This document is an update of the multidisciplinary document HEMOMAS, published in 2016 with the endorsement of the Spanish Scientific Societies of Anaesthesiology (SEDAR), Intensive Care (SEMICYUC) and Thrombosis and Haemostasis (SETH).
The aim of this document was to review and update existing recommendations on the management of massive haemorrhage. The methodology of the update was based on several elements of the ADAPTE method by searching and adapting guidelines published in the specific field of massive bleeding since 2014, plus a literature search performed in PubMed and EMBASE from January 2014 to June 2021.
Based on the review of 9 guidelines and 207 selected articles, the 47 recommendations in the original article were reviewed, maintaining, deleting, or modifying each of them and the accompanying grades of recommendation and evidence. Following a consensus process, the final wording of the article and the resulting 41 recommendations were approved by all authors.
El presente documento supone una puesta al día del documento multidisciplinar HEMOMAS, publicado en el año 2016 con el aval de las Sociedades de Anestesiología y Reanimación (SEDAR), Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC) y de Trombosis y Hemostasia (SETH).
El objetivo de este documento fue revisar y actualizar las recomendaciones existentes sobre el manejo de la hemorragia masiva. Se siguió una metodología basada en elementos del método ADAPTE (búsqueda y adaptación de guías publicadas en el ámbito específico de la hemorragia masiva desde 2014, más búsqueda bibliográfica en PubMed y EMBASE desde enero-2014 hasta junio-2021). Tras la revisión de 9 guías y 207 artículos seleccionados, se actualizaron las 47 recomendaciones existentes en el artículo original, manteniendo, suprimiendo o modificando cada una de ellas y sus grados de recomendación y evidencia. Consensuadamente, los autores aprobaron la redacción final del artículo y las 41 recomendaciones resultantes.
The management of massive hemorrhage (MH) includes a series of tools and measures for early control of bleeding and replacement of the lost blood components, minimizing associated coagulopathy that may prove life-threatening for the patient.1,2
In recent years, thanks to the introduction and diffusion of multidisciplinary protocols and the publication of excellent guides,3–5 it has become possible to improve the management of MH, particularly in the first hours of hemorrhagic shock. However, uncontrolled bleeding remains the leading cause of avoidable death following traumatism.6
A multidisciplinary group was established in 2015 with the aim of reviewing the literature and drafting a document to facilitate decision making for all those involved in the management of MH.7 A guide with 47 management recommendations or suggestions was developed, endorsed by the Spanish Society of Anesthesiology (Sociedad Española de Anestesiología y Reanimación [SEDAR]), the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]) and the Spanish Society of Thrombosis and Hemostasis (Sociedad Española de Trombosis y Hemostasia [SETH]).
In 2021, considering the time gone by and the appearance of new elements in many aspects of the management of MH, the decision was made to update the document. The same scientific societies commented above have endorsed the update, which has been carried out using specific methodology to allow review and possible modification of the recommendations contained in the original document.
In the same way as in the original version, and due to their particularities and specific treatment measures, obstetric hemorrhage, bleeding in pediatric patients, and gastrointestinal hemorrhage have been excluded from the present guide, which contemplates MH in polytraumatized patients in the global perioperative setting and in intensive care.
Materials and methodsIn order to update the guide, a group of 10 experts (the authors signing the present document) met in May 2021 to define the revision process. The decision was made to update the recommendations of the original consensus based on other existing clinical practice guides and on a review of the current literature. Updating was fundamented upon elements of the ADAPTE process, involving the search and adaptation of published articles and guides. The ADAPTE process has been described in detail elsewhere.8 In the present project it started with identification of the skills and resources needed to carry out the process, followed by the adaptation phase: search and selection of guides, compilation of the information contained in them in the form of a recommendations matrix and, where pertinent, adaptation of the collected recommendations. The criteria used for selecting the topics to be addressed by the guide were the same as those of the original document. The final phase of the ADAPTE process, in which the opinions of the decision makers affected by the updated guide are obtained, was not carried out.
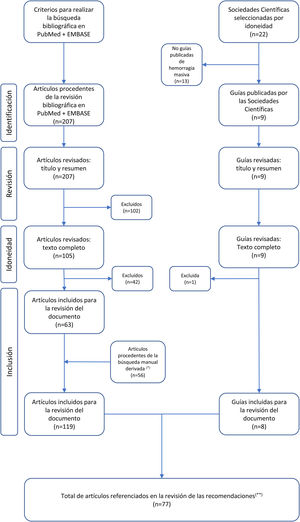
The literature review included the selection of clinical practice guides published in scientific journals, the recommendations of scientific societies related to the management of MH, and a search of the PubMed and EMBASE databases, covering the period January 2014–June 2021 (see supplementary material and Fig. 1).
