To determine the incidence, etiology, and risk factors of nosocomial urinary tract infections (nUTIs) in a second level Pediatric Intensive Care Unit (PICU).
Patients and methodsA prospective study of 104 patients admitted to the PICU with a length of stay of more than 48h was carried out over a 1 year period (January to December 2009) to study the incidence and risk factors of nUTI. Urine samples were collected and cultured in all patients admitted for more than 48h to our PICU. Those needing indwelling urinary catheters had urine samples collected upon admission and every 24h until catheter retrieval, while those who did not need catheters had samples collected upon admission and every 48h until discharge from the PICU.
ResultsSix patients (5.8% of those admitted) were diagnosed of nUTI, with an incidence density of 5/1000 patients/day and 12.2/1000 catheterization days. Four of these were caused by Escherichia coli (including a multiresistant strain), and two by Candida albicans. Patients suffering nUTIs had significantly more relevant medical antecedents and a longer period of admittance than patients without nUTI. A statistically nonsignificant tendency towards increased infection risk was also found in younger patients and in those who needed an indwelling catheter for longer periods.
ConclusionsWe found a higher incidence density of catheter associated nUTI than in other reports. This at least partially could be due to the characteristics of our patients, and to the exhaustive methodology used for detection.
Determinar la densidad de incidencia, etiología y factores de riesgo de la infección de orina nosocomial (ITUn) en una UCIP de segundo nivel.
DiseñoEstudio prospectivo descriptivo durante un periodo de 1 año que incluyó a 104 pacientes ingresados durante más de 48h en nuestra UCIP. Se recogieron urocultivos diarios a los pacientes con sonda vesical hasta su retirada y cada 48h a los no sondados hasta el alta.
ÁmbitoUnidad de cuidados intensivos pediátricos de segundo nivel.
PacientesSe incluyó a todos los pacientes que ingresaron por más de 48h en el año 2009. Se excluyó a los menores de 15 días y a los que presentaban una infección de orina o pielonefritis al ingreso o antes de las 48h tras su ingreso.
ResultadosSeis pacientes presentaron una ITUn (el 5,8% de los ingresos), con una densidad de incidencia de 5/1.000 pacientes/día y de 12,19/1.000 días de sonda. Se identificaron 4 casos por Escherichia coli (uno, multirresistente) y 2 por Candida albicans. Los niños con ITUn tuvieron significativamente más antecedentes personales y mayor estancia que los niños sin infección y, aunque sin significación estadística, menor edad y mayor número de días con sonda.
ConclusionesNuestra densidad de incidencia de infección de orina asociada a dispositivo es superior a la publicada; esto puede deberse, entre otras causas, a las características de los pacientes atendidos y al método exhaustivo empleado para su detección.
Within the range of different nosocomial infections, nosocomial urinary tract infections represent an important hospital problem, particularly in Pediatric Intensive Care Units (PICUs), causing increased morbidity and mortality, a prolongation of stay, and increased healthcare costs. The epidemiological monitorization of such infections is essential in order to improve our knowledge and these processes and to establish adequate prevention and control programs.
Urinary tract infection is the leading nosocomial infection among hospitalized adults and in critical care.1,2 However, in PICUs the epidemiology differs, and urinary infectious processes represent the second or third most common type of nosocomial infection.3–5
These infections account for 5–15% of all nosocomial infections.6 Their appearance is directly related to a series of risk factors, and the identification and evaluation of such factors can contribute to establish more effective control programs. Among all the different risk factors studied, bladder catheterization is the most important.1,2 It is estimated that 5–25% of all patients requiring a bladder catheter during hospital admission develop nosocomial urinary tract infection (nUTI).
Other risk factors in adults are the duration of catheterization, the female gender, inadequate care of the bladder catheter, or a lack of antibiotic coverage.2
Despite their importance, there are few studies of nUTIs in the PICU, and their epidemiology in this patient population has not been well established.2 The primary objective of this observational study is to determine the incidence density of nosocomial urinary infections in a second-level PICU. The secondary objectives are to define the etiology and risk factors.
Patients and methodsPopulationA prospective, descriptive observational study was carried out in a provincial reference PICU with seven beds. The Unit treats medical and surgical disorders, with the exception of cardiovascular surgery. We included all patients between 15 days and 14 years of age admitted for over 48h in the period between 1 January and 31 December 2009. The exclusion criteria were as follows: age under 15 days or over 14 years, the presence of urinary infection or pyelonephritis upon admission or in under 48h after admission, and patients remaining in the PICU for less than 48h.
