 | 14.03 | 24 | 16.90 | 0.053 |
| Urgent surgery | 205 | 14.25 | 178 | 13.72 | 27 | 19.01 | ||
| Medical | 902 | 62.68 | 829 | 63.92 | 73 | 51.41 | ||
| Trauma | 109 | 7.57 | 93 | 7.17 | 16 | 11.27 | ||
| Others | 17 | 1.18 | 15 | 1.16 | 2 | 1.41 | ||
| Disease | Ischemic heart disease | 116 | 8.01 | 113 | 8.65 | 3 | 2.10 | <0.001 |
| Abdominal surgery | 117 | 8.07 | 105 | 8.04 | 12 | 8.39 | ||
| Cardiovascular surgery | 112 | 7.73 | 90 | 6.89 | 22 | 15.38 | ||
| Thoracic surgery | 23 | 1.59 | 21 | 1.61 | 2 | 1.40 | ||
| Trauma surgery | 4 | 0.28 | 3 | 0.23 | 1 | 0.70 | ||
| Vascular surgery | 14 | 0.97 | 12 | 0.92 | 2 | 1.40 | ||
| Neurosurgery | 76 | 5.24 | 74 | 5.67 | 2 | 1.40 | ||
| Cardiovascular | 127 | 8.76 | 120 | 9.19 | 7 | 4.90 | ||
| Gastrointestinal | 90 | 6.21 | 67 | 5.13 | 23 | 16.08 | ||
| Infectious | 165 | 11.39 | 144 | 11.03 | 21 | 14.69 | ||
| Neurological | 195 | 13.46 | 190 | 14.55 | 5 | 3.50 | ||
| Renal | 21 | 1.45 | 20 | 1.53 | 1 | 0.70 | ||
| Respiratory | 181 | 12.49 | 169 | 12.94 | 12 | 8.39 | ||
| Metabolism and endocrine | 15 | 1.04 | 15 | 1.15 | 0 | 0.00 | ||
| Polytraumatism | 86 | 5.94 | 72 | 5.51 | 14 | 9.79 | ||
| Traumatic brain injury | 40 | 2.76 | 33 | 2.53 | 7 | 4.90 | ||
| Urology | 17 | 1.17 | 15 | 1.15 | 2 | 1.40 | ||
| Others | 50 | 3.45 | 43 | 3.29 | 7 | 4.90 | ||
| Days of stay (mean ± SD) | 10.62 ± 17.50 | 10.48 ± 17.58 | 11.99 ± 16.73 | 0.086 | ||||
| ECMO | No | 1.430 | 99.10 | 1.292 | 99.38 | 138 | 96.50 | 0.001 |
| Yes | 13 | 0.90 | 8 | 0.62 | 5 | 3.50 | ||
| Extrarenal replacement therapy | No | 1.326 | 91.64 | 1.210 | 92.79 | 116 | 81.12 | <0.001 |
| Yes | 121 | 8.36 | 94 | 7.21 | 27 | 18.88 | ||
| MV | No | 764 | 52.84 | 717 | 55.03 | 47 | 32.87 | 0.001 |
| Yes | 682 | 47.16 | 586 | 44.97 | 96 | 67.13 | ||
| Death | No | 1.389 | 97.34 | 1.253 | 97.59 | 136 | 95.10 | 0.050 |
| Yes | 38 | 2.66 | 31 | 2.41 | 7 | 4.90 | ||
The mean SOFA (Sequential Organ Failure Assessment) score was 4.7 ± 3.8, and was greater among the transfused patients (7.3 ± 3.8 vs. 4.4 ± 3.7; p < 0.001). Of the total study sample, 47.1% required invasive mechanical ventilation, 8.3% required continuous extrarenal replacement therapy, and 0.9% required extracorporeal membrane oxygenation (ECMO) – the rates being higher among the transfused patients, as can be seen in Table 2.
The most frequent cause of admission was medical disease (62.7%), specifically of a neurological origin (21.5%), followed by respiratory disease (20.4%) and infection (18.5%). In turn, 14.3% of the admissions came from urgent surgery, 14.3% from elective surgery, and 7.6% were admitted as a consequence of trauma.
