Traumatic brain Injury (TBI) is a major public healthcare concern, affecting people of all ages. Despite advances in the diagnosis, monitoring and clinical management of TBI, many unresolved questions remain regarding its physiopathology. In an attempt to understand the pathological features of TBI and to evaluate single potential therapeutic strategies, various animal models have been developed to simulate the mechanisms of action and the clinical manifestations of TBI patients. In turn, each model represents a specific type of trauma and evaluates a specific physiopathological aspect of the cascade triggered as a result of TBI.
This review describes the main experimental models currently available referred to TBI and their possible application to the clinical setting.
El traumatismo craneoencefálico (TCE) es una de las patologías más importantes en la actualidad, ya que afecta a un alto porcentaje de individuos de todas las edades. A pesar de los avances en el campo del diagnóstico, la monitorización y el tratamiento del TCE, quedan importantes cuestiones sin resolver alrededor de la fisiopatología de este tipo de traumatismo. Con el fin de profundizar en dicho conocimiento y poder evaluar y aplicar un posible tratamiento que resulte eficaz para estos pacientes, se han desarrollado diferentes modelos experimentales que simulan los mecanismos de acción y el cuadro clínico del TCE. A su vez, cada modelo representa un determinado tipo de traumatismo y evalúa un aspecto concreto de la cascada fisiopatológica desencadenada tras el TCE.
El objetivo de este trabajo es detallar los principales modelos experimentales que abordan la lesión cerebral tras un TCE, así como su potencial traslación a la práctica clínica diaria.
Traumatic brain injury (TBI) is one of the leading causes of death and disability worldwide.1,2 In the European Union there are over one million hospitalizations per year due to TBI.3 According to information from the RETRAUCI (SEMICYUC Registry of Trauma in ICU), in Spain the annual number of hospitalized patients due to TBI exceeds the sum of patients diagnosed with multiple sclerosis, breast cancer, and traumatic spinal cord injury.4
TBI is a complex condition due to several factors. In the first place, the injury mechanisms are variable based on the type and intensity of energy interchange. Secondly, the brain injuries unleashed, both primary and secondary, are not homogeneous in every individual (they depend on age and comorbidities, among other factors). These factors lead to a great heterogeneity in the TBI-induced pathophysiological phenomena unleashed. Also, these factors may explain why the pharmacological clinical trials conducted so far have been unsuccessful. There is no doubt that for a therapy to become successful, we need full knowledge of the pathophysiological chain of events triggered and of the time these events occur. When it comes to brain damage, this knowledge is obstructed by the very nature of the disease. That is why designing homogeneous trauma experimental models at this level gives us the opportunity to decode the molecular and cellular alterations that follow energy interchanges over the brain.
In sum, the goal of this paper is to detail the main experimental models available today and deal with TBI-induced cerebral lesions to know not only their advantages and possible applications in this field of research but also how they can be implemented in the routine clinical practice.
Pathophysiology of traumatic brain injuriesThe direct mechanical lesion over the brain, known as primary lesion, damages the structure of the axonal cytoskeleton and alters the cellular membrane patency causing oxygen supply deficit, accumulation of pathological products, and an overall alteration of brain homeostasis.5,6 Afterwards, these events activate different biochemical signaling cascades that have deleterious effects for the patient's progression that we call secondary brain injury. From the therapeutical point of view, being able to control these signaling pathways has a direct benefit on the patient since we can only implement primary prevention strategies to avoid the occurrence of primary lesions (use of helmet, vehicle safety retention systems, among others).
