To assess in individuals with traumatic spinal cord injury (TSCI) the relationship between mortality and need for ICU and early magnetic resonance imaging (MRI), analyzing spinal parenchymal alterations, disruption of vertebral ligaments (DVL) and spinal cord compression (SCC).
DesignRetrospective study.
SettingThird-level hospital, Spinal Cord Injury Unit and ICU.
PatientsIndividuals with acute TSCI between 2010 and 2019.
InterventionAnalysis of MRI performed in the first 72 h.
Variables of interestAdmission to ICU and mortality.
Results269 cases collected. The pattern that demonstrated higher mortality was cord hemorrhage (16.7%) for 12.5% of single-level edema and 6.5% of multilevel edema (p = 0.125). The same happened with ICU admissions: 69.0% in hemorrhage, 60.2% in multilevel edema and 46.3% in short edema (p = 0.018).
Analyzing CCM, mortality was 13.4% with 59.2% of ICU admissions, for 2.2% and 42.2% of individuals without cord compression (p = 0.020 and p = 0.003). The figures of death and ICU admission among cord injuries with DVL were 15.0% and 67.3%, for 6.2% and 44.4% of the individuals without DLV (p < 0.001 and p = 0.013).
ConclusionsThe presence of spinal cord hemorrhage, SCC and DVL was associated with a higher admission in ICU. A significant increase in mortality was observed in cases with SCC and DVL.
Valorar en individuos con lesión medular traumática (LMT) la relación entre la mortalidad y la necesidad de UCI y las alteraciones objetivadas mediante resonancia magnética (RM) precoz, analizando alteraciones parenquimatosas, disrupción de ligamentos vertebrales (DLV) y compresión del cordón medular (CCM).
DiseñoEstudio retrospectivo.
ÁmbitoHospital de tercer nivel, unidad de lesionados medulares y UCI.
PacientesIndividuos con LMT aguda entre los años 2010 y 2019.
IntervencionesAnálisis de RM realizada en las primeras 72 horas.
Variables de interésIngreso en UCI y mortalidad.
ResultadosRecogidos 269 casos. El patrón que se asoció a una mayor mortalidad fue la hemorragia (16,7%) por 12,5% de los edemas a un nivel y 6,5% de los edemas a múltiples niveles (p = 0,125). Lo mismo aconteció con los ingresos en UCI: 69,0% en hemorragia por 60,2% en edema múltiple y 46,3% en edemas cortos (p = 0,018).
Respecto a la CCM, la mortalidad fue del 13,4% con 59,2% de ingresos en UCI por 2,2% y 42,2% de quienes no presentaban compresión (p = 0,020 y p = 0,003). Las cifras de éxitus e ingreso en UCI en los individuos con DLV fueron respectivamente del 15,0% y el 67,3%, por un 6,2% y 44,4% de los individuos sin DLV (p < 0,001 y p = 0,013).
ConclusionesLa presencia de hemorragia medular, CCM y DLV se asoció a una mayor necesidad de UCI. Existe un significativo aumento de la mortalidad en los casos con CCM y DLV.