With regard to the risk factors favoring bleeding, anemia and transfusion, 20% of the patients had received anticoagulation (0.9% due to extracorporeal oxygenation techniques) and 22.1% had received antiplatelet medication.
With regard to mortality, only 2.7% (38/1448) of the patients died within the first 24 h of ICU stay; 4.9% (7/143) had been transfused previously while 2.4% had not been transfused previously (31/1284). Differences were observed regarding the transfused blood component and mortality. Specifically, 5.4% (7/129) of the patients that received packed red blood cells died, versus 2.4% (31/1302) of those who did not (p = 0.039). Likewise, 9.3% (5/54) of the patients that received plasma transfusions died, versus 2.4% (33/1377) of those who did not (p = 0.002). Lastly, 8.8% (5/57) of the patients that received platelet transfusions died, versus 2.4% (33/1374) of those who did not (p = 0.003).
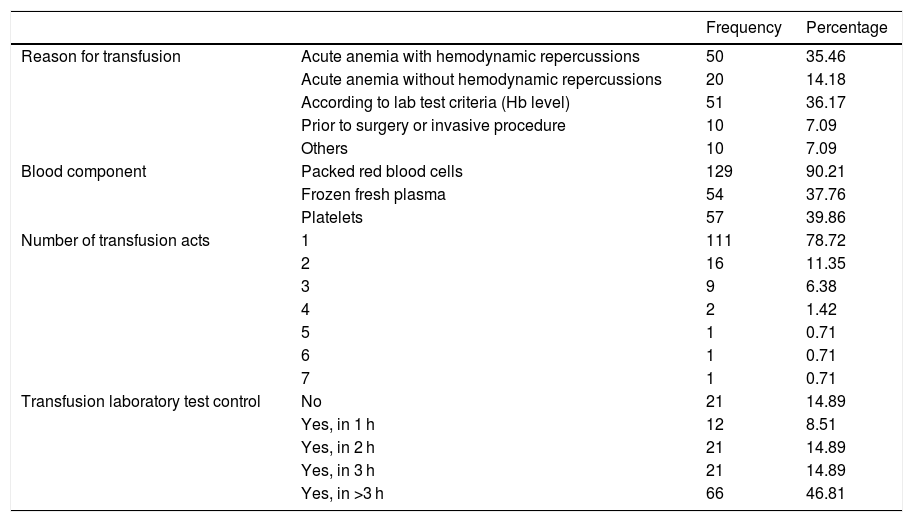
Characteristics of transfusion practiceOf the global sample, the patients who most often suffered anemia were those admitted after surgery [81.1% (219/270)], followed by septic patients [78.2% (129/165)] (Table 3).
Description of transfusion practice.
| Frequency | Percentage | ||
|---|---|---|---|
| Reason for transfusion | Acute anemia with hemodynamic repercussions | 50 | 35.46 |
| Acute anemia without hemodynamic repercussions | 20 | 14.18 | |
| According to lab test criteria (Hb level) | 51 | 36.17 | |
| Prior to surgery or invasive procedure | 10 | 7.09 | |
| Others | 10 | 7.09 | |
| Blood component | Packed red blood cells | 129 | 90.21 |
| Frozen fresh plasma | 54 | 37.76 | |
| Platelets | 57 | 39.86 | |
| Number of transfusion acts | 1 | 111 | 78.72 |
| 2 | 16 | 11.35 | |
| 3 | 9 | 6.38 | |
| 4 | 2 | 1.42 | |
| 5 | 1 | 0.71 | |
| 6 | 1 | 0.71 | |
| 7 | 1 | 0.71 | |
| Transfusion laboratory test control | No | 21 | 14.89 |
| Yes, in 1 h | 12 | 8.51 | |
| Yes, in 2 h | 21 | 14.89 | |
| Yes, in 3 h | 21 | 14.89 | |
| Yes, in >3 h | 66 | 46.81 |
Of the total 1448 patients, 143 underwent transfusion (9.9%) – the transfusional threshold based on hemoglobin being the main reason for transfusion (36.2%). The most frequently used blood component was packed red blood cells, in 90.2% of the cases (129/143), followed by platelets in 39.9% (57/143) and plasma in 37.8% (54/143).