Flowchart of the selection of articles included in the revision of the document.
(*) Derived manual search: search of articles included for revision of the document based on the literature citations in the selected articles and clinical guidelines. (**) Articles referenced in the justifications of the recommendations, without including the references of the introduction or the methodology.
After defining the strategy for review of the literature and the recommendations of the original guide (peer review), consensus was reached on the results, with agreement on the pertinent modifications of the recommendations based on the new available evidence (details of the revision protocol can be found in supplementary material).
ResultsOnly one of the 47 recommendations of the initial document was left unchanged. Modifications of the wording and/or grade of recommendation and/or level of evidence of the rest of the recommendations were considered necessary. Seven recommendations were eliminated; some were grouped; and others were developed upon, based on the available evidence. The updated guide finally contained 41 recommendations, distributed into 14 sections and involving 61 statements (38 recommendations - two with level of evidence A - and 23 suggestions). The supplementary material contains information on the differences between the documents (Tables 1SM and 2SM).
Recommendations and rationalesDefinitions and assessmentsRecommendation 1In evaluating the extent and/or severity of hemorrhage in the surgical patient, periodic visual inspection of the surgical field is recommended in search of excessive microvascular bleeding. In the trauma patient clinical examination is advised, analyzing the mechanism of trauma, the anatomical pattern and the initial response to resuscitation measures. (1C)
In patients at risk of MH, the monitoring of bleeding should be based on clinical examination and its quantification.9 In the trauma patient we also must analyze the mechanism of trauma, the anatomical pattern and the initial response to resuscitation. These measures may help to predict the need to activate massive transfusion protocols (MTPs).4,10
Recommendation 2For the early identification of trauma patients who may benefit from the activation of MTP, use is recommended of the Trauma Associated Severe Hemorrhage (TASH) score (cut-off score 15), preferably combined with the assessment of decreased blood clot firmness based on viscoelastic testing, if available. (2C)
Rapid identification of patients at risk of MH with the need for massive transfusion (MT) is crucial for the early activation of MTPs and improvement of the patient prognosis.11
In polytraumatized patients, use has been made of different scales such as the TASH score, which includes clinical parameters (blood pressure and heart rate, among others) and laboratory test data (hemoglobin, base excess), and has been shown to correctly classify 88.8% of the patients requiring MT.10
Viscoelastic tests (VETs) can also predict the need for MTP activation in trauma patients.12,13
Recommendation 3For the early identification of non-trauma patients who may benefit from the activation of MTP, the use of “resuscitation intensity” is suggested, defined as the transfusion of at least four packed red blood cell (PRC) units in one hour. (2C)
Use of the shock index (SI) is suggested in the pre-hospital setting, with a cut-off score of ≥1 for early MTP activation. (2C)
In non-trauma patients, “resuscitation intensity” has been proposed as an indicator of bleeding severity, defined as the administration of 3−4 PRC units per hour.4
The SI (heart rate divided by systolic blood pressure) is the only validated risk score to exclusively use clinical variables, and is considered to be very useful in the pre-hospital setting. Early mortality is predicted by SI ≥ 1.4,14
Recommendation 4Institutions are recommended to develop MTPs coordinated on a multidisciplinary basis with treatment algorithms supported by scientific evidence, and which include activation and deactivation criteria, and transfusion objectives, with the consideration of transition to resuscitation as soon as possible, guided by the laboratory or viscoelastic test findings. Likewise, informative and training campaigns are recommended for the teams involved, with periodic monitoring of their compliance and effectiveness, in the context of the institutional quality and safety programs. (1B)
The adoption of multidisciplinary MTPs has been shown to reduce the transfusion of blood components and mortality among trauma patients,15,16 and these protocols should be reviewed regularly within the quality and safety programs.4,17 Other MH scenarios in which coagulopathy is suspected and bleeding proves difficult to control could also benefit from these measures,18 allowing faster, coordinated and efficient MH management.5
In developing MTPs, it is advisable to transit from an algorithm based on fixed proportions of blood products to an algorithm guided by laboratory or VET data, thus making it possible to reduce the utilization of blood components, lessen patient morbidity, and improve coagulopathy.10
Recommendation 5In situations of MH, it is suggested to base initial assessment on the clinical history and anamnesis (if possible), and in assessing tissue perfusion and hypovolemic shock, sequential monitoring of blood pressure, heart rate and serum lactate or base deficit is recommended. (1C)
Dynamic monitoring of tissue perfusion includes blood pressure, heart rate, oxygen saturation and electrocardiography, in addition to clinical signs and symptoms.9 Serial serum lactate and base deficit measurements are also recommended.3,4 Although possibly not strictly correlated to the severity of injury, these measurements are effectively correlated to the degree of hypoperfusion and tissue hypoxia.19
Temperature controlRecommendation 6Systematic temperature monitoring is recommended in MH, with the rapid application of measures to avoid the loss of warmth and hypothermia, keeping the core temperature above 35 °C. (1B)
Among the measures to avoid hypothermia, mention should be made of the removal of wet clothing, covering of the patient, the elevation of room temperature, and the use of air blankets or warm water cushions. In addition, rapid infusion warming devices are advised for all fluids administered during MTP, and the use of extracorporeal warming systems should be considered in patients with severe hypothermia and a high risk of cardiac arrest. (2C)
The maintenance of normal body temperature reduces bleeding and the transfusion requirements,3 and should be a priority concern in patients with MH,4 because hypothermia (core temperature < 35 °C) is associated to acidosis, hypotension and coagulopathy. In this regard, the systematic monitoring of core temperature guides application of the most suitable measures for reaching normothermia.