In turn, cerebral lesions can be focal, consequence of an impact or due to inertia forces, diffuse or induced by a process of acceleration/deceleration. The latter, also known as diffuse axonal injuries (DAI) are characterized by being extensive and multifocal.7 We should mention here that DAIs are the most common pathological manifestations of all when talking about TBIs and their incidence rate is around 40–50% of all hospitalizations due to TBI.8 DAIs can associate cognitive impairment, motor and sensory alterations following damage to neuronal connectivity and functionality.5
Running parallel to parenchymal deformity and the inflammatory response triggered, we should mention here the blood-brain barrier (BBB) disruption that follows the energy interchange over the brain. This lack of BBB integrity due to the opening of tight junctions causes vasogenic brain edema induced by the outflow of water and plasmatic components toward the extracellular compartment.9–11 At a later stage, there is cellular (or cytotoxic) swelling following the accumulation of water in the intracellular space in compensation to the change of osmolarity due to the release of neurotoxic substances following the trauma.11,12
Animal models in the study of traumatic brain injuriesIn order to design correctly one experimental model capable of simulating TBIs the following criteria need to be met: (1) we need to be able to control the energy used; (2) the model should be easily reproducible; (3) the damage caused should be quantifiable and extrapolated to clinical cases in humans; (4) the damage caused should be the consequence of the energy interchange applied; (5) it should leave the possibility of predicting the severity of the induced brain damage open.13
In the medical literature there are different animal models that try to emulate TBIs. Each and every one of them has been designed to reproduce and simulate a certain type of traumatic brain injury. In this sense, the different models available today can be categorized into two types based on the type of energy interchange applied (indirect damage – acceleration/deceleration or direct damage – impact) and on the type of traumatic brain injury sustained (focal or diffuse lesion)14 (Table 1).
Classification of models of animal experimentation based on direct or indirect impact, type of lesion, and pathophysiology.
| Experimentation model | Type of damage | Type of lesion | Pathophysiology |
|---|---|---|---|
| Model of a weight falling over the skull | Direct | Focal | Swelling |
| Model of controlled cortical impact | Direct | Focal | Cerebral concussion, swelling |
| Model of balloon inflation | Direct | Focal | Swelling |
| Acceleration/deceleration model | Indirect | Diffuse | Diffuse axonal injury |
| Marmarou weight drop injury model | Indirect | Diffuse | Diffuse axonal injury |
| Fluid-percussion model | Direct | Focal and diffuse | Cerebral concussion, diffuse axonal injury |
| Blast models | Direct and indirect | Focal and diffuse | Cerebral concussion, diffuse axonal injury |
Lastly, in order to simplify this review, we will be focusing on models that use rodents as the animal experimentation models since stabling is easy and costs are low, all of which simplifies and makes using rodents a lot easier.
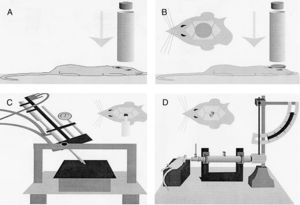
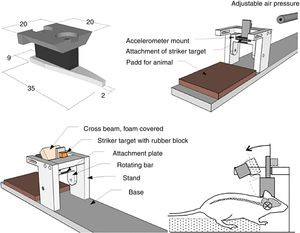
Models of focal lesionModel of a weight falling over the skullTraditionally, this model uses gravitational force and a weight of a known mass that falls over the skull of the experimentation animal (Fig. 1A). Thus, after sedating the rodent, it is placed and fixed on a horizontal surface to avoid any head movements after the weight has been dropped. The magnitude of the injury depends on the mass of the weight and the height it was dropped from.15
Models of cranioencephalic traumas in rodents. (A) Model of weight falling over the skull. (B) Impact-acceleration model due to free falling weight: Marmarou weight drop injury model. (C) Model of controlled cortical impact. (D) Fluid-percussion model.
Although this model is easy to use, inexpensive and fast, it is not widely used because it has a great variability since the skull breaks into several bone fragments of different sizes depending on the energy interchange used in the experiment. Another setback here is that there is possibility of weight rebound after impacting the animal's skull.
In order to optimize this model, several researchers have been developing a series of modifications in an attempt to increase reproducibility and achieve a specific brain damage maybe by modifying the severity of the TBI sustained.16
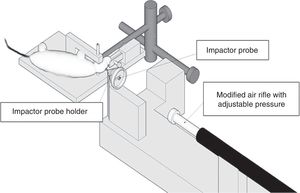
Model of controlled cortical impactThis model generates a focal-type experimental traumatic brain injury. It has been tested in rodents only. To develop it we need to conduct one craniotomy procedure in such a way that a piston pumps compressed air over the brain.13
This device consists of a pneumatic cylinder usually connected using a 4–5cm in length and 1cm in diameter threaded system to an impactor (Fig. 1C). The intensity of the traumatic brain damage sustained will depend mainly on the speed of this impactor and on the deformation depth generated. In most studies, the speed used is between 0.5m/s and 1.0m/s and the depth of cortical deformation is modulated using a bar that holds the impact system. The duration of the impact is usually adjusted between 25m/s and 250m/s.13
The histological injuries generated by this model after sustaining mechanical impact are focal, damage intracranial pressure, and compromise the cerebral perfusion pressure.17,18 In general, the brain damage achieved by this model is characterized by an area of focal necrosis in the cortex surrounded by an area of brain swelling similar to the bruises we can see in a patient with TBI.19 On the other hand, different studies have shown that, to a lesser extent and after the controlled cortical impact, it is also possible to cause, among others, DAIs at the subcortical, cerebellum, and midbrain levels.20,21
There are several advantages that this model has to offer. In the first place, it allows us to efficiently control the different mechanical variables that unfold here in such a way that we can make a more precise quantification of the damage sustained by the brain.
