Acute renal failure (ARF) is an independent risk factor associated with increased mortality during sepsis. Recent consensus definitions have allowed the standardization of research on the subject. The understanding of the physiopathology of ARF during sepsis is limited by the scarcity of histological studies and the inability to measure renal microcirculatory flows. Historically, ARF during sepsis has been considered to be a consequence of diminished renal blood flow (RBF). Indeed, in early stages of sepsis or in sepsis associated to cardiogenic shock, RBF may decrease. However, recent studies have shown that in resuscitated sepsis, in which cardiac output is characteristically normal or even elevated and there is systemic vasodilatation, RBF is normal or even increased, with no associated histological evidence of significant tubular necrosis. Thus, other factors may participate in the genesis of ARF in sepsis. These include apoptosis, glomerular and medullary microcirculatory disorders, cell changes in response to the pro-inflammatory cascade characteristic of sepsis, oxidative stress, mitochondrial dysfunction and damage induced by mechanical ventilation, among others. Sepsis-associated ARF treatment is supportive. In general, renal replacement therapies can be grouped as intermittent or continuous, and as those whose primary objective is the replacement of impaired renal function, versus those whose main objective is to secure hemodynamic stability through the clearing of pro-inflammatory mediators.
La insuficiencia renal aguda (IRA) es un factor de riesgo independiente asociado a mayor mortalidad durante la sepsis. Definiciones de consenso recientes han permitido estandarizar los trabajos de investigación en el tema. La comprensión de la fisiopatología de la IRA durante la sepsis está limitada por la escasez de estudios histológicos y por la imposibilidad de medir los flujos microcirculatorios renales. Históricamente se ha considerado a la IRA séptica como una patología dependiente de la caída del flujo sanguíneo renal (FSR). Efectivamente, en las etapas precoces de la sepsis o en la sepsis acompañada de shock cardiogénico existe compromiso del FSR; sin embargo, estudios recientes han demostrado que en la sepsis reanimada, aquella en que característicamente se observa un gasto cardiaco normal o alto y vasodilatación sistémica, el FSR es normal o incluso aumentado y no existe evidencia histológica significativa de necrosis tubular. Otros factores, distintos al puramente hemodinámico, participan en la génesis de la IRA en la sepsis. Entre éstos están la apoptosis celular, los trastornos microcirculatorios glomerulares y medulares, los cambios celulares en respuestas a la cascada proinflamatoria propia de la sepsis, el estrés oxidativo, la disfunción mitocondrial y el daño a distancia inducido por ventilación mecánica, entre otros. En la actualidad, el tratamiento de la IRA en la sepsis es de soporte. En general, las terapias de reemplazo renal pueden ser clasificadas como intermitentes o continuas, y en las que buscan primariamente el reemplazo de la función renal deteriorada, frente a aquellas cuyo objetivo principal es lograr la estabilidad hemodinámica de los pacientes mediante la remoción de mediadores proinflamatorios.
The incidence of acute renal failure (ARF) in critical patients is variable, depending on the definition used and the population studied, but ranges from 30 to 50%.1 Sepsis and its most severe presentation, septic shock, are the main causes of ARF in the Intensive Care Unit (ICU), accounting for up to 50% of all cases.2 Mortality due to sepsis remains high, particularly when associated to organ dysfunction such as ARF (with mortality rates of 20–35%) or in the presence of hemodynamic alterations (mean mortality 60%). The development of ARF during sepsis is an independent risk factor associated to increased patient mortality2; in this context, the FRAMI study, involving 43 Spanish ICUs, showed the appearance of ARF in critical patients to be independently associated to increased mortality, with an odds ratio (OR) of 2.51.3
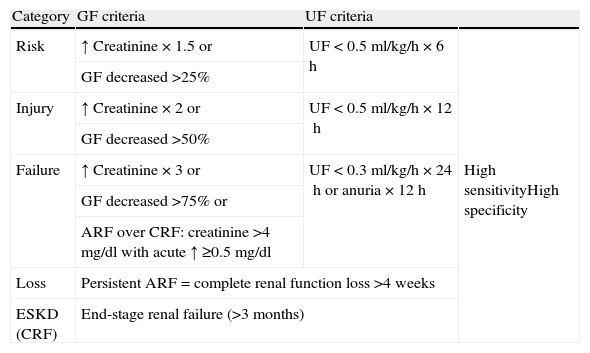
DefinitionUntil recently there was no clear consensus-based definition of ARF in sepsis. The ADQI (Acute Dialysis Quality Initiative) group has proposed a consensus-based diagnostic classification that has been favorably viewed by clinicians, and has made it possible to standardize research work in this field.4 The mentioned classification is known as the RIFLE (in reference to Risk, Injury, Failure, Loss, and End-stage renal failure) (Table 1). Patients are classified according to the loss of glomerular filtration (GF) (with respect to the baseline reference of each patient) and/or urinary flow (UF) into 5 categories (selecting the criterion yielding the poorest classification): risk (R), injury (I), failure (F), loss (L) or end-stage renal failure (E). ARF in sepsis is diagnosed in all patients meeting the criteria of sepsis,5 meeting some of the RIFLE criteria, and lacking other conditions or causes capable of accounting for ARF, such as the use of contrast media or nephrotoxic drugs.