The following data were collected from all subjects: age, gender, reason for admission, medical or surgical disease, severity in the first 24h (PRISM III and PIM II scores), and personal history of relevance (infant brain paralysis, premature delivery, spina bifida, heart disease, neuromuscular disease, liver or kidney disease, immunodeficiency or hematological disease). We documented the possible factors related to nUTI: bladder catheter, previous hospital stay, corticosteroid treatment, immunosuppressors, surgery in the 2 months prior to admission, neutropenia, and antibiotic treatment received. Clinical information indicative of UTI was collected on a daily basis in all cases. The patients with a bladder catheter were subjected to daily urine culture until discharge from the PICU or removal of the catheter. In patients without bladder catheterization, or after removal of the catheter, urine cultures were obtained upon admission and every 48h until discharge. In addition, a urine culture was obtained 48h after transfer to the hospital ward.
DefinitionNosocomial infections were defined as all infections not present at the time of admission but which appeared within 48h after admission to the PICU. The diagnosis was based on the criteria of the CDC.6,7
Contamination was defined by urine cultures with low counts (≤104cfu/ml) of microorganisms normally present in the skin or external or internal genitalia. Mixed growth cultures are usually indicative of fecal microbiota contamination, though clinical evaluation is required in such cases.
Colonization in turn was defined as the presence of microorganisms in urine culture, without clinical repercussions, and which disappear after removal of the bladder catheter.
The severity of the disease upon admission was assessed using the PRISM III and PIM II scales, both validated for pediatric patients.8
Sample collectionIn catheterized children urine cultures and systematic urine tests were made on a daily basis. In order to avoid false-positive readings due to colonization, in those children with positive urine culture findings we replaced the bladder catheter and then repeated urine culture for confirmation purposes. If bladder catheterization was discontinued during admission, sample collection was carried out as in the group described below.
In non-catheterized children with preserved sphincter control, we collected the middle portion of spontaneous micturition (preferably in the morning), after washing the genitals with water and soap, rinsing with sterile water and drying with sterile gauze. In no case were antiseptics capable of falsifying bacterial growth employed. In patients without sphincter control, the sample was collected with a perineal adhesive bag after cleaning of the zone, with replacement every 30min. In this latter case urine culture collection was systematically spaced to once every 48h in order to avoid irritation caused by the adhesive bag in the perineal region. If the urine sample proved doubtful or showed contamination, point catheterization was carried out.
The urine samples were collected under sterile conditions and transported to the laboratory in under 1h. A closed system with a 500-ml capacity was used to control hourly diuresis.
Statistical analysisNew variables were calculated for data analysis, based on differences in dates referred to patient age, days of hospital admission, and days of bladder catheterization.
The descriptive analysis was based on calculation of the mean, median and standard deviation (SD) and interval for quantitative variables, while qualitative variables were reported as frequencies and percentages.
For each categorical variable of interest we explored associations with urinary infection based on the comparison of proportions with the Mantel–Haenszel chi-squared test or the Fisher test in the case of variables including less than five individuals.
The Kolmogorov–Smirnov test was used to assess normal distribution of the study variables.
Comparisons between means of quantitative variables in the study of children with or without infection were based on the Student's t-test or the Mann–Whitney U-test in the case of variables with a non-normal distribution.
Nonparametric tests were used for the study of factors associated to bacterial or fungal nUTIs.
The level of significance was established as 95% (p<0.05). The SPSS version 15.0 statistical package was used throughout.
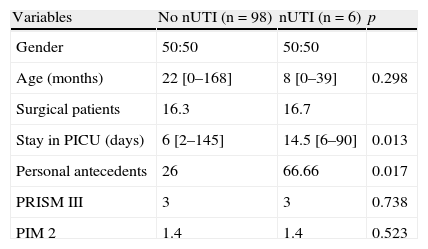
ResultsGeneral dataDuring the study period a total of 178 patients were admitted to the PICU, with a mean stay of over 48h. Seventy-four patients were excluded from the study due to some of the following reasons: age under 15 days (17 patients) or over 14 years (24 patients), presence of UTI upon admission (patients 22), or other reasons (patients 11). The final sample consisted of 104 patients. The general data are reported in Table 1. The children with nUTI were younger than the children without infection (15.8 vs 35.9 months), though the difference was not statistically significant. El group of children with nUTI showed a significantly higher percentage of personal antecedents of relevance than the group without nUTI. Upon admission, the children with and without nUTI showed similar median scores on the PRISM III and PIM II severity scales (3% and 1.4%, respectively), though the interval (range) was markedly greater in the children without infection. The children with nUTI presented significantly longer stays. The mortality rate among the children with and without nUTI was 0% and 8.7%, respectively.
