Confluence between the intrinsic and extrinsic apoptosis pathways is reached at the point of caspase-3 activation, which induces death cell. Higher serum caspase-3 levels have been recorded on day 1 of traumatic brain injury (TBI) in 30-day non-survivors compared to survivors. The objectives of this study therefore were to determine whether serum caspase-3 levels are persistently higher in non-survivors than in survivors, and whether these levels may be used to predict 30-day mortality.
DesignA prospective observational study was carried out.
SettingSix Spanish Intensive Care Units.
PatientsPatients with severe isolated TBI (defined as Glasgow Coma Scale <9 points and non-cranial Injury Severity Score <10 points).
InterventionsSerum caspase-3 concentrations were measured on days 1, 4 and 8 of TBI.
Main variables of interestThirty-day mortality was considered as the study endpoint.
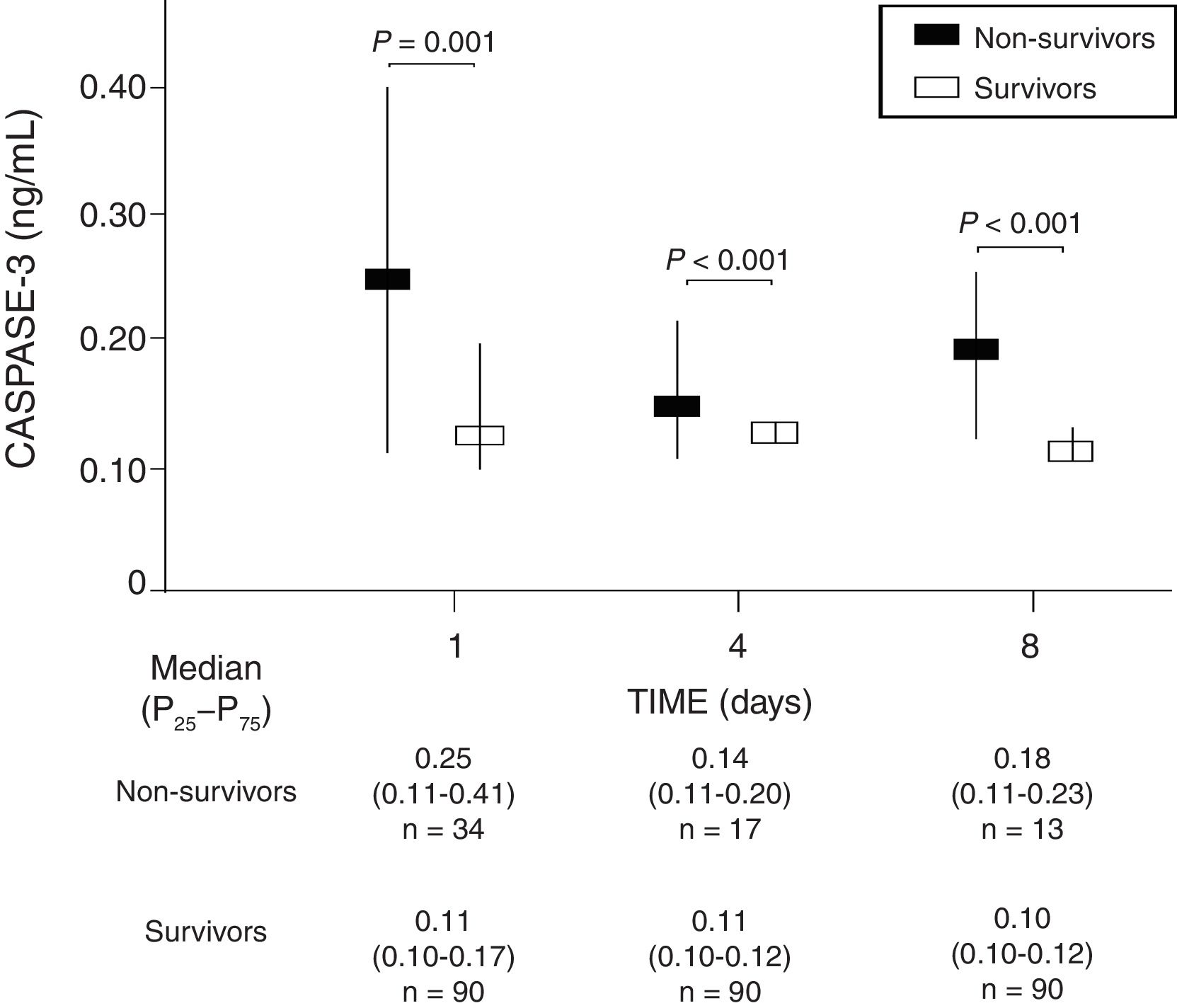
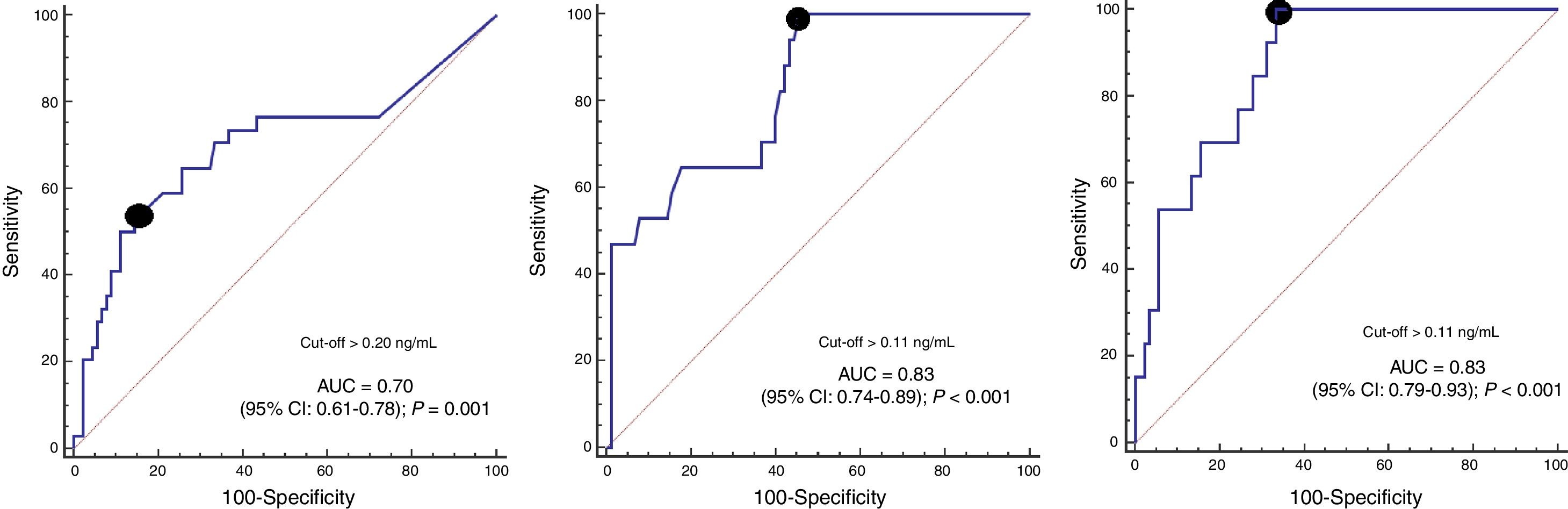
ResultsIn comparison with non-survivors (n=34), 30-day survivors (n=90) showed lower serum caspase-3 levels on days 1 (p=0.001), 4 (p<0.001) and 8 (p<0.001) of TBI. Analysis of the ROC curves showed serum caspase-3 concentrations on days 1, 4 and 8 of TBI to have an AUC (95% CI) in predicting 30-day mortality of 0.70 (0.61–0.78; p=0.001), 0.83 (0.74–0.89; p<0.001) and 0.87 (0.79–0.93; p<0.001), respectively.
ConclusionsThe novel findings of our study were that serum caspase-3 levels during the first week of TBI were lower in survivors and could predict 30-day mortality.