RIFLE criteria for classifying acute renal dysfunction.
| Category | GF criteria | UF criteria | |
| Risk | ↑ Creatinine×1.5 or | UF<0.5ml/kg/h×6h | High sensitivityHigh specificity |
| GF decreased >25% | |||
| Injury | ↑ Creatinine×2 or | UF<0.5ml/kg/h×12h | |
| GF decreased >50% | |||
| Failure | ↑ Creatinine×3 or | UF<0.3ml/kg/h×24h or anuria×12h | |
| GF decreased >75% or | |||
| ARF over CRF: creatinine >4mg/dl with acute ↑ ≥0.5mg/dl | |||
| Loss | Persistent ARF=complete renal function loss >4 weeks | ||
| ESKD (CRF) | End-stage renal failure (>3 months) | ||
GF: glomerular filtrate; UF: urinary flow; ARF: acute renal failure; CRF: chronic renal failure; ESKD: end-stage kidney disease.
The RIFLE classification has been validated by a number of studies. In a study involving 20,126 patients admitted to a university hospital, 10%, 5% and 3.5% of the subjects reached the maximum R, I and F scores in the RIFLE classification, respectively. Mortality among the patients increased linearly with the severity of the RIFLE score, making it possible to independently predict mortality.6 Another study involving 41,972 patients admitted to the ICU reported an ARF incidence of 35.8%. The mortality in the group without ARF was 8.4%, versus 20.9%, 45.6% and 56.8% in those with class R, I and F acute renal failure, respectively. The presence of ARF of any category was found to be an independent mortality risk factor.
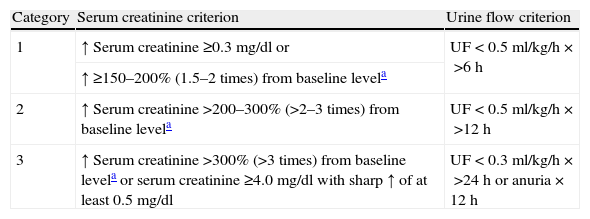
With the purpose of improving sensitivity, the RIFLE criteria were modified by the Acute Kidney Injury Network (AKIN) group, which defined ARF as an increase in serum creatinine of ≥0.3mg/dl or a percentage increase of ≥1.5 times from baseline as recorded in the previous 48h (Table 2).7 Urine output as a criterion of ARF was maintained, though the glomerular filtration rate and RIFLE L and E scores were excluded. AKIN, in contrast to RIFLE, requires two creatinine measurements spaced 48h apart in order to establish a diagnosis of ARF.
AKIN criteria for classifying acute renal dysfunction.
| Category | Serum creatinine criterion | Urine flow criterion |
| 1 | ↑ Serum creatinine ≥0.3mg/dl or | UF<0.5ml/kg/h×>6h |
| ↑ ≥150–200% (1.5–2 times) from baseline levela | ||
| 2 | ↑ Serum creatinine >200–300% (>2–3 times) from baseline levela | UF<0.5ml/kg/h×>12h |
| 3 | ↑ Serum creatinine >300% (>3 times) from baseline levela or serum creatinine ≥4.0mg/dl with sharp ↑ of at least 0.5mg/dl | UF<0.3ml/kg/h×>24h or anuria×12h |
Only one criterion (creatinine or UF) needs to be met to classify a patient. Those receiving renal replacement therapy (RRT) are considered in category 3, independently of the stage in which they are at the time of starting RRT. Categories 1, 2 and 3 correspond to R, I and F of the RIFLE classification, respectively.