Secondly, this model allows us to evaluate the effects produced by the post-traumatic brain swelling and see what role the BBB actually plays here. Basically, using this model, Kiening et al. described the role that acuaporin-4 (AQP4) plays in the development of brain swelling.22 Thirdly, this model allows us to analyze the BBB integrity after sustaining the TBI and explore different pharmacological therapies that may run through the BBB. Thus, the activated signaling pathways after sustaining the TBI are guaranteed here as it happens with melatonin or with a mimetic peptide derived from the ApoE.23–25 Fourthly, the lower mortality rate of this model since it does not damage the brainstem allows us to know what mid-term cellular changes occur when sustaining TBIs at both molecular and genetic levels.26–28 The use of microarray techniques allows us to analyze alterations in a great number of genes after applying this controlled cortical impact model. Gene sequences associated with inflammatory processes (IL1α, IL1β and COX2, among others) have been described with functions in the cytoskeleton (synaptotagmin 4, MAP2, GFAP, nestin and vimentin, among others), signal transduction pathways (ERK and MAPK, among others), functions of cell-cycle regulation pathways (such as cyclin),26 and genes regulating calcium-signaling pathways (calcium/calmodulin dependent-protein kinase C beta and protein kinase 1β).28 This model has also been used to study the pathophysiological changes associated with the GABAA receptor in cultures of dented granulosa cells.27 Lastly, from the clinical point of view, the role played by repeated brain concussions usually associated with contact sports (boxing, rugby, etc.) has been studied too, which allows us to analyze, at an experimental level, anxiety and motor issues derived and identify corticosterone as a possible biomarker of these injuries.29
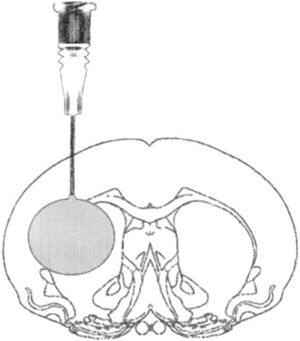
Model of focal lesion due to balloon inflationThis model generates transient intracranial hypertension by reproducing the compression effect generated by a space occupying lesion (SOL) in the brain and swelling after surgical assessment. In this model we perform a small hole through which an elastic probe is placed in the epidural space. Once inserted the probe is inflated like a balloon causing the effect of a SOL (Fig. 2).
Model of intracranial hypertension due to space occupying lesion in a rat after craniotomy procedure.
Traumatic SOLs are responsible for intracranial hypertension in between 30% and 50% of all TBIs.30 Such a sustained increase of intracranial pressure is recognized as one of the main mechanisms that casts a shadow on the prognosis of patients with TBIs and, same as it happens with the aforementioned controlled cortical impact model, there is a loss of BBB integrity that ends up leading to brain swelling.
This model is highly reproducible, inexpensive and its methodology is easy, since the variables of volume and filling/evacuation time can be easily controlled here. However, it is known that usually severe traumatic brain injuries are not a single entity given the presence of a high-volume SOL, which makes it hard to extrapolate these results to our routine clinical practice.30–33
Models of diffuse lesionAcceleration–deceleration modelOn many occasions, the lesions induced by a TBI are due to the fast acceleration of the cranioencephalic region without a direct impact from an object. The oscillation and rotation sustained by the brain inside the skull deforms the brain and leads to a DAI. The movement used here is rotational and at the beginning there is a prolonged acceleration followed by a sudden deceleration in a short period of time. Hence the expression “acceleration–deceleration” at brain level.