La vía intrínseca y extrínseca de la apoptosis confluyen en la activación de caspasa-3. Se han encontrado mayores niveles séricos de caspasa-3 en el día 1 del traumatismo craneoencefálico (TCE) en los pacientes que fallecen en los primeros 30 días que en supervivientes. Por tanto, los objetivos de este estudio es determinar si los niveles séricos de caspasa-3 se mantienen superiores en los pacientes fallecidos que en los supervivientes, y si podrían utilizarse para predecir la mortalidad a 30 días.
DiseñoEstudio observacional y prospectivo.
ÁmbitoSeis unidades de cuidados intensivos españolas.
PacientesEnfermos con un TCE grave y aislado (definido como escala de coma de Glasgow <9 y puntuación de gravedad de la lesión Score en lesiones no craneales <10).
IntervencionesSe midieron los niveles séricos de caspasa-3 en los días 1, 4 y 8 del TCE.
Variables de interés principalesMortalidad a los 30 días.
ResultadosLos pacientes supervivientes a los 30 días (n=90) presentan menores niveles séricos de caspasa-3 en los días 1 (p=0,001), 4 (p<0,001) y 8 (p<0,001) del TCE que los fallecidos (n=34). Los niveles séricos de caspasa-3 en los días 1, 4 y 8 del TCE tenían un área bajo la curva (intervalo de confianza del 95%) para predecir la mortalidad de 0,70 (0,61-0,78; p=0,001), 0,83 (0,74-0,89; p<0,001) y 0,87 (0,79-0,93; p<0,001), respectivamente.
ConclusionesLos nuevos hallazgos de nuestro estudio fueron que los niveles séricos de caspasa-3 durante la primera semana del TCE fueron menores en los pacientes supervivientes, y que pueden predecir la mortalidad a los 30 días.
Many disabilities, deaths, and health resource consumption are due to traumatic brain injury (TBI).1–3 In TBI appears a cerebral damage due to the primary brain injury produced by the physical forces in the moment of trauma, and also a cerebral damage due to the secondary brain injury produced by the activation in the following hours or days of different physiopathological ways such as apoptosis.4–7 The programed cell death by apoptosis occurs mainly through the activation of the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway, and both pathways (intrinsic and extrinsic) activate caspase-3 which motivate the cell changes that leads to death cell.4–7
There have been found increased caspase-3 in brain tissues of TBI animal models,8–20 in cerebrospinal fluid (CSF) of TBI patients,21–23 and in brain tissues of TBI patients.24,25 In addition, higher brain tissue caspase-3 concentrations in non-surviving than in surviving TBI patients have been found.25 Besides, higher serum caspase-3 on day 1 of TBI have been found in 30-day non-survivor compared to survivor patients.26 Thus, the objectives of this study were to determine whether serum caspase-3 levels are maintainly higher in non-survivor than in survivor patients and whether those levels could be used to predict 30-day mortality.
MethodsDesign and subjectsSix spanish hospitals participated in this observational and prospective study. The Institutional Board of all hospitals approved the protocol before to start the study: H. General of La Palma, H. Universitario de Canarias of La Laguna, H. Insular of Las Palmas de Gran Canaria, H. Clínico Universitario of Valencia, H. Universitario Nuestra Señora de Candelaria of Santa Cruz de Tenerife, and H. Universitario Dr. Negrín of Las Palmas de Gran Canaria. A relative of each patient signed the written informed consent for the inclusion of the patient in the study.
We included patients with a severe isolated TBI. The criteria used for the definition of isolated was <10 points in non-cranial aspects of Injury Severity Score (ISS),27 and the criteria for used for the definition of severe was <9 points in Glasgow Coma Scale (GCS).28 We excluded patients with inflammatory disease, pregnancy, age less than 18 years, malignant disease, or comfort measures only.
We registered sex, age, temperature, GCS, ISS, glycemia, creatinine, lactic acid, bilirrubin, fraction inspired oxygen (FIO2), pressure of arterial oxygen (PaO2), sodium, leukocytes, platelets, hemoglobin, activated partial thromboplastin time (aPTT), fibrinogen, international normalized ratio (INR), cerebral perfusion pressure (CPP), intracranial pressure (ICP), Acute Physiology and Chronic Health Evaluation II (APACHE II) score,29 and brain lesions according to Marshall computer tomography classification (CT).30 Thirty-day mortality from the ICU admission was considered as the end-point study. Clinical variables, CT findings and blood parameters were taken on admission, and ICP and CPP correspond to the values peak and small in the first 24h, respectively.