The measures required to deal with hypothermia include fluid-warming units, air-warming systems or warm water cushions.4 In addition, it is important to ensure adequate control of the temperature of the PRCs and other blood products to be administered.
Fluid therapyRecommendation 7Early therapy with fluids is recommended in patients with MH and hypotension, preferably using isotonic crystalloids. (1A)
In patients with MH secondary to trauma, balanced crystalloid solutions instead of saline solutions are recommended. (1B)
The correction of hypovolemia through fluid therapy is the first measure to be applied in cases of MH,20 due to lesser patient tolerability of hypovolemia than anemia.
Crystalloids (Table 3SM, supplementary material) are the fluids of choice, due to their clinical efficacy, moderate adverse effects and low cost. There are not enough data to suggest that colloid fluids improve the prognosis of patients with hemorrhagic shock,19 and adverse effects have been described in the form of allergies (especially when using gelatins), coagulation disorders and renal failure.
In relation to the use of balanced solutions, improvements in acid-base status and lesser hyperchloremia at 24 h versus 0.9% saline solution have been reported in polytraumatized patients,21 with a superior cost-benefit ratio.22 Although more recent studies published after completion of the review to update the present guide suggest that both solutions could be adequate in critical patients,23,24 we favor the preferential use of balanced solutions versus saline solutions in the management of MH in polytraumatized patients. If saline solution is chosen, administration should be limited to a maximum of 1–1.5 liters.4,19
Recommendation 8The avoidance of hypotonic crystalloids such as Ringer lactate is recommended in patients with severe traumatic brain injury (TBI). (1B)
Hypotonic solutions have lesser expanding capacity than isotonic and hypertonic solutions, with an increase in free water volume, and thus should be avoided in patients with severe TBI, as mortality may be increased compared with 0.9% saline solution.3,4,25,26
Recommendation 9The avoidance of hypertonic fluids for resuscitation in patients with severe TBI is recommended. (2C)
Hypertonic saline is a first-line treatment for temporarily reducing elevated intracranial pressure, though it should not be used as main resuscitation fluid for hemorrhagic shock, since it is unable to improve survival or the cognitive outcome.4
Recommendation 10Restriction of synthetic colloids is recommended. (1C)
The administration of synthetic colloids can worsen coagulopathy due to their negative effect upon hemostasis.3,4
They have been used due to presumed greater efficacy in restoring intravascular volume, allowing a lesser infusion of crystalloids.27 As a result, synthetic colloids have been proposed when the combination of crystalloids and vasopressors is unable to maintain basic tissue perfusion, though affording no demonstrated benefit in terms of mortality,28 and with no established preferential indication of use.
At present, with the withdrawal of hydroxyethyl starches, the eventual use of synthetic colloids is limited to gelatins.
Recommendation 11It is advisable to avoid the systematic use of albumin for volume replacement in patients with MH. (1C)
Due to the lack of data on its efficacy with respect to other solutes, and considering its high cost and potential risks, albumin is not the fluid of choice in the resuscitation of patients with MH.19 Although its use has been suggested as a second line option fluid in septic patients after the initial administration of large amounts of crystalloids,29 this recommendation cannot be extended to MH.
A subgroups analysis of the SAFE trial30 suggests an increased mortality risk with the use of 4% albumin in patients with TBI, possibly due to the hyperosmolarity of this formulation.