General characteristics of the patients admitted for over 48h to the PICU. Comparison between the presence and absence of nosocomial urinary tract infection.
| Variables | No nUTI (n=98) | nUTI (n=6) | p |
| Gender | 50:50 | 50:50 | |
| Age (months) | 22 [0–168] | 8 [0–39] | 0.298 |
| Surgical patients | 16.3 | 16.7 | |
| Stay in PICU (days) | 6 [2–145] | 14.5 [6–90] | 0.013 |
| Personal antecedents | 26 | 66.66 | 0.017 |
| PRISM III | 3 | 3 | 0.738 |
| PIM 2 | 1.4 | 1.4 | 0.523 |
Data expressed as median [interval] or percentages.
A total of 68% of the patients carried a bladder catheter, with a mean of 7 days and a total duration of 492 days. The bladder catheter utilization rate was 0.41. The mean time from admission to the appearance of nUTI was 3.83 days. The urine cultures showed negative conversion after 7 days on average.
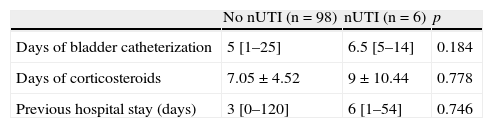
All the patients with nUTI had received a bladder catheter, and the catheterization period was longer than in the children without infection, though the difference was not statistically significant (6.5 vs 5 days). Likewise, the days of corticosteroid treatment and of previous hospital stay showed no significant differences. These data, compared with the patients without nUTI, are shown in Table 2. Three of the six cases of nUTI (50% vs 88.5% of the patients without nUTI) were receiving antibiotic treatment at the time of diagnosis, though only in the two documented cases of fungal infection did the treatment have to be changed. In turn, 4.9% of the patients without nUTI had undergone surgery in the previous 60 days, 1% presented neutropenia, and 1.9% suffered immune deficiency.
Risk factors of the patients admitted for over 48h to the PICU. Comparison between the presence and absence of nosocomial urinary tract infection.
| No nUTI (n=98) | nUTI (n=6) | p | |
| Days of bladder catheterization | 5 [1–25] | 6.5 [5–14] | 0.184 |
| Days of corticosteroids | 7.05±4.52 | 9±10.44 | 0.778 |
| Previous hospital stay (days) | 3 [0–120] | 6 [1–54] | 0.746 |
A total of 27 patients showed positive urine cultures (26%). Two of these cases were classified as colonization (1.9%), 19 as contamination (18.3%), and six as nosocomial urinary tract infection (5.8%).
The six cases of nUTI presented fever, with no identification of urinary symptoms (urgency, abdominal pain, pollakiuria, dysuria, or others). The colonized patients suffered no symptoms, while one of the 19 cases of contamination coincided with a fever process that did not meet the CDC criteria of nUTI.
Nosocomial urinary tract infectionWe documented six cases of nUTI, all in catheterized children—this representing 5.8% of all the admissions for over 48h, and 8.6% of all children with bladder catheters. A total of 1188 days of hospital stay were registered (5.05/1000 patients/day), together with 492 days of bladder catheterization (incidence density 12.19/1000 days of catheterization).
Two of the urinary infections were recorded in one same girl with spina bifida, admitted on two occasions for different reasons.
Regarding the etiology of nUTI, we identified four cases attributable to Escherichia coli (multiresistant (BLEE) in once case) and two to Candida albicans.
DiscussionIn contrast to the data published on adult patients,2,9,10 there are few literature references to nUTI in the critical pediatric population, and the existing information refers mainly to third-level PICUs or multicenter registries comprising numerous Units, without specifying their characteristics.3,4 The diseases seen in a second-level PICU are different, and it is therefore reasonable to assume that the incidence and epidemiology of the nosocomial infections are also different. We have found no specific publications on nUTIs in second-level PICUs. In addition, the few pediatric studies identified contain practically no prospective studies with a duration of over 6 months.