Traumatic spinal cord injury (TSCI) constitutes an important challenge for healthcare professionals. In addition to motor and sensory alterations, disruption of the spinal cord tracts has a strong impact on cardiorespiratory function. It is therefore considered crucial to include specialists in intensive care within the multidisciplinary care team, particularly during the acute phase.1
The middle and long-term mortality risk factors in TSCI have been widely investigated, though few studies have assessed the risk of complications during the first post-injury hours.2 Based on longer-term research, it is known that advanced age, injuries at cervical level and lesion grade3,4 constitute the main indicators of a poor prognosis.5
Considering the above, it is important to perform a correct and early neurological evaluation in order to establish the lesion level and grade. Protocolized physical examination based on the guidelines of the American Spinal Injury Association (ASIA) has been shown to be the most reliable method for the diagnosis and classification of TSCI.6 However, such evaluation requires the collaboration of the patient, which is not always possible during the acute phase of the lesion. It is therefore necessary to have alternative methods in order to at least be able to estimate the characteristics of the lesion - with imaging techniques being an interesting option in this sense. Of the different techniques available, magnetic resonance imaging (MRI) allows us to assess the spinal cord parenchyma together with the ligaments and vertebral discs.7 Indeed, MRI is considered to be the gold standard in TSCI, and is thus recommended in all cases.8
The relationship between the MRI image and the prognosis of TSCI has been established for decades.9 In recent years, several studies have related the existence of certain parenchymal lesion patterns to a poorer patient outcome.10–14 The following classification can be made in which the prognosis worsens as we move down the list:
- -
Pattern without spinal cord damage
- -
Single-level edema
- -
Multi-level edema
- -
Spinal cord hemorrhage
- -
Sectioning of the spinal cord
In addition to the parenchymal pattern, different authors have analyzed other possible factors indicative of a poor prognosis and that can be evaluated by MRI. Of these factors, the most widely investigated has been the presence of spinal cord compression (SCC),15 which is generally associated with a poorer outcome. On the other hand, and although less widely studied, several articles have been published in which the disruption of vertebral ligaments (DVL) in the context of TSCI has been associated with a poorer outcome. In this regard, lesions of the ligamenta flava and the anterior and posterior longitudinal ligaments have been found to be of prognostic value.16–18
The present study evaluates the influence of the alterations identified by MRI performed in the first hours following TSCI upon the patient’s vital prognosis and the need for critical care, to offer a new tool to facilitate acute management of the disorder.
Patients and methodsStudy settingThe present study was carried out in a tertiary hospital, with the collection of data on patients admitted to the Spinal Cord Injury Unit (SCIU).
Characteristics of the studyA retrospective, descriptive observational study was made.
PatientsWe selected those individuals admitted to the SCIU between January 2010 and December 2019.
Inclusion criteria: patient age > 18 years, the presence of acute TSCI, and MRI evaluation performed in the first 72 h.
Exclusion criteria: a history of neurological disease implying important functional loss before TSCI, or resulting in altered spinal cord MRI signal characteristics, and the presence of vertebral instrumentation in the injured zone.
Data compilationThe following variables were recorded: age, gender, etiology, time to MRI, pattern of the lesion in MRI, presence of SCC, presence of DVL, level and grade of TSCI upon admission, presence of vertebral lesions or other associated lesions, Charlson comorbidity index, admission to the Intensive Care Unit (ICU), and in-hospital mortality.
Age was recorded as the mean ± standard deviation (SD), and was also stratified as either over or under 65 years, in concordance with the practice of most published studies on TSCI.19,20
The patients with TSCI were evaluated with the cooperation of an expert in radiology of the central nervous system. The classification of the lesion pattern was based on the elements described above: normal pattern, single-level edema, multilevel edema, spinal cord hemorrhage and complete sectioning of the spinal cord.10–14 Single-level edema was defined as edema with a sagittal extent inferior to the size of an adjacent disc-vertebra complex. The disruption of vertebral ligaments (DVL) in turn was defined as the alteration of at least one of the following components of the vertebral ligament system: ligamenta flava, anterior longitudinal ligament and posterior longitudinal ligament.16–18
The characteristics of the spinal cord injury were evaluated based on the international standards for the classification of TSCI according to the ASIA.21 To simplify the analysis, the lesion levels were grouped into high tetraplegia (levels C1 to C4), low tetraplegia (C5 to C8) and paraplegia.
Statistical analysisA descriptive study was made, reporting quantitative variables as the mean ± standard deviation (SD), and qualitative variables as absolute values and percentages. The comparison of means was carried out using the Student-test, while multiple comparisons of means were performed with the Kruskal-Wallis test. Associations between qualitative variables were explored using the chi-square test. The IBM® SPSS® version 20 statistical package (IBM Corp., 2010, Armonk, NY, USA) was used throughout.
Ethical and legal aspectsThe data were obtained from the SCIU admissions registry and the electronic case histories, followed by coding and anonymization, and were processed following the guidelines of the Research Ethics Committee, complying with Spanish legislation (Ley Orgánica 3/2018, of 5 December) referred to personal data protection and digital rights. The study was approved by the Research Ethics Committee (Ref.: 2020/370).