It has recently been suggested that albumin could exert a protective effect upon the glycocalyx, with a certain benefit in terms of vascular permeability.31 Lastly, the use of albumin could be adequate for bleeding resuscitation in cirrhotic patients, during liver surgery32 or heart surgery.33
Recommendation 12A restrictive volume replacement strategy is recommended to reach the target blood pressure until bleeding is controlled. (1B)
Until bleeding can be controlled, it seems logical to tolerate a degree of arterial hypotension, considering the individual particularities of the patient (advanced age, history of arterial hypertension, and the tissue perfusion maintenance target). On the other hand, an excessive increase in blood pressure can contribute to further bleeding.19
In liver surgery, a decrease in bleeding and in the transfusion requirements has been demonstrated with the adoption of a restrictive volume replacement strategy.3,4,19
Hypotensive resuscitationRecommendation 13In trauma patients with bleeding, hypotension and no TBI, a systolic blood pressure target of 80−90 mmHg is recommended. This should be a temporary measure until the cause of bleeding has been controlled, particularly in elderly or hypertensive patients. (1C)
In trauma patients, the concept of “damage control resuscitation”, involving a restrictive volume replacement strategy and permissive hypotension, has been shown to offer benefit.4,19,34,35
However, the recommended mean blood pressure (50−60 mmHg) and systolic blood pressure (80−90 mmHg) values are arbitrary, and might not be safe for all trauma patients, particularly in individuals with blunt and penetrating injuries.36 The use of this approach should be considered with caution in elderly patients37 and chronic hypertensive individuals.38
Recommendation 14In trauma patients with bleeding, hypotension and TBI, a transient target systolic blood pressure of at least 110 mmHg is recommended until the cause of the bleeding has been controlled, particularly in concrete scenarios such as elderly patients, hypertensive individuals, etc. (1C)
Restrictive volume replacement strategies and permissive hypotension are contraindicated in patients with severe TBI and acute spinal cord injuries. In such situations, the maintenance of tissue perfusion pressure and autoregulation are crucial in order to guarantee oxygenation.4,38
Recommendation 15The early use of norepinephrine should be considered an option for maintaining blood pressure in the absence of an adequate response to fluid therapy. Ideally, a central venous access should be used, provided it does not delay the start of treatment. (1C)
Vasopressors temporarily may be needed to maintain tissue perfusion, and in this regard, norepinephrine is considered to be the drug of choice.
A comparison of volume replacement plus vasopressor drugs versus volume replacement alone showed the vasopressor group to receive less volume.39 Although early treatment with vasopressor drugs has been shown to offer benefit,40 observational studies have found greater mortality in trauma patients that receive such treatment. Therefore, the use of these drugs has been suggested in the context of volume replacement strategies in the presence of hypotension refractory to treatment.4,19
The administration of norepinephrine is typically performed via a central venous access, due to the risk of subcutaneous diffusion and necrosis, though peripheral venous administration is possible and effective.
Damage controlRecommendation 16Application of the concept of “damage control surgery” is recommended in trauma patients with anatomical lesions that are complex or difficult to access, with associated bleeding that proves very difficult to control, with long surgery times and/or accompanied by acidosis and/or hypothermia. (1B)
Damage control resuscitation is defined as a strategy for rapid bleeding control and intravascular volume replacement. It includes aspects such as the minimization of blood loss, hypotensive resuscitation, balanced hemostatic treatment with a high red cell: plasma: platelet proportion (1:1:1 in the original concept),41 the application of MTP, restricted crystalloid infusion, and the eventual use of hemostatic complements.4,42,43
One of the key elements in the resuscitation of specific patients is damage control surgery, which is based on rapid laparotomy to control the abdominal bleeding via a visceral “packing” approach to compress lacerations where hemostasis proves difficult, together with the restoration of local blood flow where needed, and the control of contamination from the abdominal organs.4 Second surgery is performed at least 48 h later to revert the visceral packing, and leaving definitive abdominal repair for third surgery, if necessary.
The concept of “damage control orthopedics” has been described in polytraumatized patients with severe bone fractures.44 This approach is likewise based on initial stabilization of the fractures, leaving definitive osteosynthesis for posterior second surgery.