Our incidence density of device-related UTIs (12.19/1000 days of bladder catheter) is greater than in other series. The National Nosocomial Infections Surveillance (NNIS) System Report compiles information on 52 PICUs in the United States, and publishes an average of four nUTIs/1000 days of bladder catheter4—though no specification is provided of the characteristics of the participating Units (medical, surgical, medical–surgical, traumatologic, burn units, with cardiovascular surgery or with transplants). The International Nosocomial Infection Control Consortium (INICC) in turn compiles data on 22 PICUs in Latin America, Asia, Africa, and Europe, with the publication of an average of 4.4 infections/1000 days of bladder catheter—and likewise no specification is provided of the characteristics of the participating PICUs.3 In contrast, in adult ICUs, a division is made according to the characteristics of each Unit, with the recording of quite different incidence densities. In the Spanish national setting, we have a registry system (VINCIP-SECIP) with a rate of 8.2 infections/1000 days of bladder catheter,11 though here again the types of Units are not specified. Other authors such as Richards et al., analyzed the epidemiology in 61 PICUs in the United States, with an average of 5.9 episodes/1000 days of bladder catheterization.12 In turn, Urrea et al., in a study of a third-level PICU, reported a rate of 10.7 infections/1000 days of catheterization.13 Among the few studies specific of nUTIs, mention must be made of the work of Matlow et al.—a retrospective 10-year study in a third-level PICU. However, since these authors reflect the incidence per percentage of admissions (0.95/100 admissions), not per days of catheterization, comparisons are difficult to establish.14
We consider that the greater incidence density of nUTIs in our PICU may be due to a number of reasons: (a) Our bladder catheter utilization rate is greater than in other studies: 0.41 versus 0.3 in the NNIS, 0.17 in the INICC, and 0.32 in the article published by Richards et al.12 In this context, protocols designed to lessen catheter use and its duration are the strategies with the greatest impact in terms of reducing the incidence of infections; (b) In our PICU there is a greater percentage of children admitted with invasive devices and without antibiotic treatment than in other higher-level Units, and these patients are more susceptible to nosocomial infection. In fact, none of the patients with UTIs of bacterial origin received antibiotics, or alternatively such drugs had only been prescribed for under 24h; (c) The high volume of our collector systems makes it necessary to manipulate the latter for the measurement of hourly diuresis in small children—thereby increasing the risk of contamination; (d) nUTIs associated to bladder catheter are rarely symptomatic, especially in critical patients9,15; such infections therefore may be underdiagnosed. In contrast to other studies, we applied an exhaustive protocol of daily culture collection, independently of the patient clinical or laboratory test condition, and this may have led us to diagnose infections which otherwise would have gone undetected or been treated on an empirical basis. On the other hand, some studies have reported an increase in the rate of nUTIs (VINCIP-SECIP study in the year 2007: 5.5 infections/1000 days of catheterization to 8.2 infections/1000 days in 2009); (e) The risk implied by repeated manipulation of the system for obtaining urine cultures in catheterized patients cannot be discarded, despite the adoption of maximum aseptic measures.
Our recorded distribution of etiological agents differs with respect to the data found in the literature, though the limited sample size makes it necessary to view the data with caution. In 66.6% of the cases the isolated organisms were gramnegative bacilli (E. coli), though a high percentage of fungal agents was also recorded (44.4%), corresponding to C. albicans. In turn, 16% of the isolated germs were multiresistant (BLEE).
Among the risk factors studied in our series, and in addition to the presence of a bladder catheter, mention must be made of the association of nUTI to the duration of stay in the PICU (p=0.013) and the presence of patient antecedents of relevance (p=0.017). A younger patient age, the severity scores, and the duration of bladder catheterization were not significantly correlated to infection. In contrast to other authors, we found no relationship between the severity scores and the risk of nosocomial infection.16,17 This could be explained by a greater percentage of patients in less serious condition admitted without antibiotic treatment despite the need for external devices. This aspect has already been commented by Platt et al.18
In conclusion, our incidence density of device-related urinary infections is greater than in other studies published in the literature. Among the risk factors studied, and in addition to the need for a bladder catheter, mention must be made of the presence of patient antecedents of relevance and a longer duration of stay in the PICU. The days of bladder catheterization and a younger patient age failed to reach statistical significance.
It would be necessary to establish multicenter registry systems referred to nosocomial infections in critical pediatric patients, similar to those already found in adults,19 in order to compare PICUs with different characteristics and/or corresponding to different care levels.
The authors wish to thank the personnel of the Pediatric Intensive Care Unit of Puerta del Mar University Hospital (Cádiz, Spain), and especially the auxiliary nursing staff, for making this study possible.
Please cite this article as: Flores-González JC, et al. Infección nosocomial del tracto urinario en niños críticos. Med Intensiva. 2011;35:344–8.