ResultsA total of 720 patients were admitted with acute spinal cord injury during the study period. Of these cases, 489 were of traumatic origin – with 269 cases meeting the study inclusion criteria. In 77.3% of the cases, MRI was performed in the first 24 h after TSCI.
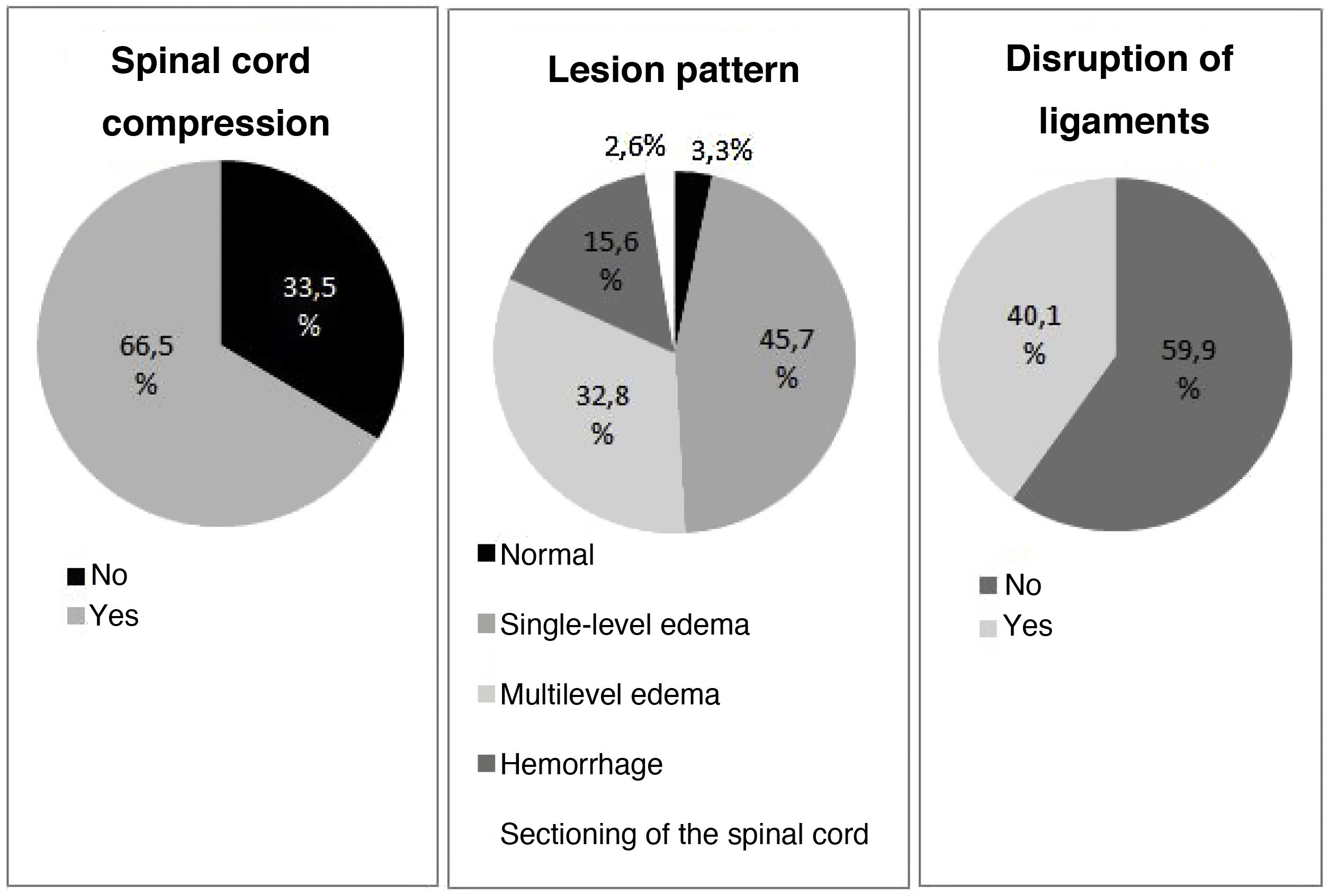
A total of 30.3% of the patients (n = 80) that could be correctly evaluated (in 5 cases patient collaboration was not adequate) suffered complete lesions, and in 69.3% of the cases (n = 183) the lesion was located at cervical level. The data are summarized in Table 1.