MonitoringRecommendation 17The use of dynamic variables such as systemic volume variation (SVV) and pulse pressure variation (PPV) is recommended versus static variables such as central venous pressure (CVP) or pulmonary artery occlusion pressure (PAOP) to guide fluid administration in patients with MH subjected to controlled mechanical ventilation (MV) and with sinus rhythm that fail to respond to initial resuscitation therapy. (1B)
Dynamic indicators are to be preferred over static indicators because they allow the assessment of preload to guide the response to fluids in patients subjected to controlled mechanical ventilation and with sinus rhythm. The most widely used dynamic variables and with the greatest predictive value are SVV and PPV,3 though in addition to dynamic volume parameters (SVV and PPV), the use of CO2 gap and central venous oxygen saturation has also been proposed.3
Recommendation 18The early and serial determination of lactate and base deficit is recommended to estimate and monitor the severity of bleeding, the degree of hypoperfusion, tissue hypoxia and shock status. (1B)
The most useful laboratory test parameters for evaluating the evolution of patients with MH include the early and serial determination of lactate and base deficit. These parameters are altered in an early phase and appear to be good prognostic indicators in patients with MH, allowing us to estimate and monitor the degree of hypoperfusion and tissue hypoxia.3,4,19
Recommendation 19In patients with MH, early monitoring of hemostasis is recommended in order to optimize the administration of blood products and hemostatic drugs, with algorithms being used to guide individualized treatment preferentially based on the results of viscoelastic testing (1B) or alternatively using conventional coagulation tests. (1C)
In monitoring coagulation, the most widely used VETs are Rotem® (Instrumentation Laboratory, Bedford, MA, USA) and TEG® (Haemonetics Corporation, Boston, MA, USA). Conventional coagulation tests in turn should include prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma fibrinogen using the Clauss method, and platelet count. There is not enough evidence to confirm the superiority of one approach versus the rest,42,45 though VETs offer rapid results and additional information on hyperfibrinolysis, clot firmness and hypofibrinogenemia.4
In concrete settings such as cardiac surgery and liver transplantation, VETs have been shown to be useful in reducing bleeding and the transfusion requirements (1B in cardiac surgery).4,46 The International Society on Thrombosis and Hemostasis (ISTH) suggests their use in liver transplantation,47 and the British Society for Hematology proposes such tests in cardiac and liver surgery, traumatisms and postpartum hemorrhage as a guide for transfusion practice.13
Recommendation 20The serial monitoring of hemoglobin is recommended as a basis for indicating red cell transfusion, with due consideration of both clinical and laboratory test parameters. (1C)
In deciding transfusion, and in addition to clinical parameters such as blood pressure, heart rate or SI and laboratory test parameters such as plasma lactate or base deficit, due consideration is required of the influence exerted by the clinical condition of the patient. In this regard, we must take into account whether the patient presents multiple trauma (fractures or injuries), constitutes a surgical case, or involves any other scenario.48,49
Packed red blood cell transfusionRecommendation 21In the context of MH, it is advisable to consider the early transfusion of packed red blood cells (PRC) within a global restrictive strategy. (1B)
The use of leukocyte-depleted blood components is suggested. (2B)
The transfusion of PRC is generally needed when the blood volume losses are between 30%–40%. In addition to considering clinical and laboratory test parameters, with individualization of the patient situation, the transfusion of PRC is to be avoided on the basis of isolated hemoglobin determinations. A restrictive strategy reduces the need for PRC transfusion, though it has no impact upon patient morbidity-mortality versus the adoption of a liberal strategy.50
The use of blood components subjected to a leukocyte depletion process seeks to reduce the immune complications associated to allogenic blood transfusion.
Recommendation 22The administration of erythrocytes to reach a hemoglobin target of 7−9 g/dl is recommended. (1B)
A transfusion target of 7−9 g/dl is suggested in patients at risk with associated conditions such as chronic cardiovascular disease, acute coronary syndrome, stroke, thrombocytopenia or cancer. (2C)
The hemoglobin concentration generally used to define the restrictive transfusion group is 7−8 g/dl; it therefore should be avoided in most patients with hemoglobin values above this range. In certain patient subgroups and clinical contexts, it may be advisable to secure a plasma hemoglobin concentration of over 9 g/dl (chronic cardiovascular disease, acute coronary syndrome, stroke, or low-weight patients and elderly individuals), though there are not enough data to establish recommendations regarding the optimum transfusion strategy in these subgroups.50,51
Recommendation 23It is advisable to manage MH during the initial empirical phase based on the hemostatic resuscitation concept with a high proportion (at least 1:2) of fresh plasma in relation to PRC in patients with MH secondary to severe trauma. No specific recommendations can be made in the case of non-trauma patients. (1C)
Benefits have been suggested with the use of transfusion strategies characterized by fixed and high plasma/PRC administration ratios, particularly in polytraumatized patients, though it has not been possible to demonstrate that the 1:1:1 proportion offers a better risk-benefit profile than the 1:1:2 proportion.2,52–54
Plasma transfusionRecommendation 24In the monitoring of MH it is advisable for the fresh plasma dose to be fundamented upon a “target-guided strategy” based on clinical (microvascular bleeding, bleeding control) and laboratory test parameters (evidence of prolonged clotting time [CT] or reaction rate [R] in VETs, or increased prothrombin time [PT] and/or activated partial thromboplastin time [aPTT] ratios in conventional tests). (1C)
Plasma transfusion remains the standard choice for preventing and treating coagulopathy in MH, despite its known inconveniences of use or the time required for it to become available.17
It has been reported that a prefixed blood components protocol, involving high doses, affords greater plasma and platelet volumes than a target-guided strategy.2
Platelet transfusionRecommendation 25The administration of platelet concentrates to keep the count above 50 × 109/l is recommended in all patients with active bleeding. (1C)
Platelet transfusion to keep the count above 100 × 109/l is advised in patients with MH and traumatic brain or eye injury, as well as in those requiring neurosurgery or surgery of the posterior pole of the eye, and in patients with active bleeding when hemorrhage does not cease with counts above 50 × 109/l. (2C)
The transfusion of platelet concentrates is suggested in order to reach a count of 50 × 109/l in patients requiring major invasive procedures or in polytraumatized individuals even in the absence of hemorrhage. (2C)
Despite the lack of solid scientific evidence, there appears to be consensus in establishing a minimum platelet count of 50 × 109/l in patients with acute hemorrhage.4,7,19,55–57
In patients with TBI or ocular hemorrhage, it is advisable to maintain the platelet count above 100 × 109/l,4,19,56 with higher values in cases characterized by persistent bleeding.4,19,56,57 There are no solid recommendations regarding platelet transfusion for bleeding control in patients with platelet dysfunction.57
As a prophylactic measure, it has been suggested that platelet transfusion may be indicated when major surgery is needed, or in polytraumatized patients, if the count is below 50 × 109/l.4,19,58,59
Recommendation 26The avoidance of platelet transfusion is recommended in intracranial hemorrhage (spontaneous or secondary to trauma) in patients under antiplatelet medication, except when neurosurgery is needed. (2C)
This recommendation is endorsed by the PATCH trial,60 where the clinical outcomes and three-month mortality rate were seen to be poorer in the transfused patients (see recommendation 41).