Determinations of caspase-3 serum levelsWe obtained and frozen at −80°C until the moment of determination, serum samples on day 1, day 4 and day 8 of TBI. Serum caspase-3 levels on day 1 of TBI had been previously determined by our team in some of those patients26; and in this study we determined serum caspase-3 levels on day 1, day 4 and day 8 of TBI. Caspase-3 levels determination were performed in the Laboratory Department of the Hospital Universitario de Canarias (La Laguna Spain) by means of Human Caspase-3 Elisa BlueGene Biotech® (Shanghai, China). The assay detection limit was 0.10ng/mL, the intra-assay variation coefficient (VC) was <5.6%, and the inter-assay VC was <7.9%.
Statistical methodsWe reported continuous variables as medians (and percentile 25 and 75) and were compared between both groups of patients (30-day survivor and non-survivor) using Wilcoxon–Mann–Whitney test. We reported categorical variables as frequencies (and percentages) and were compared between both groups of patients using chi-square test. To test the capacity of serum caspase-3 levels at day 1, 4 and 8 of TBI to predict 30-day mortality were performed receiver operating characteristic (ROC) analyses. We reported area under curve (AUC) and its 95% confidence intervals (CI). In addition, we reported sensitivity and specificity, negative and positive likelihood ratios, and negative and positive predicted values and its 95% CI for the cut-offs of serum caspase-3 levels at days 1, 4 and 8 of TBI (which were selected according to Youden J index). Multiple logistic regression was used to determine the association between serum caspase-3 levels and 30 day-mortality controlling for APACHE-II, CT, sex, age and CGS. For statistical analyses, we used the programs SPSS 17.0 (SPSS Inc., Chicago, IL, USA), NCSS 2000 (Kaysville, Utah), and LogXact 4.1 (Cytel Co., Cambridge, MA). To consider a difference as statistically significant, we used the cut-off of p-value <0.05.
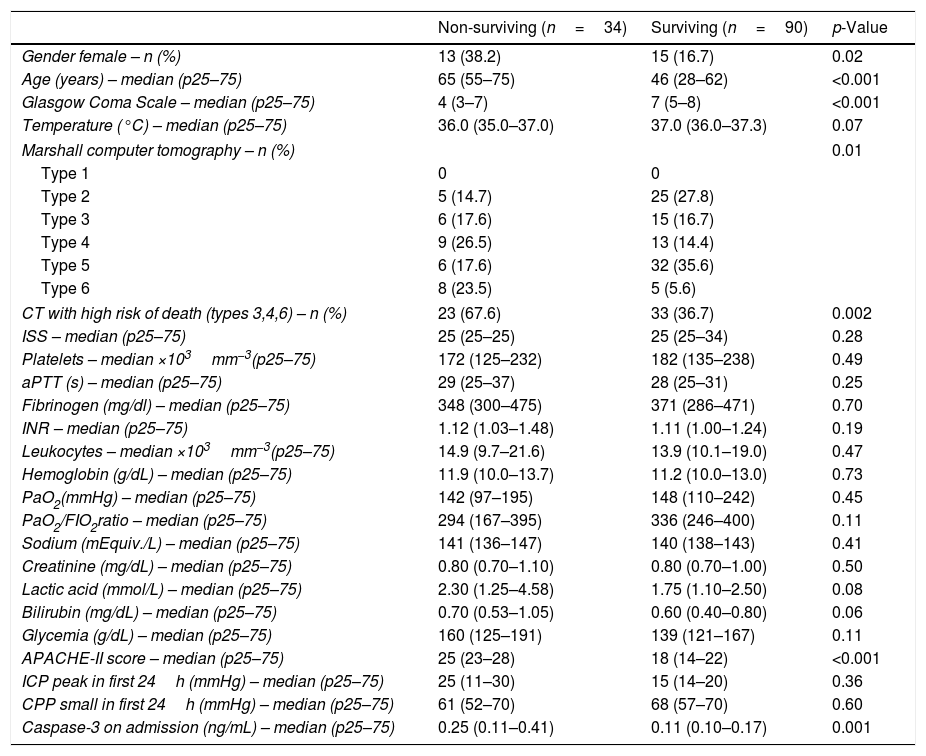
ResultsThirty-day survivor TBI patients (n=90) and non-survivors patients (n=34) were not statistically different in temperature, lactic acid, sodium, glycemia, bilirubin, PaO2, PaO2/FIO2 ratio, hemoglobin, creatinine, platelets, leukocytes, INR, fibrinogen, aPTT, ISS, ICP, and CPP. We found that survivor and non-survivor patients showed different brain computer tomography findings, and that survivor compared to non-survivor patients showed lower female rate, lower age, higher GCS, and lower APACHE-II score (Table 1). All patients were undergoing to mechanical ventilation and monitorization of ICP. The causes of death were brain death in 25 patients, cardiac arrest in 5 patients, and nosocomial pneumonia in 4 patients.