AKIN requires two creatinine measurements spaced 48h apart–the first being the baseline value.
Some authors have compared RIFLE versus AKIN in patients subjected to heart surgery8 or admitted to the ICU.9 In general, mortality is comparable with both methods and tends to increase with the severity of ARF–thus confirming that acute renal damage is correlated to patient mortality.
PathogenesisThe study of the mechanisms involved in the development of ARF in sepsis is limited by the few histological studies in humans, due to the risk involved in the process and its frequently irreversible nature, and by the impossibility of measuring renal microcirculatory flow values.
Renal blood flow in sepsisThe classical position in septic patients is that the principal mechanism underlying ARF is ischemia or hypoperfusion–suggesting that the decrease in renal blood flow (RBF) and renal vasoconstriction are the characteristic events of sepsis. Furthermore, the main interventions for the management of ARF in sepsis have been volume replacement in already resuscitated patients,10 and the use of renal vasodilators such as dopamine and fenoldapam–though there is little evidence of their usefulness.11
In effect, the physiopathological processes inherent to sepsis, such as absolute and relative hypovolemia due to vasoplegia (pathological vasodilatation) and capillary leakage, myocardial dysfunction and impaired oxygenation, among other aspects, suggest that decreased oxygen transportation may be a relevant mechanism in ARF–mainly in the early stages or in sepsis accompanied by cardiogenic shock. However, most studies suggesting an ischemic etiology for ARF in sepsis are derived from animal models of ischemia and reperfusion.12,13 These models are not consistent with the classical physiopathology of resuscitated sepsis, characterized by high cardiac output (CO) and low peripheral resistance.
The study of RBF in human sepsis is complex, due to the difficulty of measuring it on a continuous basis. Studies in septic animals have yielded contradictory results in relation to RBF. Some studies indicate that during the early phases of sepsis, or after a bolus dose of endotoxin, RBF decreases.14,15 These models of endotoxemia induce an initial proinflammatory state that is not found in true sepsis, where the increase in inflammatory mediators is gradual rather than explosive as in the mentioned models.16 Other more recent studies underscore the fact that under normal conditions, RBF is several times greater than that required by the actual renal metabolic needs – since RBF is destined more to glomerular filtration than to renal oxygen transport. These studies show that in resuscitated sepsis, i.e., where a normal or high cardiac output and systemic vasodilatation are characteristically observed, RBF is normal or even increased.17,18 A study involving a porcine model of hyperdynamic sepsis found RBF to be generally increased, and particularly increased towards the renal medulla.19 Another study in 8 septic patients in which RBF was estimated invasively via thermodilution showed ARF to develop in the absence of alterations in RBF.20 A systematic review of 160 experimental studies of sepsis and ARF found the principal determinant factor of the normality of RBF in sepsis to be cardiac output (CO). A high or normal CO is associated to preserved RBF, while a low CO–i.e., non-resuscitated sepsis or sepsis associated to cardiogenic shock–is associated to low RBF.18
Thus, even though renal hypoperfusion may play a role in low-flow states such as non-resuscitated sepsis, recent studies show that once the hyperdynamic state characteristic of sepsis has been established, hypoperfusion or renal ischemia are not relevant mechanisms.17
Renal histology in sepsisThe renal histological changes observed in sepsis are few and nonspecific.15 A systematic review found only 22% of 184 patients to show evidence of acute tubular necrosis (ATN), and concluded that the existing experimental and human clinical evidence does not support the idea of ATN as the manifestation or mechanism characteristic of septic ARF.21 The histology of septic ARF is heterogeneous–relevant findings being leukocyte infiltration (predominantly mononuclear cells), some degree of tubular cell vacuolization, loss of the brush border, and apoptosis.22,23 Other described alterations are dysfunction of the intercellular tight junctions, favoring tubular fluid reflux through the epithelium,24 and dysfunction of the basal membrane–with the consequent detachment of cells into the tubular lumen. This in turn is associated to the appearance of tubular cells or cylinders in the urine sediment. These cellular cylinders produce micro-obstruction of tubular urinary flow (UF), with cessation of GF in the affected nephron unit. The absence of necrosis in 70% of the patients is compatible with the existing evidence that other mechanisms different from ischemia contribute to the development of ARF during sepsis.8,10
Apoptosis, or programmed cell death, which in contrast to necrosis does not induce local inflammation,25 has been described as one of the physiopathological phenomena present during ARF in sepsis.21,22,26 Apoptosis is observed in 2–3% of the tubular cells during sepsis, and is more frequent in the distal tubules.22 Tumor necrosis factor-alpha (TNF-α) plays an important role in the induction of renal tubular apoptosis; however, the relevance of apoptosis as a mechanism of ARF in vivo remains the subject of study.