Recommendation 27An initial dose of 4–8 platelet concentrates (or their equivalent: 1–2 pools), or one apheresis unit, is suggested, with adjustment of the administration rate according to persistence of the bleeding, the levels reached with the initial dose, the response to other measures for the control of bleeding, and the results of the VETs once they become available. (2C)
The platelet doses to be transfused were already suggested in the previous version of this guide. If donors are available, one apheresis unit (equivalent to 4−6 platelet concentrates) raises the platelet count by 30−50 × 109/l, reducing the exposure to multiple donors.4,58,59
Prothrombin complex concentrateRecommendation 28In the context of MH, the use of prothrombin complex concentrate (PCC) and vitamin K is recommended in patients receiving antivitamin K-type oral anticoagulants for rapid reversal of their effect. (1B)
In this context, the preferential administration of PCC is recommended for urgent reversal of the anticoagulant effect of antivitamin K drugs.3–5,9,17
In relation to the administration of PCC, it is advisable to use four-factor PCC (Tables 4SM and 5SM, supplementary material).5,19,61–64 Although no optimum dosing strategy has been established, it is advisable to base the dose on patient weight and the international normalized ratio (INR).61
Recommendation 29In the context of MH, in patients not receiving antivitamin K-type oral anticoagulants, it is not advisable to use PCC as first option for correcting coagulopathy. Such treatment may be used in selected patients conditioned to the urgency of therapy and the availability of fresh plasma. (1C)
Not enough evidence has been found to recommend PCC as the hemostatic option of first choice in MH.
However, in specific scenarios such as refractory coagulopathy, cardiac surgery,63,64 liver surgery and polytraumatized patients,65 the off-label use of PCC has been suggested, in accordance with the established legal references,3–5,9,17 in the context of the multimodal management of MH.
Recommendation 30The use of activated PCC or rFVIIa has been suggested only for hemophiliac patients with inhibitors in the context of MH. (2C)
Activated PCC (FEIBA®) and rFVIIa have specific indications in congenital hemophilia with inhibitor and in acquired hemophilia.66,67
Recommendation 31Cautious use of PCC is suggested in patients with life-threatening MH or requiring urgent surgery that cannot be delayed, and with a high thrombotic risk. (2C)
Although PCC is considered to be safe, thrombotic complications have been reported in 2%–4% of the treated MH patients. The use of such products therefore must take the risk-benefit ratio into account in each individual case in hemorrhagic patients presenting a high risk of thrombosis.
FibrinogenRecommendation 32It is advisable to determine fibrinogen using the Clauss method or FIBTEM® in ROTEM® or Functional Fibrinogen in TEG® for diagnostic purposes or when clinical management decisions must be made in the context of MH. (1C)
This recommendation, contemplated in the previous version of the guide,7 remains fully valid.4,9,13,68,69 The Clauss method should be used for the quantification of fibrinogen.17
Recommendation 33In patients with active bleeding, the administration of fibrinogen concentrate is recommended if the plasma levels (Clauss functional assay) are below 1.5–2.0 g/l or A5 in FIBTEM is under 7−9 mm or - through equivalence - the maximum amplitude (MACFF) is under 10 mm. (1C)
In the initial phase of MH, particularly if due to severe trauma, the administration of fibrinogen concentrate is recommended along with PRC as an alternative to the hemostatic resuscitation strategy, even without the need for the VET or coagulation test results. (1C)
The fibrinogen administration threshold is controversial. The plasma level should not be under 1.5 g/l,3,4,13,19,45,68–70 corresponding to A5-FIBTEM < 7 mm, though a threshold of 8−9 mm has been proposed in some clinical scenarios.70 By equivalence, in TEG®, this would correspond to a maximum amplitude (MACFF) of 10 mm.