Among the transfused patients, only a small proportion received blood components. Specifically, 4.9% (7/143) received tranexamic acid, 4.2% (6/143) prothrombin complex concentrate, and 3.5% (5/143) fibrinogen concentrate. The percentage of patients subjected to more than three transfusion acts during ICU stay was minimal (<3%).
The mean Hb concentration prior to transfusion among the transfused patients was 7.8 g/dl (95%CI: 7.4–8.1). The mean INR (international normalized ratio) value was 1.2 (95%CI: 1.13–1.23), while the mean platelet count was 148,000 (95%CI: 137,6369–169,000).
An average of 2.7 packed red blood cell units were transfused (95%CI: 2.2–3.1); in the case of fresh plasma, an average of 3.8 units were administered (95%CI: 2.8–4.8); and in the case of platelet transfusion, an average of 3.6 pools were used (95%CI: 2.7–4.6). With regard to transfusion control, i.e., laboratory testing to detect possible transfusion reactions, it should be noted than in 14.89% of the transfusions no post-transfusion controls were made.
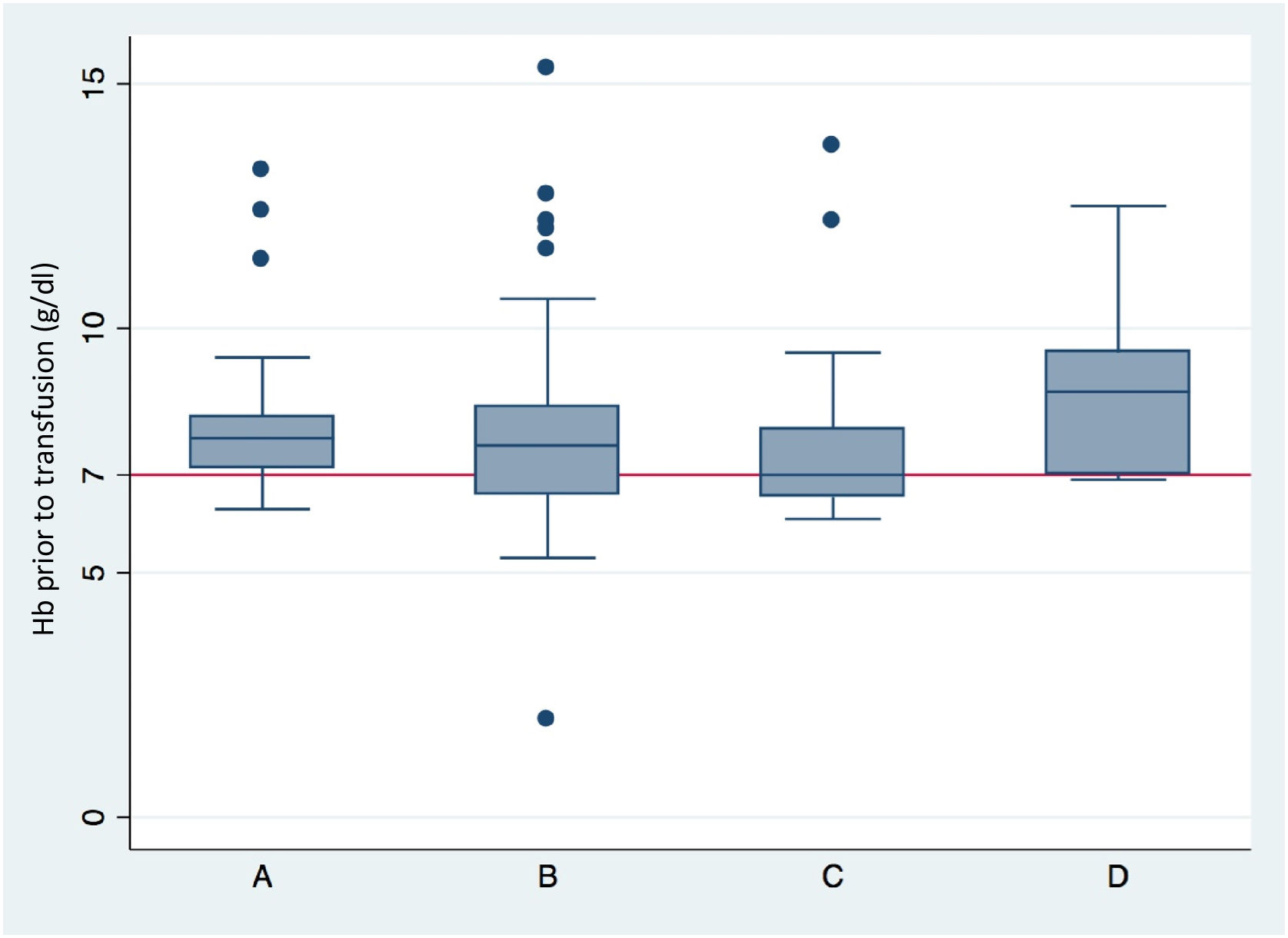