American Spinal Injury Association (ASIA) classification of lesion grade and MRI imaging characteristics of the sample according to lesion level.
| Level | Tetraplegia C1−4 | Tetraplegia C5−8 | Paraplegia | TOTAL |
|---|---|---|---|---|
| ASIA | N (%) | N (%) | N (%) | N (%) |
| A | 21 (20.4) | 17 (21.2) | 42 (51.9) | 80 (30.3) |
| B | 6 (5.8) | 8 (10.0) | 15 (18.5) | 29 (11.0) |
| C | 39 (37.9) | 16 (20.0) | 15 (18.5) | 70 (26.5) |
| D | 37 (35.9) | 39 (48.8) | 9 (11.1) | 85 (32.2) |
| Pattern | N (%) | N (%) | N (%) | N (%) |
| Normal | 3 (2.9) | 4 (4.9) | 1 (1.2) | 8 (3.0) |
| Single-level edema | 44 (41.9) | 45 (54.9) | 34 (42.0) | 123 (45.9) |
| Multilevel edema | 46 (43.8) | 27 (32.9) | 15 (18.5) | 88 (32.8) |
| Hemorrhage | 10 (9.5) | 6 (7.3) | 26 (32.1) | 42 (15.7) |
| Complete sectioning | 2 (1.9) | 0 | 5 (6.2) | 7 (2.6) |
| Compression | N (%) | N (%) | N (%) | N (%) |
| Yes | 69 (65.7) | 52 (63.4) | 58 (71.6) | 179 (66.8) |
| No | 36 (34.3) | 30 (36.6) | 23 (28.4) | 89 (33.2) |
| Disruption of ligaments | N (%) | N (%) | N (%) | N (%) |
| Yes | 39 (37.5) | 26 (31.7) | 42 (52.5) | 107 (40.2) |
| No | 65 (62.5) | 56 (68.3) | 38 (47.5) | 159 (59.8) |
MRI: magnetic resonance imaging.
The alterations documented by the MRI scans are detailed in Fig. 1. Few cases were classified as corresponding to a normal pattern or complete sectioning, with edema being the most frequent pattern. The distributions according to lesion level, percentage hemorrhage, SCC and DVL were similar between low and high tetraplegia, but not so in establishing comparisons with paraplegia.
The most common cause of injury was falls (n = 183). Of these cases, 63.4% corresponded to falls from a height (defined as over one meter). Traffic accidents were the second most common cause of TSCI (n = 56). Three diving injuries were also recorded, as well as 27 cases of TSCI due to other causes (mostly direct traumatisms). The percentage of tetraplegia cases corresponding to the different etiologies was similar among the three predominant groups: 74.0% in the case of traffic accidents, 68.3% in the case of falls (on considering only falls on the same level, tetraplegia represented 89.6%), and 66.7% in the case of other trauma origins. All diving injuries corresponded to tetraplegia cases.
Stratified by etiology, the MRI patterns yielded similar data, as can be seen in Table 2, with a slightly greater percentage of traffic accident cases among the hemorrhagic and spinal cord section patterns. Likewise, DVL was more frequent in the case of traffic accidents, though no differences were recorded in terms of the presence of SCC.