Recommendation 34An initial fibrinogen concentrate dose of 25−50 mg/kg is suggested when the recommended plasma threshold is not reached, and/or a second dose guided by VET can be repeated. (2C)
An initial minimum dose of 3−4 g is suggested in adult trauma patients with bleeding. (2C)
The use of plasma as preferential source of fibrinogen is not recommended, though this option can be used if fibrinogen concentrate or cryoprecipitate is not available. (1C)
Although an initial dose of 3−4 g is recommended in polytraumatized patients, followed by doses guided by the VET results,4,19 it seems best to calculate the dosage according to patient weight3,4,19,68 (Table 6SM).
Cryoprecipitate could be used as an alternative, at doses of 4−6 ml/kg (approximately 1 U/5−10 kg).3,4,9,71
Factor VIIaRecommendation 35It is advisable to avoid the use of FVIIa as routine first level choice in the treatment of MH. (1B)
It is suggested that FVIIa should only be considered in cases where the conventional hemostatic measures have been ineffective. (2B)
The treatment of MH using FVIIa is an off-label practice. The available studies are few, with contradictory results, and describe a greater incidence of thrombotic events (particularly arterial).3,4,9,17,19,72
We suggest the use of FVIIa only if all other options for controlling bleeding have proven to be ineffective, and once fibrinogen, platelet count, pH and temperature have been optimized4,68,72 (Table 7SM).
Antifibrinolytic agentsRecommendation 36The early administration (in the first 3 h) of tranexamic acid (TXA) is recommended in patients with MH secondary to trauma, and in patients with TBI. (1A)
The use of tranexamic acid in severe perioperative bleeding is suggested, preferably based on the VET results. (2B)
The recommendation to use TXA in polytraumatized patients is based on the CRASH-2 trial73 and on patients with TBI in CRASH-3. In any case, it must be noted that “non-early” administration affords no benefits and increases the risk of complications.74 The recommended dose is 1 g administered in 10 min, plus a continuous perfusion of 1 g over the next 8 h.
In the perioperative setting, the administration of TXA is suggested in active bleeding, though there are no randomized studies capable of offering sufficient evidence. Administration of the drug should be based on the documentation of hyperfibrinolysis. An exception is cardiac surgery, where its prophylactic use is recommended, with a high level of evidence (1A).33
Other measuresRecommendation 37Consideration of the use of desmopressin (0.3 μg/kg) is suggested in bleeding patients with Von Willebrand disease. (2C)
Consideration of the use of desmopressin (0.3 μg/kg) is suggested in bleeding uremic patients, individuals receiving aspirin or with critical bleeding, and patients with documented platelet dysfunction. (2C)
Desmopressin acetate (DDAVP) is effective in the treatment and prevention of mild to moderate bleeding in patients with congenital or acquired primary hemostasis defects.3,4,9
Recommendation 38Recommendation is made of the use of topical hemostatic agents combined with conventional measures associated to different surgical procedures in the context of MH. (1B)
Topical hemostatic agents are effective in cases where direct access to the bleeding point is possible.4,7,9
Recommendation 39The assessment of mechanical control measures such as vascular embolization is recommended. (1B)
When available, the use of endovascular procedures (resuscitative endovascular balloon occlusion of the aorta [REBOA] for example) is suggested in the treatment of MH in selected patients. (2C)
Vascular embolization is effective in selected patients (non-varicose upper gastrointestinal bleeding, lower gastrointestinal bleeding, or arterial bleeding complications of pancreatitis) as an alternative to open surgery on an early basis,3 or in pelvic fracture patients.4
The use of balloon occlusion of the aorta can be considered in cases of severe polytraumatism in extreme circumstances to gain time until adequate bleeding control is achieved,4 in patients with abdominal aortic aneurysm rupture and in severe gastrointestinal or peripartum bleeding.41
Recommendation 40Monitoring of plasma calcium levels in bleeding patients is recommended (1C), particularly if massive transfusion is required. (1B)
It is advisable to keep the plasma calcium levels within normal ranges, administering calcium in the event of hypocalcemia (ionic calcium < 3.6 mg/dl, or corrected serum calcium < 7.5 mg/dl). (1B)
Calcemia control is relevant particularly in situations involving the abundant administration of blood components.75–77
Recommendation 41In severe or massive hemorrhage, it is advisable to determine whether the patient is receiving antiplatelet drugs or anticoagulants, in order to take specific measures to revert such treatment - weighing the benefit of reversal against the risk of thrombosis in each individual case. (1B)
The use of PCC plus vitamin K is recommended in patients receiving anticoagulation with antivitamin K drugs. (1B)
Early platelet transfusion is suggested in patients with bleeding clearly related to antiplatelet medication, preferably after monitoring platelet function. (2C)