This type of indirect energy interchange has been recreated in different animal models of TBI such as pigs, sheep, and primates.7,34 Several studies with small pigs of the Hanford type have shown that the rotational acceleration of the head leads to DAIs with possible damage to the brainstem and even a state of coma following damage to the ascending reticular system.35,36
In general, these models have been able to reproduce effectively the pathophysiological mechanisms that occur in patients with DAI following TBIs. However, their main setback is their high cost since they use big animals, have many technical requirements and require state-of-the art facilities. Lastly, we should mention here that, due to the different brain mass of the different species available, extrapolating the results obtained in each study depends on the animal used. Also, it is difficult to translate these findings into our routine clinical practice.
Marmarou weight drop injury modelThe model developed by Marmarou et al. is one of the most widely used in rodents due to its low cost and high reproducibility.37 It is an impact-acceleration model of a free-falling weight over the skull. As Fig. 1B shows the animal is placed on a foam rubber surface with its skull exposed. Then, one metallic disk is fixed to the rodent in order to reduce the possibility of cranial fracture and appearance of focal injuries. The degree of brain injury sustained is closely related to the size of the weight used and the height the weight was dropped from.
One of the biggest advantages of this model is that it simulates traumatic DAIs and subsequent brain swelling.13,38 The process of swelling is associated with BBB tears that trigger neuroinflammatory responses inside the intracranial compartment.39 That is why this model is used to study the BBB integrity and functionality and to assess all possible molecules or therapies to improve functional results following a TBI.40 In sum, the Marmarou weight drop injury model appropriately simulates the TBIs sustained in a car crash and has proven useful for the study of repeated TBIs.38,41
The setbacks of this model have to do with how limited the researcher is when it comes to controlling the impact biomechanical forces at stake, which can lead to a weight rebound effect over the skull, thus generating uncontrolled injuries after the first energy interchange.
Models of mixed lesionFluid-percussion modelThis model was initially designed to mimic focal brain injuries. But even though the theoretical mechanics of this model is oriented toward the origin of focal damage, in some cases, the energy released in the impact can produce DAIs, which is why it is considered a mixed traumatic brain damage model.42–44
The use of this model requires a prior craniotomy in such a way that energy interchange occurs following the exertion of pressure through a fluid directly over the dura mater of the experimentation animal. In order to exert this pressure, a pendulum linked to a piston is used to move a certain mass of fluid originating the pressure pre-established in the experiment (Fig. 1D). This impact causes displacement and damage due to the deformation of neuronal tissue. The magnitude of the damage depends on the amount of fluid used.13,45
In the first models used, the impact occurred at brain midline level.46 Then, other working groups started to cause the injury in one of the two hemispheres of the brain, the so-called “fluid-induced lateral perfusion model”. It has been confirmed that the lateralization of damage allows us to study and compare the extent of the injury sustained in both hemispheres separately. Also, small variations in the position of craniotomy can cause significant differences in the severity of brain damage.47 This fluid-induced lateral perfusion model produces focal cortical concussions and diffuse subcortical damage. Using this mode, Hicks et al. showed the persistence of a series of degenerative cascades following a TBI in vulnerable areas of the brain such as the thalamus, the ipsilateral hippocampus, the septum pellucidum, the striated septum, and the amygdala.48
The histological manifestations obtained with the fluid-percussion model are multiple and it can reproduce the appearance of intracerebral hemorrhages, swelling, and gradual damage to gray matter, all common injuries of traumatic brain damage in humans.49 Also, in this model we have been able to see an increase of blood pressure and also an increase of intracranial pressure. Although, as we said before, this model tries to simulate focal lesions, it also causes mixed lesions since it triggers processes such as DAIs, neuroinflammation and swelling in the cerebral parenchyma.50,51 This model has been used in studies of memory, cognitive, learning, and behavioral impairment, at experimental stages.50–54
The fluid-percussion model has a series of advantages such as being inexpensive, being capable of recreating cerebral concussions similar to the ones sustained by humans and being highly reproducible. But even though the use of this model is relatively common, its main setback is how difficult it is to maintain an adequate control of calibration and the percussion system since air bubbles may originate inside the fluid. This can lead to a certain variability in the degree of lesion caused. On the other hand, this model does not exclude multiple damage and it can affect other areas such as the brainstem.45,46
Blast injury model (explosive model)This model simulates TBIs induced by the detonation of an explosive. The brain damage induced by this type of traumas is multiple and varied. Explosions can associate multiple inert elements that are launched or make a projectile-like impact in the skull. Also, brain damage can be caused by the shock waves that follow the detonation. That is why when it comes to designing blast-like experimental models, we need to make sure that they are reproducible and take all these aspects into consideration both in isolation and simultaneously. In these blast-like experimental models, TBIs are both focal and diffuse and damage is mixed.55
Within the different blast-like experimental models available today, one of the most widely used ones is ballistic penetration-induced brain damage. This model has been designed to simulate the damage caused by a projectile or any other type of object to the brain55–57 (Fig. 3). Also, there are other blast-like experimental models that specifically combine DAI damage and development of cerebral concussions55,57 (Fig. 4).