Characteristics on admission of 30-day surviving and non-surviving patients.
| Non-surviving (n=34) | Surviving (n=90) | p-Value | |
|---|---|---|---|
| Gender female – n (%) | 13 (38.2) | 15 (16.7) | 0.02 |
| Age (years) – median (p25–75) | 65 (55–75) | 46 (28–62) | <0.001 |
| Glasgow Coma Scale – median (p25–75) | 4 (3–7) | 7 (5–8) | <0.001 |
| Temperature (°C) – median (p25–75) | 36.0 (35.0–37.0) | 37.0 (36.0–37.3) | 0.07 |
| Marshall computer tomography – n (%) | 0.01 | ||
| Type 1 | 0 | 0 | |
| Type 2 | 5 (14.7) | 25 (27.8) | |
| Type 3 | 6 (17.6) | 15 (16.7) | |
| Type 4 | 9 (26.5) | 13 (14.4) | |
| Type 5 | 6 (17.6) | 32 (35.6) | |
| Type 6 | 8 (23.5) | 5 (5.6) | |
| CT with high risk of death (types 3,4,6) – n (%) | 23 (67.6) | 33 (36.7) | 0.002 |
| ISS – median (p25–75) | 25 (25–25) | 25 (25–34) | 0.28 |
| Platelets – median ×103mm−3(p25–75) | 172 (125–232) | 182 (135–238) | 0.49 |
| aPTT (s) – median (p25–75) | 29 (25–37) | 28 (25–31) | 0.25 |
| Fibrinogen (mg/dl) – median (p25–75) | 348 (300–475) | 371 (286–471) | 0.70 |
| INR – median (p25–75) | 1.12 (1.03–1.48) | 1.11 (1.00–1.24) | 0.19 |
| Leukocytes – median ×103mm−3(p25–75) | 14.9 (9.7–21.6) | 13.9 (10.1–19.0) | 0.47 |
| Hemoglobin (g/dL) – median (p25–75) | 11.9 (10.0–13.7) | 11.2 (10.0–13.0) | 0.73 |
| PaO2(mmHg) – median (p25–75) | 142 (97–195) | 148 (110–242) | 0.45 |
| PaO2/FIO2ratio – median (p25–75) | 294 (167–395) | 336 (246–400) | 0.11 |
| Sodium (mEquiv./L) – median (p25–75) | 141 (136–147) | 140 (138–143) | 0.41 |
| Creatinine (mg/dL) – median (p25–75) | 0.80 (0.70–1.10) | 0.80 (0.70–1.00) | 0.50 |
| Lactic acid (mmol/L) – median (p25–75) | 2.30 (1.25–4.58) | 1.75 (1.10–2.50) | 0.08 |
| Bilirubin (mg/dL) – median (p25–75) | 0.70 (0.53–1.05) | 0.60 (0.40–0.80) | 0.06 |
| Glycemia (g/dL) – median (p25–75) | 160 (125–191) | 139 (121–167) | 0.11 |
| APACHE-II score – median (p25–75) | 25 (23–28) | 18 (14–22) | <0.001 |
| ICP peak in first 24h (mmHg) – median (p25–75) | 25 (11–30) | 15 (14–20) | 0.36 |
| CPP small in first 24h (mmHg) – median (p25–75) | 61 (52–70) | 68 (57–70) | 0.60 |
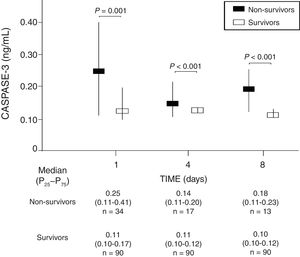
| Caspase-3 on admission (ng/mL) – median (p25–75) | 0.25 (0.11–0.41) | 0.11 (0.10–0.17) | 0.001 |