The administration of idarucizumab is suggested in patients treated with dabigatran, instead of using nonspecific hemostatic agents such as PCC. (2A)
The administration of andexanet alfa or PCC is suggested in patients receiving oral anti-FXa drugs (“xabans”) and with uncontrolled or potentially fatal hemorrhage. (2C)
The administration of platelets may be regarded as the best option in patients with bleeding clearly related to antiplatelet drug prescription.3,4 Efficacy depends on factors such as the drug involved, the time since the last dose, the etiology and anatomical location of hemorrhage, and the efficacy criterion used.78 Dosing is also subject to controversy, with the suggestion of 0.7 × 1011 platelets/10 kg (equivalent to one concentrate unit per 10 kg approximately, or two pools in a patient weighing 70−80 kg) in cases related to aspirin, up to double in the case of clopidogrel, and even higher in the case of prasugrel.3,78
In cerebral hemorrhage the results of a systematic review were inconclusive, due to the methodological limitations of the published studies.58,79,80
In patients receiving anticoagulants, the use of specific antidotes is recommended3,4 (Table 8SM). The use of idarucizumab for specific reversal of anticoagulation induced by dabigatran does not appear to be related to any clear decrease in patient mortality.81 The recommended dosage is 5 g in two intravenous doses of 2.5 g each, administered in 15 min. Andexanet alfa has been approved for the reversal of anticoagulation in patients treated with apixaban and rivaroxaban.
DiscussionThe management of MH is extremely complex and includes the use of multiple tools and strategies contemplated in clinical guides, consensus documents and massive transfusion protocols, with the fundamental aim of improving patient survival.
The present multidisciplinary document, endorsed by the SEDAR, SEMICYUC and SETH, offers an update on the recommendations of the original HEMOMAS document.7 As in the previous edition, this updated document does not contemplate specific scenarios such as obstetrics, pediatrics or gastrointestinal hemorrhage, which require the adoption of specific measures and would have fallen beyond the objective and scope of the present guide.
Drafting of the document was based on a review of the literature published since the last edition of the guide. This strategy allowed the previous recommendations to be updated without modifying the fundamental structure of the original document.
In relation to the previous guide of 2016, we have reduced the final number of recommendations to 41, comprising a total of 61 statements, of which 38 are recommendations and 23 are suggestions. The only two recommendations with maximum level of evidence are referred to the early use of isotonic crystalloids and tranexamic acid use in patients with MH secondary to trauma. Updating the document has implied changes in the grade of recommendation or level of evidence, and in the wording of a number of statements. Table 2SM describes all these modifications.
The document highlights the importance gained by damage control surgery, the transfusion of blood components based on appropriate PRC/plasma/platelet transfusion rates, prohemostatic therapy guided by VETs or conventional coagulation tests allowing improved characterization of coagulopathy and an individualized approach, and the specific management of patients with antiplatelet medication or with antiplatelet medication and bleeding.
ConclusionsMassive hemorrhage is associated with a high mortality rate. Periodic updating of the recommendations for the multidisciplinary management of MS is crucial in order to secure the early diagnosis of patients with MH and of those at a high risk of suffering MH, initiating the strategies allowing bleeding control, replacing the lost blood components, and avoiding associated coagulopathy.
Author contributionsJuan V. Llau: general coordination, review of the recommendations, writing of the manuscript, review and approval of the manuscript.
César Aldecoa, Emilia Guasch, Pascual Marco, Pilar Marcos-Neira, Pilar Paniagua, José A. Páramo, Manuel Quintana, Francisco Javier Rodríguez-Martorell y Ainhoa Serrano: review of the recommendations, writing of the manuscript, review and approval of the manuscript.
Financial supportThe present study has been funded by an unrestricted grant from CSL-Behring.
Conflict of interestNone.
The authors wish to thank Fernando Rico-Vilademoros for methodological support, Isabel San Andrés for the literature search, and Ampersand Consulting for logistics in preparing the manuscript.
In the years since publication of the first document, some of the original authors have retired from professional practice. Their generosity in giving way to other colleagues who have joined the panel of experts of the present document must be acknowledged. All are aware that this new version would not have been possible without the publication of the first HEMOMAS document.
This article is published simultaneously, within the framework of the corresponding publication and copyright agreement, in: Medicina Intensiva (https://doi.org/10.1016/j.medin.2023.03.007) and in the Spanish Journal of Anesthesiology and Resuscitation (https://doi.org/10.1016/j.redar.2023.05.001).