Mortality associated with mechanical ventilation in patients with ventilatory failure due to Covid 19 is around 50%–60%,1,2 often associated with many complications. With the use of HFNO and prone position, some patients can improve the hypoxemia and fatigue, avoiding in some cases invasive ventilation.3,4 Even though the guidelines for the treatment of respiratory failure secondary to covid-19 do not include the use of HFNO as first-line therapy, our institution used this as an initial strategy for these patients in the ICU.5,8 The primary goal of the intervention was to reduce the need for mechanical ventilation and its associated mortality.
This is a retrospective cohort of patients admitted to Hospital Manuel Uribe Angel (HMUA) in Envigado, Colombia. The study was approved by the hospital's ethics committee and informed consent was signed by each patient. Patients were admitted from May 1 to October 31, 2020. Eligible participants were 18 years or older, with COVID-19 infection confirmed by reverse transcriptase PCR admitted to the ICU, and treated with HFNO. Patients were placed in HFNO if they had ventilatory failure. Patients that had severely altered consciousness at admission, severe work of breathing (exhaustion), or shock were placed on invasive mechanical ventilation. The awake prone position was encouraged.
Support was given by a high-flow oxygen system (Fisher and Paykel RT-330). All patients treated with HFNO were admitted to the ICU, as they required a high standard of care and supervision due to the risk of treatment-refractory hypoxemia or severe respiratory failure requiring rescue invasive ventilation. The flow was initiated at 40L/min and titrated up by 5L increments depending on the patient's tolerance up to 60L/min with an initial FIO2 at 100% and titrated depending on the patient's clinical evaluation and ROX index.
The primary endpoint was success measured as weaning of HFNO without the need for intubation or mechanical ventilation. Failure was defined as the need for intubation, mechanical ventilation, or death. The secondary endpoint was finding the predictors associated with failure or success.
Categorical variables were compared using Chi-square tests or Fisher's exact tests. Continuous variables were expressed as medians with interquartile ranges. The Mann–Whitney U test was used for the mean difference. The crude cumulative proportion of HFNO success was calculated. Predictors of intubation were analyzed using a Cox proportional hazards model. The index date was the date of initiation of HFNO, with censoring occurring upon intubation, death, or the end of the study. Data were analyzed by the Statistical Package for Social Sciences software.
We analyzed 90 patients that were admitted to the ICU with ventilatory failure secondary to Covid 19 infection confirmed by imaging and RT-PCR. Of this group, 30 (33%) received invasive mechanical ventilation as first-line therapy, and 60 (67%) patients received HFNO as first-line therapy. In the HNFO group, the median age was 59 years, 80% male, mean BMI of 26.95. The most common comorbidities were hypertension 50% and diabetes 28%. The mean APACHE II scores for the success group was 7.11 and the failure group was 10.95. Full doses of anticoagulation were given to 28 patients (66%), 38% required vasopressor support with norepinephrine. Superinfection was a complication in 69% of the patients that failed HFNO. Every patient received steroids and thromboprophylaxis.
The survival of the group that initially received mechanical ventilation and did not receive at any point HFNO was 42.2%; the overall survival of the HNFO group was 76%. Of the 60 patients who received HFNO, 35 (58%) were successfully weaned off the device and eventually discharged from the hospital. Failure of the intervention was seen in 25 patients (42%). Of these patients, 24 required subsequent mechanical ventilation, and 13 died. The mortality of the group that received exclusive oxygenation via HFNO was 0%, for the entire HFNO group it was 23% and for the group that received mechanical ventilation it was 57.8%.
The mean PaO2/FIO2 at the admission of patients in the success group was 188 and the failure group was 160. The Curb 65 median for the success group was 1 and for the failure, group was 2. The median duration of high-flow nasal cannula was 6 days IQR (4–8) in the success group and 17 days IQR 9.25–24 in the failure group, p value of =0.000.