Relationship between the cause of spinal cord injury and the MRI findings.
| Traffic | Fall | Dive | Others | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Normal pattern | 0 | 7 (3.85) | 1 (33.3) | 1 (3.7) |
| Single-level edema | 26 (46.4) | 84 (45.9) | 0 | 13 (48.2) |
| Multilevel edema | 17 (30.4) | 65 (35.5) | 0 | 6 (22.2) |
| Spinal cord hemorrhage | 11 (19.6) | 23 (12.6) | 2 (66.7) | 6 (22.2) |
| Cord sectioning | 2 (3.6) | 4 (2.2) | 0 | 1 (3.7) |
| Compression: no | 17 (30.4) | 62 (33.9) | 2 (66.7) | 9 (33.3) |
| Compression: yes | 39 (69.6) | 121 (66.1) | 1 (33.3) | 18 (66.7) |
| Intact ligaments | 25 (44.6) | 120 (66.3) | 1 (33.3) | 14 (51.8) |
| Disruption of ligaments | 31 (55.4) | 61 (33.7) | 2 (66.6) | 13 (48.2) |
The groups of cases were analyzed to detect population differences that might constitute a source of bias (Table 3). The analysis excluded normal images and spinal cord sectioning due to the small sample size. Among the parenchymal lesion patterns, significant differences were recorded in relation to age (p = 0.006), which was greater among the patients with extensive edema (63.7 ± 18.2 years), while the individuals with spinal cord hemorrhage were the youngest (51.4 ± 21.7 years). However, on analyzing the sample by age groups, no differences were observed according to whether the patients were under or over 65 years of age (p = 0.070). On the other hand, age was found to be significantly lower among the individuals with DVL versus those without ligament damage (p = 0.013). With regard to gender, none of the groups presented important differences.
General characteristics of the different groups.
| Age | +65 years | Gender: male | MRI within 24 h | |||||
|---|---|---|---|---|---|---|---|---|
| mean ± SD | p | N (%) | p | N (%) | p | N (%) | p | |
| Normal pattern | 54.7 ± 18.6 | 3 (33.3) | 8 (88.9) | 8 (88.9) | ||||
| Single-level edema | 59.0 ± 19.3 | 53 (43.1) | 95 (77.2) | 97 (78.9) | ||||
| Multilevel edema | 63.7 ± 18.2 | 0.006 | 44 (50) | 0.070 | 38 (57.6) | 0.262 | 63 (71.6) | 0.266 |
| Hemorrhage | 51.4 ± 21.7 | 12 (28.6) | 33 (78.6) | 35 (83.3) | ||||
| Cord sectioning | 53.9 ± 24.1 | 2 (28.6) | 5 (71.4) | 5 (71.4) | ||||
| Compression: no | 57.4 ± 18.3 | 0.330 | 32 (35.6) | 0.131 | 70 (77.8) | 0.576 | 68 (75.6) | 0.632 |
| Compression: yes | 59.9 ± 20.5 | 82 (45.8) | 131 (73.2) | 140 (78.2) | ||||
| Intact ligaments | 61.4 ± 19.0 | 0.017 | 73 (45.6) | 0.353 | 119 (74.4) | 0.911 | 118 (73.8) | 0.023 |
| Disruption of ligaments | 55.8 ± 20.5 | 41 (38.3) | 80 (74.8) | 90 (84.1) | ||||
On considering comorbidities (Table 4), the Charlson index scores were similar in all the groups, with no significant differences among them. With regard to concomitant lesions, we detected a significantly greater percentage of fractures in the group of hemorrhagic injuries (p < 0.001) and in the cases presenting DVL (p < 0.001) and SCC (p = 0.016). Associated lesions were also more frequent in the individuals with hemorrhagic injuries (p = 0.007) and DVL (p = 0.022), though no differences were recorded according to whether SCC was present or not (p = 0.518).
Comorbidities in the different groups.