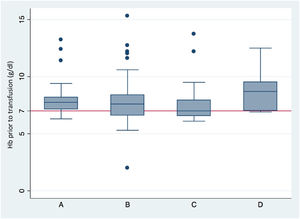
The mean post-transfusion Hb concentration was 9 g/dl (95%CI: 8.4–10), which proved significantly higher (p < 0.001) than the pre-transfusion Hb concentration, which was 7.8 g/dl (95%CI: 6.9–8.5). With regard to pre-transfusion Hb according to the reason for transfusion, we found that the Hb concentration in the patients subjected to transfusion due to laboratory test criteria was 7.8 g/dl (95%CI: 7.5–8.1), while in those transfused because of acute anemia with hemodynamic repercussions the concentration was 7.6 g/dl (95%CI: 7–8.1), versus 7 g/dl in those presenting acute anemia without hemodynamic repercussions (95%CI: 6.6–7.8). Lastly, in the patients transfused before surgery or some invasive procedure, the Hb concentration was 8.9 g/dl (6.9–11.7) (p = 0.005) (Fig. 2).
Hemoglobin concentration prior to transfusion, according to the indication of transfusion.
(A) Transfusion according to hemoglobin-based transfusion threshold. (B) Transfusion due to acute anemia with hemodynamic repercussions. (C) Transfusion due to acute anemia without hemodynamic repercussions. (D) Transfusion prior to surgery or invasive procedures (p = 0.005).