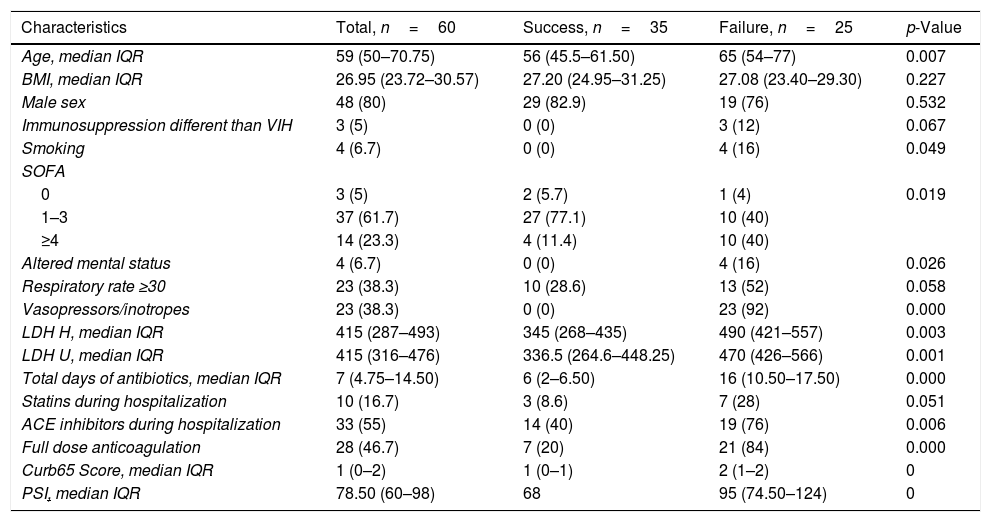
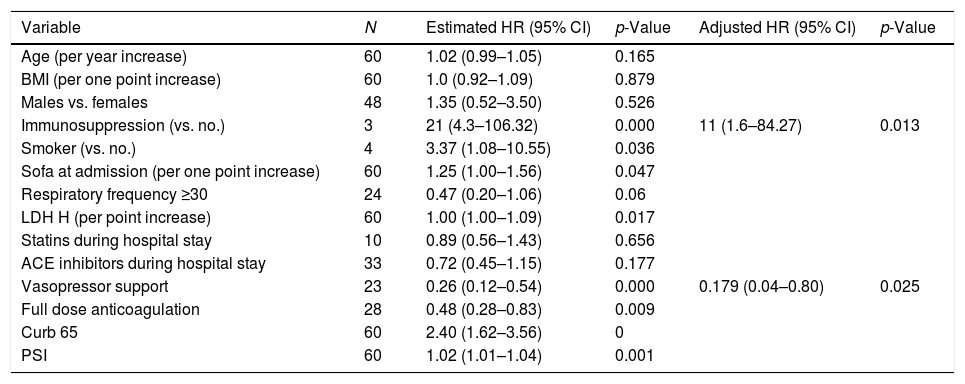
Table 1 summarizes the predictors of failure of HFNO. Higher PSI, CURB-65, SOFA scores, and LDH levels at ICU admission were significantly associated with failure. Vasopressor use and a full dose of anticoagulation were associated with worse overall outcomes. Table 2 summarizes the factors associated with failure over time.
Factors related to success vs failure of HFNO.