Glomerular filtration in sepsisSince in most cases of sepsis cardiac output is either normal or elevated, RBF is seen to be normal. A recent study in septic sheep has shown that, in effect, RBF is elevated in association to hyperdynamic CO, though renal vascular resistance (RVR) is decreased, with a secondary reduction in glomerular filtration rate and an associated rise in plasma creatinine concentration.27 The drop in RVR may be explained by an increase in nitric oxide (NO) release. The proinflammatory cascade induces expression of inducible nitric oxide synthetase (iNOS) in the renal medulla,28 in the glomerular mesangial cells and in the endothelial cells of the renal blood vessels28 – resulting in intense and prolonged NO release. On the other hand, the acidosis inherent to septic shock, and the decrease in ATP levels in the vascular smooth muscle cells, favor cellular hyperpolarization as a result of potassium release from the cell through ATP-dependent membrane potassium channels–this in turn contributing to renal vasodilatation through resistance to catecholamines and angiotensin II. Likewise, the recovery of renal function was associated to a recovery of RVR associated to a decrease in RBF. This study suggests that the loss of GF pressure regulation participates as a mechanism of ARF in sepsis, even in the presence of increased RBF.
Glomerular filtration pressure depends on the diameter of the afferent and efferent arterioles. Constriction of the afferent arteriole and/or vasodilatation of the efferent arteriole can give rise to reductions in GF and in UF. Afferent vasodilatation participates as a mechanism of ARF in sepsis, though the efferent arteriole plays an even greater role (hyperemic ARF)–generating a drop in GF and in UF. However, the lack of direct measurements of RBF in human sepsis limits the drawing of conclusions.
Intrarenal hemodynamics during sepsisDespite preserved RBF in resuscitated sepsis, the intrarenal distribution of the blood flow may be altered, with a predominance of cortical flow over medullary blood flow–a situation known as “corticomedullary redistribution”, and which is responsible for medullary hypoxia.29 A recent study in animals established differentiated measurements of critical and medullary blood flow using intrarenal laser Doppler flowmetry during sepsis. Both flows remained stable, and the use of noradrenalin–an adrenergic vasoconstrictor–significantly increased the flow in both regions. This suggests that the compensation mechanisms are active during hyperdynamic sepsis.30 There probably are modifications in intrarenal blood flow during sepsis, but the evidence suggests that the compensatory mechanisms are active, and that such modifications do not represent a predominant mechanism.
Inflammation and oxidative stressOther mechanisms, apart from the hemodynamic mechanisms, also participate in the genesis of ARF in sepsis. The inflammatory response inherent to sepsis has been examined as a direct mechanism of ARF. Different mediators implicated in sepsis, together with the neuroendocrine response, participate in the pathogenesis of septic ARF.31,32 The kidneys are particularly sensitive to mediator-induced damage. Both the mesangial cells and the tubular cells are able to express proinflammatory cytokines such as interleukin (IL)-1, IL-6 and TNF-α.33 Both IL-1 and TNF-α have been found to act as inducers of ARF in sepsis.34 Mice with TNF-α receptor deficiency are resistant to the development of endotoxin-mediated ARF, and exhibit less tubular apoptosis and lesser mononuclear cell infiltration.35 However, the use of anti-TNF-α antibodies during sepsis has not been able to improve survival or prevent the development of ARF.36
The mechanisms proposed to explain how IL-1 and TNF-α produce ARF during sepsis include the induction of increased cytokine release, amplifying the inflammatory cascade; favoring of tissue factor expression, which promotes local thrombosis37; the induction of tubular cell apoptosis38; and principally the elevation of regional oxidative stress through an increased production of reactive oxygen species (ROS).