In another version of this blast model, damage is caused by the shock waves released following the detonation of an explosive. To this end, one metallic cylinder is used with one of its edges closed and in contact with the detonation. On the other side of the tube, the sedated rodent is introduced in a compartment to prevent any movement from happening after the detonation.58,59 Different types of histological phenomena have been recorded following this injury such as DAI, damage to white matter, neurodegeneration and glia activation.60
Blast-like experimental models are usually highly reproducible and they allow us to study multiple responses after sustaining the TBI such as secondary inflammatory responses, concussions or axonal injuries.55,57,58 Depending on the model used, the greatest setback here is that it may be very expensive and depending on the case we are dealing with, very complex due to the type of materials required to implement it.
Combined animal modelsUsually, in the pathology and development of TBI, other phenomena such as hypoxia, ischemia, hypotension, and hypovolemia are all present and contribute to developing secondary brain damage. The combo of one TBI plus any of these situations worsens the progression of the patient and increases his morbimortality rate.61 In order to be able to understand and assess the consequences in this context, these types of animal models have been developed in an attempt to evaluate more complex real situations so that we can test more effective therapies to improve the prognosis of patients.62
Some of the most widely used models are those that evaluate hypoxia and hypotension. When it comes to hypoxia, the animals sustain low oxygenation conditions (PO2 of 30–40mmHg). In TBI models under conditions of hypotension hemorrhages are induced or drugs are administered to bring blood pressure down to values between 30 and 50mmHg.62 Another methodology widely used here is to occlude the carotid artery, which leads to a cerebral ischemia that simulates severe blood hypotension. This type of interventions has been used in combination with TBI models of controlled cortical impact.63
Added to the aforementioned models, there are other mixed models associated with processes of hyperthermia, hyperglycemia, hyponatremia and even models that simulate polytraumas.62
In vitro models for the study of traumatic brain injuriesThese models are the tool used to understand the mechanisms involved in a TBI at tissue level once the injury has occurred. Where possible, the goal of in vitro clinical trials is to simulate the pathophysiology of TBIs through experiments aimed at controlling physical and environmental parameters so that we can study the response of a certain area of the brain to a single or combined damaged sustained. These models are based not only on the acute injuries sustained at a given time, but also on the study of how these injuries evolve from the beginning until a few days later in order to obtain more chrono-pathological information and know what the optimal time may be for the administration of this or that therapy. For this reason, several mechanisms are studied here such as apoptotic rates or cell signaling cascades, among others.64,65
Within in vitro models, there is the possibility of working with complete brain tissue or with dissociated cells. The advantages of using brain tissue are numerous since the anatomical structure made up by one heterogeneous cell population is preserved here. The results can be extrapolated to TBIs in humans in a more direct way. However, there are some limitations since it has no circulatory system and there is no inflammatory response with activation signals such as those mediated by the cytokines. Another technical limitation is that, when performing these experiments, we should always take into consideration the damage sustained by the tissue while it is being extracted and during manipulation. Another limitation is the short period of time to conduct these trials, being the viability of the tissue of just eight (8) hours.66 In in vitro clinical trials with dissociated cells, instead of using a sample from the brain of an animal, cells obtained through dissociation following mechanical and enzymatic processes in tissues extracted from the brain are used. This provides us with specific cell groups such as cortical neurons67 or astrocytes and neurons; this is how we can evaluate the individual response of every cell subtype.68
In vitro model of traumatic brain injury due to direct mechanical damageThis model studies the traumatic damage sustained by the cerebral parenchyma following the impact and penetration of an object. This model can be used directly over brain tissue or isolated cell cultures. Damage is generated using a needle, knife or any other sharp object which causes primary axotomy and cell loss induced by direct damage. Once the lesion has been caused in a given area, a series of metabolic pathways become activated, the most significant ones being the caspase-3 mediated apoptotic pathways and the epidermal growth factor-dependent proliferation pathways.64,65 This model has been tested with therapeutic goals in mind by testing different peptides with neuroprotective capabilities.69
One limitation of this model is that the mechanical parameters required to execute it have not been established properly. Therefore, the severity of this model is heterogeneous and quantified by the number of cells damaged only. Also, this type of damage is only clinically relevant in a small percentage of patients after the trauma.70
In vitro model of compression-induced traumatic brain injuryThis model shows the clinical manifestation of TBI induced in the model of animal experimentation of a weight being dropped over the skull of the animal.15 To this end, certain pressures are exerted on cell or tissue cultures that cause damage to the impacted area that eventually spreads to peripheral regions.71 The setback of this model is that it is really difficult to measure the degree of tissue deformation.