| Charlson | Associated lesion | Vertebral lesion | ||||
|---|---|---|---|---|---|---|
| mean ± SD | p | N (%) | p | N (%) | p | |
| Normal pattern | 0.7 ± 0.9 | 2 (22.2) | 5 (55.6) | |||
| Single-level edema | 0.7 ± 1.2 | 45 (36.6) | 68 (55.3) | |||
| Multilevel edema | 0.7 ± 1.4 | 0.623 | 40 (45.4) | 0.007 | 58 (65.9) | <0.001 |
| Hemorrhage | 0.4 ± 0.7 | 27 (64.3) | 39 (92.8) | |||
| Cord sectioning | 0.4 ± 0.8 | 4 (57.1) | 6 (85.7) | |||
| Compression: no | 0.5 ± 1.2 | 0.180 | 37 (41.1) | 0.518 | 50 (55.6) | 0.016 |
| Compression: yes | 0.7 ± 1.1 | 81 (45.2) | 126 (70.4) | |||
| Intact ligaments | 0.7 ± 1.3 | 0.169 | 61 (38.1) | 0.022 | 77 (48.1) | <0.001 |
| Disruption of ligaments | 0.5 ± 1.0 | 56 (52.3) | 98 (91.6) | |||
In our study sample, 53.5% of the patients (n = 144) required admission to the ICU. Of these, 47.9% were admitted in the first 24 h after injury, and 35.4% between 24−48 h post-injury. A total of 26 patients died during hospital admission, representing a global mortality rate of 9.7%, with four deaths in the first 24 h and three between the first and seventh day.
Based on the collected data, we examined the alterations associated with increased mortality and the need for admission to the ICU (Table 5). In this regard, although the mortality risk was found to be greater among the individuals with hemorrhagic injuries, no significant differences were observed (p = 0.125). We did record a significant increase in mortality among the patients with SCC (p = 0.003) and DVL (p = 0.013) compared with those without such lesions. The percentage of individuals admitted to the ICU was also significantly higher in the cases of DVL (p < 0.001) and SCC (p = 0.020) versus the cases without such lesions. Likewise, a lesser need for ICU admission was observed among the subjects with single-level edema versus those with more extensive edema and with spinal cord hemorrhage (p = 0.018).
Analysis of the risk of admission to the ICU and mortality according to the data obtained from the MRI scan.
| ICU no | ICU yes | p | Death | No death | p | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |||
| Normal pattern | 8 (88.9) | 1 (11.1) | 0 | 9 (100) | ||
| Single-level edema | 66 (53.7) | 57 (46.3) | 8 (6.5) | 115 (93.5) | ||
| Multilevel edema | 35 (39.8) | 53 (60.2) | 0.018 | 11 (12.5) | 77 (87.5) | 0.125 |
| Hemorrhage | 13 (31.0) | 29 (69.0) | 7 (16.7) | 35 (83.3) | ||
| Cord sectioning | 3 (42.9) | 4 (57.1) | 0 | 7 (100) | ||
| Compression: no | 52 (57.8) | 38 (42.2) | 0.020 | 2 (2.2) | 88 (97.2) | 0.003 |
| Compression: yes | 73 (41.1) | 106 (58.9) | 24 (13.4) | 155 (86.6) | ||
| Intact ligaments | 89 (55.6) | 71 (44.4) | < 0.001 | 10 (6.2) | 150 (93.8) | 0.013 |
| Disruption of ligaments | 35 (32.7) | 72 (67.3) | 16 (15.0) | 91 (85.0) |
ICU: Intensive Care Unit; MRI: magnetic resonance imaging.
The present study was designed to evaluate the usefulness of alterations identified by MRI performed in the first hours following TSCI, with a view to offering a new tool capable of complementing the already known prognostic factors, and specifically analyzing the parenchymal lesion patterns and extramedullary alterations.
In our sample, the most frequent parenchymal pattern was single-level edema (45.7%), in contrast to the findings of other studies in which greater percentages of multilevel edema were observed. This could be explained by the fact that the mentioned studies performed MRI in later stages of spinal cord injury. It is now known that the development of changes in MRI signal following TSCI takes place in the course of the first few days, with an increase in most cases of the length of edema after the first 24 h.22 In this regard, the meta-analysis conducted in 2011 by Bozzo8 documented higher percentages of hemorrhagic lesions than in our sample (between 22–32%), with the exception of one of the documented studies.23
Incomplete cervical injuries were the most common presentation, in coincidence with the findings of other studies - this circumstance being related to the increase in TSCI secondary to low-energy falls among elderly individuals.24,25 This could account for the older mean age of the patients with spinal cord edema compared with those presenting a hemorrhagic pattern. In effect, while edema is more commonly seen in the elderly, hemorrhagic patterns are generally associated with greater energy traumatisms such as falls from a height or traffic accidents, which are more frequent in younger individuals.26
In the present sample, the patients were admitted to the ICU always after due assessment by the SCIU at our center. The time elapsed from injury to ICU admission may seem long, though it must be pointed out that since ours is a reference center, the recorded period covers care at the site of the accident, transfer to the nearest hospital, the care received at that hospital, and subsequent transfer to our center.