| Characteristics | Total, n=60 | Success, n=35 | Failure, n=25 | p-Value |
|---|---|---|---|---|
| Age, median IQR | 59 (50–70.75) | 56 (45.5–61.50) | 65 (54–77) | 0.007 |
| BMI, median IQR | 26.95 (23.72–30.57) | 27.20 (24.95–31.25) | 27.08 (23.40–29.30) | 0.227 |
| Male sex | 48 (80) | 29 (82.9) | 19 (76) | 0.532 |
| Immunosuppression different than VIH | 3 (5) | 0 (0) | 3 (12) | 0.067 |
| Smoking | 4 (6.7) | 0 (0) | 4 (16) | 0.049 |
| SOFA | ||||
| 0 | 3 (5) | 2 (5.7) | 1 (4) | 0.019 |
| 1–3 | 37 (61.7) | 27 (77.1) | 10 (40) | |
| ≥4 | 14 (23.3) | 4 (11.4) | 10 (40) | |
| Altered mental status | 4 (6.7) | 0 (0) | 4 (16) | 0.026 |
| Respiratory rate ≥30 | 23 (38.3) | 10 (28.6) | 13 (52) | 0.058 |
| Vasopressors/inotropes | 23 (38.3) | 0 (0) | 23 (92) | 0.000 |
| LDH H, median IQR | 415 (287–493) | 345 (268–435) | 490 (421–557) | 0.003 |
| LDH U, median IQR | 415 (316–476) | 336.5 (264.6–448.25) | 470 (426–566) | 0.001 |
| Total days of antibiotics, median IQR | 7 (4.75–14.50) | 6 (2–6.50) | 16 (10.50–17.50) | 0.000 |
| Statins during hospitalization | 10 (16.7) | 3 (8.6) | 7 (28) | 0.051 |
| ACE inhibitors during hospitalization | 33 (55) | 14 (40) | 19 (76) | 0.006 |
| Full dose anticoagulation | 28 (46.7) | 7 (20) | 21 (84) | 0.000 |
| Curb65 Score, median IQR | 1 (0–2) | 1 (0–1) | 2 (1–2) | 0 |
| PSI, median IQR | 78.50 (60–98) | 68 | 95 (74.50–124) | 0 |
Factors related to failure over time.
| Variable | N | Estimated HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age (per year increase) | 60 | 1.02 (0.99–1.05) | 0.165 | ||
| BMI (per one point increase) | 60 | 1.0 (0.92–1.09) | 0.879 | ||
| Males vs. females | 48 | 1.35 (0.52–3.50) | 0.526 | ||
| Immunosuppression (vs. no.) | 3 | 21 (4.3–106.32) | 0.000 | 11 (1.6–84.27) | 0.013 |
| Smoker (vs. no.) | 4 | 3.37 (1.08–10.55) | 0.036 | ||
| Sofa at admission (per one point increase) | 60 | 1.25 (1.00–1.56) | 0.047 | ||
| Respiratory frequency ≥30 | 24 | 0.47 (0.20–1.06) | 0.06 | ||
| LDH H (per point increase) | 60 | 1.00 (1.00–1.09) | 0.017 | ||
| Statins during hospital stay | 10 | 0.89 (0.56–1.43) | 0.656 | ||
| ACE inhibitors during hospital stay | 33 | 0.72 (0.45–1.15) | 0.177 | ||
| Vasopressor support | 23 | 0.26 (0.12–0.54) | 0.000 | 0.179 (0.04–0.80) | 0.025 |
| Full dose anticoagulation | 28 | 0.48 (0.28–0.83) | 0.009 | ||
| Curb 65 | 60 | 2.40 (1.62–3.56) | 0 | ||
| PSI | 60 | 1.02 (1.01–1.04) | 0.001 |
Of the 60 patients that required HFNO, 46 were eventually discharged. Mortality associated with covid 19 in the ICU of our institution was lower than other institutions in our geographical area (28% vs. 55%).
We show evidence of the utility of HFNO in severe covid 19 patients that required ICU admission for the treatment of severe respiratory failure. We achieved a high survival and posterior discharge rate. Several severe hypoxemic patients that were treated with HFNO avoided mechanical ventilation and were eventually discharged.
The use of HFNO has been evaluated in multiple studies, including promising results from a multicenter prospective study in South Africa that had a success rate of 47%, and overall survival of 52%, and a mortality of 48%.6 Rochwerg et al. evidenced a 15% relative risk reduction of the need for mechanical ventilation in HFNO compared to conventional oxygen therapy. This benefit is related to the alveolar recruitment and possibly the reduction of the airway and alveolar collapse, acting as a continuous positive airway pressure (CPAP).7
We believe that the use of HFNO as an initial strategy to treat respiratory failure in these patients has greatly impacted this result. Limitations in our study include the small number of patients participating. HFNO is a simple intervention and does not require extensive training for safe and appropriate use, but does require close patient monitoring.