Those patients that met criteria of anemia [13% (188/1448)] were transfused more often than those who did not meet such criteria [2.6% (37/1448)] (p < 0.001). Likewise, surgical patients were more often transfused (14.4%) than those admitted due to medical conditions (8.9%) (p = 0.006).
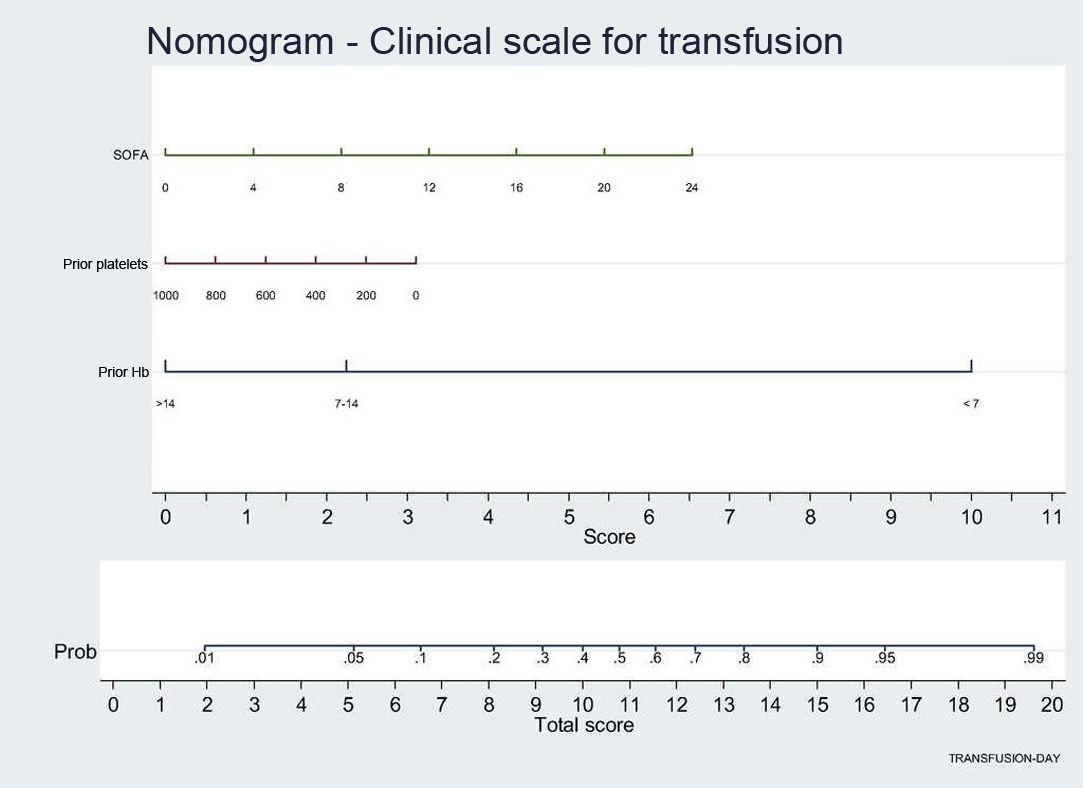
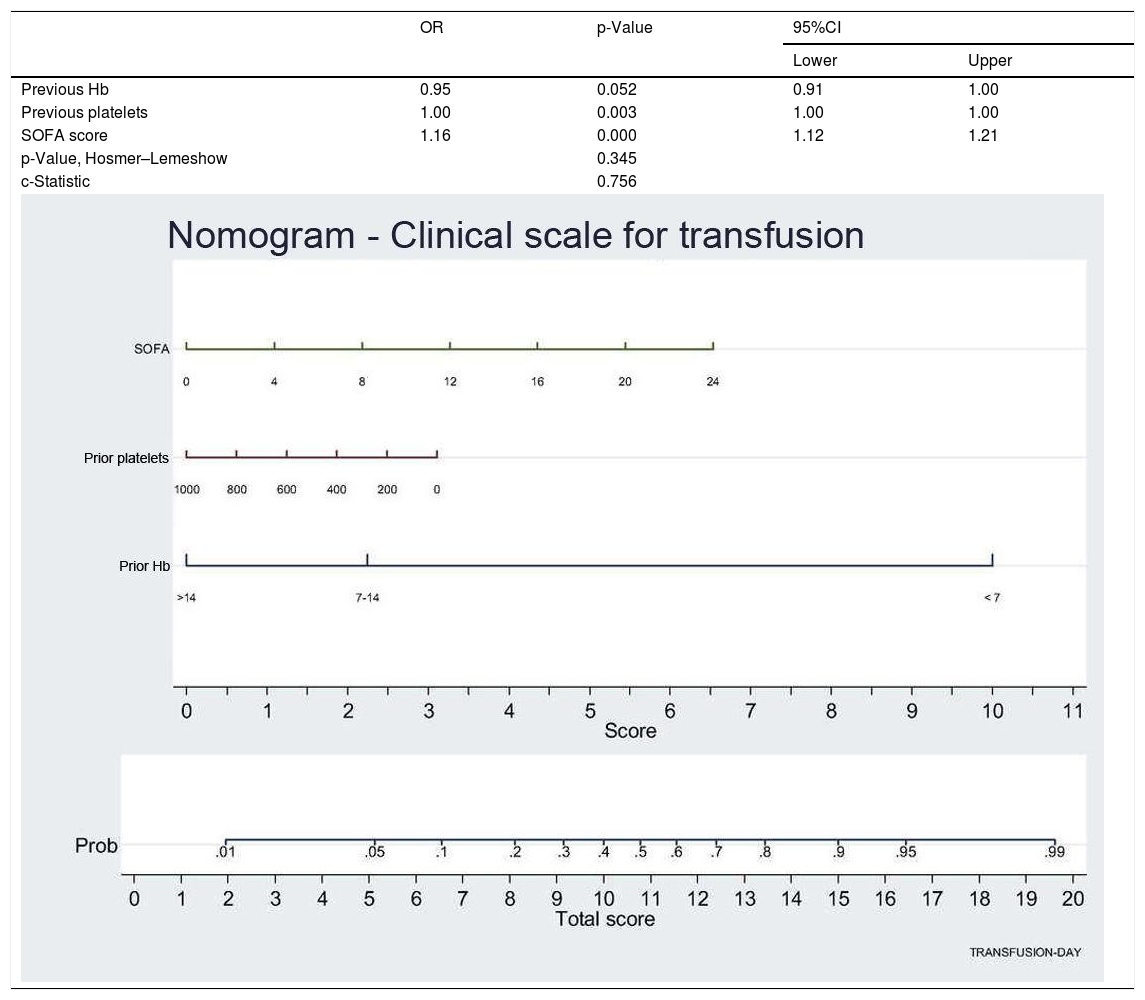
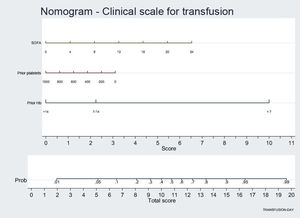
Lastly, in the logistic regression model, we found that the factors influencing the decision to provide transfusion were laboratory criteria referred to Hb and platelets, where the OR < 1 indicates that lower values imply a greater risk of transfusion (Table 4). However, severity as measured by the SOFA score is a factor associated to transfusion. Other parameters, such as ECMO, extrarenal replacement therapy or surgical disease were not found to be significant in the final model. The nomogram presents the results of the regression analysis as a clinical scale, with each factor being associated to a score within the scale.