aPTT=activated partial thromboplastin time; INR=international normalized ratio; PaO2=pressure of arterial oxygen; FIO2=fraction inspired oxygen; ISS=Injury Severity Score; APACHE II=Acute Physiology and Chronic Health Evaluation; ICP=intracranial pressure; CPP=cerebral perfusion pressure; p25–75=percentile 25th–75th.
We found that survivor patients in comparison with non-survivors showed lower serum caspase-3 levels at days 1 (p=0.001), 4 (p<0.001), and 8 (p<0.001) of TBI (Fig. 1). We found that non-survivor patients showed higher serum caspase-3 levels at day 1 than at day 4 (p=0.005) and at day 8 (p<0.001) of TBI, and that survivor patients showed higher serum caspase-3 levels at day 1 than at day 4 (p=0.005) but not at day 8 (p=0.15) of TBI.
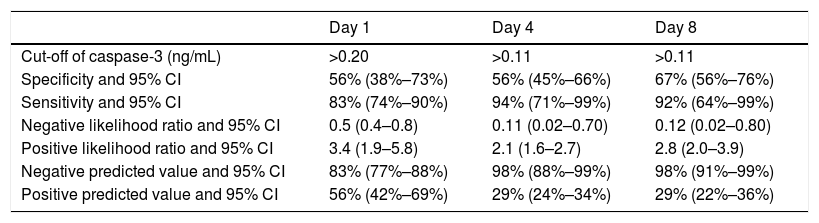
ROC curve analyses showed that serum caspase-3 concentrations at days 1, 4, and 8 of TBI had an AUC (95% CI) to predict 30-day mortality of 0.70 (0.61–0.78; p=0.001), 0.83 (0.74–0.89; p<0.001) and 0.87 (0.79–0.93; p<0.001) respectively (Fig. 2). Table 2 showed sensitivity and specificity, negative and positive likelihood ratios, and negative and positive predicted values of cut-offs of serum caspase-3 levels at day 1 (>0.20ng/mL), day 4 (>0.11ng/mL) and day 8 (>0.11ng/mL) for 30-day mortality prediction.
Thirty-day mortality prognostic capability of serum caspase-3 levels at day 1, 4 and 8 of trauma brain injury.
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
| Cut-off of caspase-3 (ng/mL) | >0.20 | >0.11 | >0.11 |
| Specificity and 95% CI | 56% (38%–73%) | 56% (45%–66%) | 67% (56%–76%) |
| Sensitivity and 95% CI | 83% (74%–90%) | 94% (71%–99%) | 92% (64%–99%) |
| Negative likelihood ratio and 95% CI | 0.5 (0.4–0.8) | 0.11 (0.02–0.70) | 0.12 (0.02–0.80) |
| Positive likelihood ratio and 95% CI | 3.4 (1.9–5.8) | 2.1 (1.6–2.7) | 2.8 (2.0–3.9) |
| Negative predicted value and 95% CI | 83% (77%–88%) | 98% (88%–99%) | 98% (91%–99%) |
| Positive predicted value and 95% CI | 56% (42%–69%) | 29% (24%–34%) | 29% (22%–36%) |
We found an AUC for 30-day mortality prediction from GCS of 0.75 (0.66–0.83), age of 0.74 (0.65–0.81) and APACHE-II of 0.86 (0.79–0.92). We did not find significant differences in the comparison of AUC for 30-day mortality prediction of serum caspase-3 levels with GCS (p=0.59) and with age (p=0.75); but the AUC was lower with serum caspase-3 levels that with APACHE-II (p=0.04).