An important aspect drawn from the analysis is the greater need for ICU admission among the patients with DVL, SCC and spinal cord hemorrhage. The reason for this is largely the fact that these disorders are usually associated with greater energy traumatisms, and therefore more often involve concomitant injuries that increase the severity of the patient. Nevertheless, although the presence of SCC and DVL was effectively seen to increase the mortality rate, the same cannot be said of the hemorrhagic parenchymal lesions. This observation seems to be related to the younger mean age of the individuals with hemorrhagic lesions compared with the other patterns, especially multilevel spinal cord edema, where the mean patient age was 10 years older - since in TSCI, as in any other disease condition, advancing age significantly increases the mortality rate.
It could be postulated that the energy of the traumatism and not the MRI alterations as such would justify the increased mortality. However, this hypothesis was evaluated by McCarthy et al.,27 who found no significant differences – though their study assessed the long-term outcome in a small sample of patients. The authors themselves pointed out that classification of the injuries as corresponding to high/low energy is rather arbitrary – making it difficult to carry out studies in this respect. Our review of the literature yielded no other studies evaluating this hypothesis.
The mortality rate in our sample was 9.7%, with only 1.5% corresponding to the first day. These figures are lower than those described in other studies, though we have no information corresponding to the pre-hospital phase. Studies such as those included in the review by Hagen et al.28 have reported diverse pre- and in-hospital mortality figures, and in all cases these figures were higher than in our study. However, the different characteristics of the samples and the different epochs and healthcare systems involved make it difficult to establish comparisons.
An interesting article was published by Lalwani et al.29 involving cases from a Forensic Medicine Department, with the selection of deceased individuals presenting spinal cord damage. The authors evaluated mortality during the different phases of medical care. A total of 16.13% of the deaths occurred during phase 2, which covered the period between patient arrival in the Emergency Department and admission to the ICU / hospital ward. The greatest proportion of deaths (over 70%) occurred during patient stay in the hospital ward. This study moreover found that mortality during the first phases was largely due to hemodynamic instability and head injuries, while in the later phases it was secondary to respiratory disorders.
We have found practically no morbidity-mortality publications with objectives comparable to those of our study, since most articles involve longer-term follow-up and few have analyzed the relationship with MRI characteristics. In the same way as in our study, Selden et al.,30 with a sample smaller than our own, recorded a poorer prognosis among individuals with multilevel spinal cord edema, hemorrhage and SCC. However, the main aim of their study was to assess neurological recovery, not morbidity-mortality.
LimitationsThe present study has focused on MRI imaging analysis. Although other prognostic factors could have been investigated, such as those described in the Introduction, we have preferred to limit ourselves to the objectives of the study in order to avoid confounding factors.
The criterion for acute TSCI patient admission to the ICU is not regulated by strict protocols, and in some centers it is conditioned by the acute TSCI care model in force; the results therefore might not be fully extrapolatable to samples from other centers. In our case, we followed the recommendations of the international organisms.
ConclusionsThe MRI imaging patterns of parenchymal lesions afford information on the need for patient admission to the ICU – the risk being higher among individuals with multilevel edema and intraparenchymal hemorrhage. Likewise, the presence of other extramedullary MRI alterations (DVL and SCC) implies an increased risk of mortality and the need for admission to the ICU. In this regard, it has been seen that MRI performed in the first 72 h after TSCI affords information on the vital prognosis of the patient, and is of help in planning management during the acute phase.
Contribution of the authorsRMB contributed to the conception and design of the study, and to the drafting of the manuscript.
OVM contributed to data acquisition and interpretation.
SPG performed the statistical analysis.
RMMF and ARS approved the final draft of the manuscript.
MEFV and SSB contributed to data acquisition.
AMM contributed to the conception of the study and critical review.
Financial supportNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.