Oxidative stress in sepsis is related to an increase in the production of ROS, and to the concomitant reduction of antioxidant levels through either consumption or diminished intake.39–41 The proinflammatory cascade induces the expression of iNOS in the renal medulla,28 in the glomerular mesangial cells, and in the renal vascular endothelial cells28–with the consequent rise in NO levels during sepsis. NO has both beneficial and deleterious effects during sepsis. Baseline levels of NO are necessary to maintain RBF and intrarenal flow during sepsis, particularly at afferent arteriolar level,28 and to favor cellular mitochondrial biogenesis (re-synthesis).42,43 However, NO is also a free radical, and when produced in excess is able to inhibit the oxidative phosphorylation chain and reduce oxygen consumption.44 NO, moreover, can interact with other ROS to form more toxic reactive species such as peroxynitrite,45–47 which can cause damage to DNA, proteins and membranes–resulting in an increase in mitochondrial permeability.48,49 Increased mitochondrial permeability is associated to a decrease in electrochemical gradient and in ATP synthesis, as well as to the activation of apoptosis pathways.50 The intensity of oxidative damage is correlated to the intensity of mitochondrial damage and to survival.48,51 A number of studies, including one by our own group, have shown that there is not only an increase in ROS during sepsis but also a decrease in antioxidant levels, related to the intensity of the septic process.52–55
Coagulation and microcirculationSepsis is characterized by a prothrombotic and antifibrinolytic state,56 and the associated microcirculatory dysfunction has been described as a relevant mechanism in the development of multiorgan failure in sepsis, with an association to mortality.57 Endothelial dysfunction is induced by the inflammatory cascade, and is characterized by an increase in the expression of tissue factor–which in turn activates the coagulation cascade. At renal level, fibrin deposits have been described in the glomerular capillaries during sepsis, though a recent study has shown that renal arterial/arteriolar thrombosis is not frequent in sepsis, and is not associated to the presence of disseminated intravascular coagulation.22
Mitochondrial dysfunctionMitochondrial dysfunction is described as the incapacity of the cell to maintain its metabolic functions despite adequate oxygen transport, due to the impossibility of using the available oxygen for ATP synthesis.58 Briefly, mitochondria must couple the transport of energy-rich substrates to the generation of a transmembrane electrochemical gradient allowing the synthesis of ATP. In order for this process to be efficient, there must be adequate function of the oxidative phosphorylation complexes (complexes I–IV plus ATP synthase),59,60 structural integrity of the mitochondrial membrane (fundamentally the internal membrane),61,62 a sufficient substrate supply,63,64 and a sufficient number of mitochondria.65,66 Few studies have evaluated cell function in septic ARF. Based on the continuous perfusion of lipopolysaccharide (LPS), one study observed no alterations in renal mitochondrial function,67 though a more recent study in pigs with sepsis of intraabdominal origin reported an alteration in renal mitochondrial function, associated to an increase in oxidative stress marker levels.68
Distant damage caused by mechanical ventilationThe use of small tidal volumes (TV) (6ml/kg ideal weight) in mechanical ventilation (MV) during acute respiratory distress syndrome (ARDS) reduces mortality among these patients.69 One of the mechanisms proposed for explaining mortality associated to ARDS and MV is the release of systemic mediators generated at lung level in situations of high TV. An interesting study showed that animals ventilated with high TV values show greater tubular apoptosis and associated renal dysfunction. In fact, on cultivating renal cells in vitro with plasma from animals subjected to high TV, the cells likewise showed a higher apoptosis rate.70
Biomarkers in sepsis and ARFThe use of creatinine and UF for the diagnosis and prognosis of ARF during sepsis (RIFLE and AKIN criteria) poses several limitations. The rise in plasma creatinine is a late phenomenon, and in order for such an elevation to occur, it must be associated with an important decrease in GF capacity. The RIFLE classification does not clearly define the baseline value of patient renal function, in contrast to the AKIN classification, which requires the obtainment of two creatinine measurements spaced 48h apart. On the other hand, UF as a diagnostic criterion of ARF is conditioned by patient volemia and the use of diuretics. Most studies included in the RIFLE and AKIN analyses are retrospective and did not measure UF every 6 or 12h; accordingly, only 12% of them made use of both criteria (increase in creatinine and UF) for diagnosing ARF. The studies that used both criteria reported lesser mortality than those which used only creatinine as diagnostic criterion–this suggesting that the decrease in UF is more benign and/or reversible than the increase in creatinine.
The need to establish markers allowing an earlier and more sensitive diagnosis of ARF than creatinine elevation or a decrease in UF has led to the search for biomarkers of renal origin reflecting cellular damage in early stages of the disease.