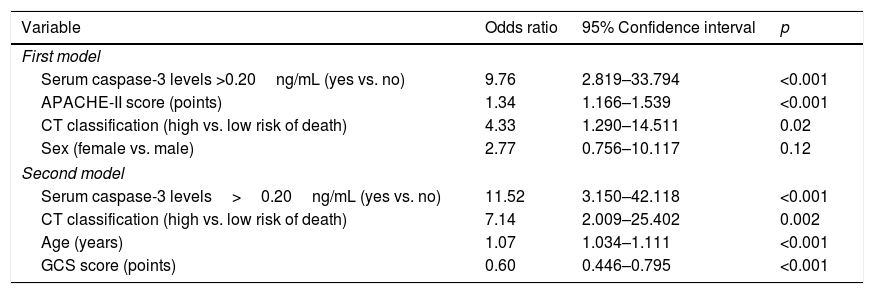
In multiple logistic regression we found an association between serum caspase-3 concentrations >20ng/mL and mortality controlling for sex, CT and APACHE-II (OR=9.76; 95% CI=2.819–33.794; p<0.001), and controlling for age, CT and CGS (OR=11.52; 95% CI=3.150–42.118; p<0.001) (Table 3). We found a mortality rate of 15.8% (6/38) in patients with CT on admission type 5, 16.7% (5/30) with type 2, 28.6% (6/21) with type 3, 40.9% (9/22) with type 4, and 61.5% (8/13) with type 6. Therefore, we recoded the patients according to CT findings in death low risk (including CT types 2 and 5) and death high risk (including CT types 3, 4 and 6), and that variable was included in logistic regression analyses.
Multiple logistic regression analysis to predict 30-day mortality.
| Variable | Odds ratio | 95% Confidence interval | p |
|---|---|---|---|
| First model | |||
| Serum caspase-3 levels >0.20ng/mL (yes vs. no) | 9.76 | 2.819–33.794 | <0.001 |
| APACHE-II score (points) | 1.34 | 1.166–1.539 | <0.001 |
| CT classification (high vs. low risk of death) | 4.33 | 1.290–14.511 | 0.02 |
| Sex (female vs. male) | 2.77 | 0.756–10.117 | 0.12 |
| Second model | |||
| Serum caspase-3 levels>0.20ng/mL (yes vs. no) | 11.52 | 3.150–42.118 | <0.001 |
| CT classification (high vs. low risk of death) | 7.14 | 2.009–25.402 | 0.002 |
| Age (years) | 1.07 | 1.034–1.111 | <0.001 |
| GCS score (points) | 0.60 | 0.446–0.795 | <0.001 |
APACHE II=Acute Physiology and Chronic Health Evaluation; CT=computed tomography; GCS=Glasgow Coma Scale.
The novel findings of our study were that non-surviving TBI patients showed higher serum caspase-3 levels during the first week of TBI than survivors, and that those levels could be used to predict 30-day mortality.
In a previous study by our team were determined serum caspase-3 levels on day 1 of TBI, and we found higher serum caspase-3 levels in non-surviving than in surviving patients.20 The novel aspect of the current study was that those levels have been not only measured on day 1 of TBI but also on days 4 and 8 of TBI, and we found that serum caspase-3 levels at different moments of the first week of TBI could be used as biomarkers for the prediction of 30-day mortality according the results of ROC analyses. However, we think that the findings of our study do not imply a causal relationship between serum caspase-3 levels and mortality.