Logistic regression analysis (with nomogram) to identify factors associated to blood component transfusion.
| OR | p-Value | 95%CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Previous Hb | 0.95 | 0.052 | 0.91 | 1.00 |
| Previous platelets | 1.00 | 0.003 | 1.00 | 1.00 |
| SOFA score | 1.16 | 0.000 | 1.12 | 1.21 |
| p-Value, Hosmer–Lemeshow | 0.345 | |||
| c-Statistic | 0.756 | |||
Nomogram: displays weight of each variable (SOFA score, previous Hb, previous platelets) in transfusion probability. This graphically shows the score a patient would have on this scale according to the values of each variable. The final score is calculated as the sum of the score obtained for each variable, and the corresponding transfusion probability is shown at the lower part of the nomogram.
Vincent et al.12 reported a 26% transfusion rate among patients over 16 years of age, and other earlier studies have documented higher rates, such as the European ABC study, where the estimated transfusion rate among adults was 37%.13–21 In the present study, the blood transfusion rate did not exceed 10%. This low percentage may be explained in part by the bias inherent to our cross-sectional study design, where there is no patient follow-up from ICU admission to discharge – though the large number of patients included from an extensive sample of Spanish ICUs tends to minimize this bias. Furthermore, the transfusional threshold was very close to 7 g/dl of Hb, which suggests that this low transfusion rate may also be a consequence of a better transfusion policy in our country, thanks to correct operation of the committees that regulate the transfusion act as a result of implementation of the Royal Decree of 2005.7 On the other hand, an interesting finding in our series is that the highest transfusion rate did not correspond to the surgical patients, despite the fact that the scientific evidence points to these patients as those requiring more transfusions. In any case, it is true that 79.28% of the ICUs in our study were polyvalent Units, and the structure corresponding to the type of ICU may be related to the low transfusion rate in cardiological patients – where specialized ICUs represented 9.01% – or surgical patients.
Anemia was the most frequent laboratory test finding in the ICUs. On the third day of admission, anemia was present in a full 95% of the critical patients, and this caused a considerable proportion of them (30–75%) to receive at least one allogenic blood transfusion during admission.13–16 In any case, in clinical practice, blood transfusion seeks to increase oxygen supply and therefore alleviate tissue hypoxia. However, the hypothetical benefit of blood transfusion has not been firmly demonstrated.17 Few studies have described an increase in oxygen consumption following transfusion, and most of the articles in this field only document an increase in oxygen transport,18 reflecting independence between consumption and transport before blood transfusion. However, in patients with a positive dobutamine test,19 lactic acidosis20and septic cases,21,22 where oxygen consumption – transport dependency may exist, transfusion does not increase tissue oxygen consumption. Therefore, if there is no clear evidence of the efficacy of allogenic blood transfusion in reducing tissue hypoxia, there is likewise no clear evidence that transfusion improves patient survival. In our study, an increase in Hb concentration was recorded after blood transfusion, and the patients with anemia had a higher transfusion rate. However, the 24-h mortality rate was similar in transfused patients and non-transfused individuals (4.90% versus 2.41%; p = 0.070) – these results being consistent with the data described above.