Neutrophil gelatinase-associated lipocalin (NGAL) is a 24kDa protein normally expressed in low concentrations in different human tissues (kidneys, lungs, stomach and colon), and is found in the secondary granules of neutrophils. NGAL is released when these cells are activated, particularly in response to bacterial infections. NGAL transcription and release is intensely induced in the presence of epithelial damage.
In ARF, NGAL is promptly released from the proximal renal tubules following ischemic71 or toxic damage,72 and its levels can be measured in plasma and urine. A recent review73 involving over 4000 patients at risk of ARF due to sepsis, heart surgery, exposure to contrast media or transplantation, found NGAL to be significantly elevated in those individuals who develop ARF, and that this elevation significantly precedes the clinical diagnosis of ARF. Elevations in plasma and urine levels of NGAL have also been described in septic patients.74 The plasma and urine concentrations of NGAL are correlated to the degree of renal dysfunction established by the RIFLE or AKIN.75,76 However, a recent study suggests that urine NGAL elevation is a better predictor of ARF in sepsis than plasma NGAL elevation, which is less specific–possibly because of the activation of circulating neutrophils.74
Interleukin-18 is a proinflammatory cytokine transcribed and released in the proximal renal tubules, and which can easily be detected in urine following ischemic damage.77 It does not appear to increase under conditions of infection, prerenal ARF or chronic renal failure. This marker was initially described in heart surgery patients in which IL-18 was seen to rise early before the clinical diagnosis of ARF, with an area under the curve (AUC-ROC) of 0.75.78 IL-18 has also been described as a good predictor of ARF in critical patients in general, and in septic patients.79
KIM-1 (kidney injury molecule-1) is a transmembrane glycoprotein that shows a marked increase in expression on the part of the cell of the proximal renal tubules in response to ischemic or toxic stimuli. Its concentrations can be detected in urine and are seen to increase in patients with ARF. This marker might be useful for predicting the need for dialysis or in-hospital mortality in patients with ARF of different origins and severity.80
TreatmentThe limitations in establishing a physiopathological model of ARF have delayed the development of successful drug treatments, and at present much of the treatment of ARF in sepsis focuses on the support of kidney function. The management of ARF in septic patients is complicated, due to the existing hemodynamic instability and associated multiorgan dysfunction. As a result, in recent years many, both continuous and intermittent renal replacement therapy (RRT), techniques have been developed–though a lack of evidence in favor of one technique over the rest has largely precluded their clinical applicability.81–85
The different techniques developed are fundamentally based on two principles: diffusion and convection, or a combination of both. While diffusion techniques (hemodialysis) are preferentially used as non-antiinflammatory replacement therapy, and in hemodynamically stable patients, the convection techniques (hemofiltration) allow greater hemodynamic stability and the achievement of negative water balances, with lesser systemic repercussion.86–88 However, more extended hemodialysis allows the replacement of renal function and the achievement of negative balances even in unstable patients. On the other hand, hemofiltration techniques not only allow renal support but also the possibility of modulating the inflammatory response through the removal of inflammatory compounds (cytokines) of greater molecular weight.89,90 Hemofiltration with higher ultrafiltrate doses, referred to as high-volume hemofiltration (ultrafiltration rate >35ml/kg/h), is mainly associated with a reduction in the need for vasopressors,91–93 though some studies have also related it to improvements in microcirculation94 and survival.92
However, although some studies suggest benefits from the use of continuous hemofiltration in hemodynamically unstable patients beyond those offered by intermittent hemodialysis techniques,95 there is still not sufficient evidence of the superiority of continuous RRT over intermittent hemodialysis (IHD) in terms of mortality or the recovery of renal function.96,97 The use of peritoneal dialysis is related to increased mortality, and thus is not recommended in ARF associated to sepsis.98
ConclusionsAcute renal failure associated to sepsis is frequent, and implies increased management complexity and mortality. A series of still poorly understood pathogenic mechanisms are involved–a fact that has limited the strategies for dealing with the disease. At present, renal support techniques make it possible to replace kidney function efficiently, and there is evidence that they can modulate the inflammatory response.
Financial supportFondecyt 11100247 (Tomas Regueira).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Regueira T, et al. Fisiopatología de la insuficiencia renal aguda durante la sepsis. Med Intensiva. 2011;35:424–32.