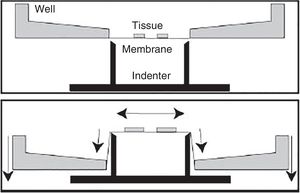
In vitro model of traumatic brain injury due to spasticityWithin the different in vitro models available today, this is the most widely used for the study of traumatic brain injuries. It was designed to reproduce the process of deformation of the cerebral parenchyma since this is one of the most common TBIs.
Ever since the decade of 1990, different models have been developed that use compressed air or even processes of aspiration.72,73 However, the setbacks here are the high variability and heterogeneity of the results obtained and the difficulty when trying to estimate the magnitude of deformation in the experiment. In time, other models of spasticity have been developed that are much more efficient and of great reproducibility.74 These models exert an opposing force from different angles (Fig. 5). In these cases, we have the possibility of controlling mechanical parameters using computer software and microscopy techniques, which results in greater control for the researcher of the different variables and also in more results being recorded.74–76
This model is highly reproducible, and it can be applied on very specific histological sections such as the hippocampus.76–78 On the other hand, it allows us to test different extracts or drugs.76–78
In vitro model of traumatic brain injury due to dynamic displacement or fluidThese in vitro models deform cell or tissue cultures by exerting pressure through a fluid. Thus, they can deform using one micropipette or by orienting the deformation in one direction through the dynamic displacement generated by hydrogels in cell cultures.79,80 With this model we can study cell deformation very thoroughly by using different microscopy techniques; among these, the scanning electron microscopy (SEM).79 One of the main advantages of this model is that it allows us to study cell response on real time.
ConclusionsAlthough they were developed in the decades of the 1970 and 1980, some of the models exposed here are still used today with some modifications that make them more complex and reliable. We should mention here that due to the increasing global phenomenon of terrorism and armed conflicts, today blast-type models are being developed and optimized by several working teams in an effort to provide relevant information and improve the management of patients who sustain this type of TBI.
In vitro models, however, are more useful for the study of TBIs at cell or tissue level since they allow us to go deeper from a molecular standpoint more easily than in vitro models do. Thanks to microscopy techniques, in vitro clinical trials also allow us to see, clearly and directly, the morphological changes following a TBI. Other more complex and real models are being developed today that, in some cases, require computers and software. In the coming future, the development of new systems of genetic editing will simplify obtaining transgenic animal models specifically designed for these studies.
In sum, animal models for TBI research are very useful because they let us to go deeper, at experimental level, in the knowledge of how these phenomena triggered after sustaining a TBI actually happen. Getting to know the pathophysiological cascades and the time frame of secondary brain injuries will also help identify new possible therapeutic targets that will improve the prognosis of TBI patients. We should emphasize here that during the early care of a TBI patient, it is very difficult to have first-hand real time information on these phenomena at the patient's bedside due to the nature and peculiarities of clinical manifestations associated to TBIs.
Conflicts of interestThe authors declared no conflicts of interest whatsoever.
Please cite this article as: Sempere L, Rodríguez-Rodríguez A, Boyero L, Egea-Guerrero JJ. Principales modelos experimentales de traumatismo craneoencefálico: de la preclínica a los modelos in vitro. Med Intensiva. 2019;43:362–372.