On the other hand, variability in the transfusion of blood components is a situation constantly reflected in the literature. This circumstance may be explained by the fact that the patients differ in terms of severity or comorbidities, thus generating differences in the transfusion criteria, which are based fundamentally upon laboratory test data (Hb concentration), but also on patient age, reason for admission, hospital stay or the severity of the disease condition leading to admission in the first place.12
According to the findings of a national survey on transfusion practices in the ICU,23 patients usually receive 2–4 packed red blood cell units. While these figures may be adequate in the context of acute bleeding, they are excessive in critical patients with non-hemorrhagic anemia, as is seen in our own figures, with an average of 3.6 units. We also observe that there is not much confidence in the use of transfusion alternatives or in assuming a clear relationship between transfusion and mortality. With regard to such inadequacy of red cell transfusion (indicator 87) and red cell over-transfusion (indicator 88), work has been done in the intensive care setting (book of indicators of the SEMICYUC, 2017),24 though with scant real evidencing of the theory.
In the present study, differences were observed in the transfusion rates among the 111 hospitals conforming the sample. In effect, some hospitals had no patients subjected to transfusion in the 24-h period of the study, while in contrast other hospitals reported transfusion among all the patients admitted to the ICU – though in most centers the transfusion rates ranged between 5–20%. In this regard, the SANGUIS (Safer And Good Use of blood In Surgery) project has audited the transfusion practices in 7195 patients in 43 hospitals in 10 European countries, evidencing extreme variability of transfusion practice – not only between hospitals but also between Departments and also between physicians from one same Department. This evidences the need to unify transfusion criteria in order to minimize needless patient exposure to allogenic blood transfusions.22 In this scenario, PBMs appear with the purpose not of improving transfusion practice but of improving the patient clinical outcomes – though the optimization of transfusion practice is one of the many tools available to reach this objective. In our sample, many hospitals still have not implemented a PBM program; specifically, of the 111 hospitals in the study, 64.86% did not contemplate programs of this kind. Furthermore, 38.74% do not even have a transfusion protocol. On the other hand, the group of ICUs that exceed a transfusion rate of 20% in our study (representing 16.21% of the global ICUs) largely correspond to specialized polytrauma patient Units. As a result, specialized Units of this kind are those that conform a special group with large transfusion requirements, representing approximately 81% of the blood units, compared with the rest of the ICUs.23
However, variability is observed not only between hospitals. In our study we also evidenced differences in transfusion rates within one same hospital, specifically according to whether the patient meets criteria of anemia or not, or whether the patient constitutes a medical or a surgical case. This may be due to a range of factors, such as the training of physicians in this field in the Department of Intensive Care Medicine, but not to the number of residents in training, since the staff physician – alone or with the resident – is the professional that usually decides transfusion based on personal experience and the consensus recommendations. Residents in training make fewer than 2% of all transfusion decisions in Spain.23
A limitation of this study is the design involved, which is adequate for describing transfusion practices in this country and the variability between hospitals, though none of the patients were followed-up on beyond the 24-h duration of the study. Accordingly, the number of patients that died in the ICU was very low, and no evaluation was made of the duration of ICU stay. Likewise, the rest of patients were not monitored in order to thoroughly assess mortality associated to the transfused individuals. In turn, the study design did not contemplate the evaluation of adverse effects or reactions to blood transfusion. In this respect, we are conducting a new data compilation process covering a longer period, in order to conduct a follow-up of the ICU stay of patients with and without blood transfusion, with the purpose of analyzing the safety of the transfusion act in the real-life clinical setting.
AuthorshipStudy conception and design: A. Robles and M. Quintana.
Data acquisition: M.A. Henríquez and García-Olmos.
Data analysis and interpretation: R. Juárez and S. Yus.
Drafting of the article: all the authors.
Final approval of the submitted manuscript version: all the authors.
Conflicts of interestThe present study has received no financial support, and the authors declare that they have no conflicts of interest.
Please cite this article as: Quintana-Diaz M, Nanwani-Nanwani K, Marcos-Neira P, Serrano-Lázaro A, Juarez-Vela R, Andrés-Esteban EM. Epidemiología de la transfusión sanguínea en los Servicios de Medicina Intensiva en España: «Transfusion Day». Med Intensiva. 2022;46:123–131.