We have not found differences in the capacity of mortality prediction between serum caspase-3 levels and age and GCS, and was higher with APACHE-II than with serum caspase-3 levels; however, the main objective of this study was to determine whether serum caspase-3 levels could play a role in the mortality prediction and not whether its potential capacity of mortality prediction is better than that of other biomarkers such as age, GCS and APACHE-II.31,32
We found in the bivariate analysis that women showed higher mortality rate that men; however, there we did not find an association between sex and mortality in multiple logistic regression. In a recent review has been found that the most of studies showed worse outcomes in females than males, in other better outcomes in females, and in other no sex differences. The authors concluded that sex differences in mitochondrial function might contribute to possible sex differences in TBI outcomes.33
Apoptotic cell death can occur mainly through the extrinsic or death receptor pathway (type I cells) and the intrinsic or mitochondrial pathway (type II cells).2–5 The intrinsic pathway is initiated in type II cells by action of oxygen free radicals and of cytokines such as interleukin (IL)-1 and IL-6; all of them releasing cytochrome c from mitochondria to cytoplasma and then activating caspase-3. The extrinsic pathway is initiated in type I cells by the action of tumor necrosis factor superfamily (TNFSF) that activates a surface death receptor of tumor necrosis factor receptor superfamily (TNFRSF), then a death signal is created and caspase-8 activates caspase-3. Therefore, intrinsic and extrinsic apoptosis pathways come together to caspase-3 which motivate death cell. We found that serum caspase-3 levels decreased from day 1 to day 4 and day 8 of TBI in non-survivor patients, and also from day 1 to day 4 in survivor patients. We think that this decreased in serum caspase-3 levels could be due to a decrease in apoptosis degree with the time.
We must to recognize as limitations of our study that we have not analyze brain apoptosis and levels of the potential activator agents of apoptosis pathways (such as TNFSF, IL-1, IL-6 or oxygen free radicals). Besides, we have registered some variables only on admission and not also the worst value, and we have not registered the patients excluded and the exclusion cause. Another limitation was that we did not establish a protocol for patient management and we cannot ensure that patients were treated homogeneously in all hospitals, in fact one of the hospitals has not neurosurgery and some of its patients had to move to other hospitals that do have it; however, these patients were excluded from the study and we do not find differences in the mortality rate between different hospitals (p=0.99). Another limitation was that the assay detection limit was 0.10ng/mL, the percentile 25 of serum caspase-3 levels in non-surviving and surviving patients was 0.10ng/mL, and the range of serum caspase-3 levels in non-surviving and surviving patients were 0.10–1.21 and 0.10–0.59, respectively. Besides, we have not validated our findings in other patients cohort.
The sample size of our study could be relatively small; however, we have found higher caspase-3 levels in non-surviving than in surviving patients in the three moments that were determined (days 1, 4 and 8 of TBI) and we believe that this consistence in the findings is a strength of our study. Thus, we think that those levels could help in the mortality prediction together with other markers (as age, GCS, APACHE-II) and that additional studies are needed to confirm our findings.
We think that the interest of our study lies in that is the first one reporting higher serum caspase-3 levels during the first week of TBI in non-survivor than in survivor TBI patients. Those results could originate more research to determine the role of serum caspase-3 levels for TBI mortality prediction. In addition, in TBI rat models have been found a reduction of caspase-3 activity and apoptosis in brain tissues with the administration of caspase-3 inhibitors.13–20 Thus, the results of our study and those in animal models could originate more research to explore the use of caspase-3 inhibitors in TBI patients.
ConclusionsThe novels findings of our study were that serum caspase-3 levels during the first week of TBI were lower in survivor patients and could predict 30-day mortality.
Author contributionsLL conceived, designed and coordinated the study, participated in acquisition of data, and drafted the manuscript.
MMM, MA, LR, JSV, JJC, VGM participated in acquisition of data and provided useful suggestions.
APC and AGR carried out the immunoassays and provided useful suggestions.
AJ interpreted the data and provided useful suggestions.
All authors read and approved the final version of the manuscript.
FundingsThis study was supported by a grant (OA18/011) from Fundación DISA a la Investigación Médica 2017 (Santa Cruz de Tenerife, Spain). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestsThe authors declare that they have no competing interests.
This study was supported by a grant (OA18/011) from Fundación DISA a la Investigación Médica 2017 (Santa Cruz de Tenerife. Spain). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.