Edited by: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Last update: December 2023
More infoPseudomonas aeruginosa is the microorganism most frequently involved in the main ICU-acquired infections, with special importance in ventilator associated pneumonia. Its importance lies, in addition to its high incidence in critically ill patients, in the severity of the infections it causes and in the difficulty of its antimicrobial treatment, directly related to the high percentage of resistance to antibiotics classically considered first-line. New active antibiotics have recently been developed against Pseudomonas aeruginosa, even against multi-drug resistant strains. This review analyzes both the differential characteristics of Pseudomonas aeruginosa infections and the new therapeutic options, focusing on multi-drug resistant Pseudomonas aeruginosa.
Pseudomonas aeruginosa es el microorganismo que participa con mayor frecuencia en las principales infecciones adquiridas en la UCI, con especial importancia en la neumonía asociada a ventilación mecánica. Su importancia radica, además de en su elevada incidencia en el paciente crítico, en la gravedad de las infecciones que causa y en la dificultad de su tratamiento antimicrobiano, directamente relacionada con el elevado porcentaje de resistencias a los antibióticos considerados clásicamente de primera línea. Recientemente se han desarrollado nuevos antibióticos activos frente a Pseudomonas aeruginosa, incluso frente a cepas multirresistentes. La presente revisión analiza tanto las características diferenciales de las infecciones por Pseudomonas aeruginosa como las nuevas opciones terapéuticas, centrando el foco en la Pseudomonas aeruginosa multirresistente.
Pseudomonas aeruginosa (PA) is intrinsically resistant to various families of antibiotics, and moreover can acquire resistance to practically any antibiotic. On occasion of a meeting of experts of the World Health Organization (WHO) held in Geneva in 2017, a list of priorities in the investigation of certain pathogens was established. On this list, characterized by three levels of priority, the highest-ranking position included carbapenem-resistant PA1.
EpidemiologyPseudomonas aeruginosa is a motile, gram-negative aerobic bacterium that does not ferment lactose. It is widely distributed both in and out of hospitals, and is often found in water or contaminating aqueous zones. Pseudomonas aeruginosa is an opportunistic pathogen that can cause infection in patients with altered defense mechanisms. Thus, it is a very frequent pathogen in lung infections among patients with cystic fibrosis. In addition, regarding serious infections or in critically ill patients, PA is one of the most common etiological agents in nosocomial pneumonia (including particularly ventilator-associated pneumonia [VAP]), bacteremia and bladder catheter-related urinary tract infection (BC-UTI).
According to the ENVIN report of 20202, PA with a prevalence of 16.2% was the pathogen most frequently isolated among the main infections in the Intensive Care Unit (ICU). Pseudomonas aeruginosa was the leading causal pathogen of VAP in Spain in 2020, representing over 30% of all cases2.
When speaking of PA, it is very important to consider the episodes caused by multi-drug resistant (MDR) strains. According to the definitions derived from the consensus between the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC)3, multi-drug resistant PA (PA-MDR) can be defined as a strain that is not sensitive to at least one antimicrobial agent in three or more groups of antimicrobials with activity against PA. In turn, extensively drug-resistant PA (PA-XDR) is defined as a strain that is not sensitive to some antimicrobial in all but ≤ 2 drug groups. On the other hand, pan-drug resistant PA (PA-PDR) is defined as a strain that is not sensitive to any antimicrobial agent. We use the term not sensitive because it conceptually encompasses the terms “resistant” and “intermediate”. The concept of “difficult to treat resistance” PA (PA-DTR) has also been proposed and includes those PA strains not sensitive to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin and levofloxacin4.
The ENVIN report of 2020 described 255 episodes of colonization/infection due to PA-MDR, of which approximately 25% were detected at the time of admission to the ICU2. It should be noted that in hospital wards, resistance to betalactams – including carbapenems – is only 5–9 percentage points lower than in the ICU5. Among patients admitted to the ICU with non-ventilator-associated pneumonia due to PA, up to one-half of the episodes can be caused by multi-drug resistant strains6.
Resistances and in vitro activity of the different antimicrobials against PAAs has been mentioned, antimicrobial resistance is one of the most relevant aspects of infections due to PA. The resistance mechanisms of the pathogen can be classified as intrinsic, acquired and adaptive.
Intrinsic resistance is due to the expression of efflux pumps that expel antibiotics from the cell; the production of enzymes that inactivate or hydrolyze antibiotics (such as extended-spectrum betalactamases [ESBLs], AmpC or carbapenemases); and low permeability of the outer membrane (a particularly relevant factor in this respect being down-regulation of the OprD outer membrane protein, which reduces membrane permeability to certain antibiotics, and is usually responsible for resistance to carbapenem).
Resistance in turn may be acquired via both horizontal transference (through plasmids, transposons, integrons and prophages of the same or different bacterial species) and mutational changes that can offer advantages for the bacterium, such as reduction of antimicrobial uptake, modification of the antibiotic target, over-expression of efflux pumps, or the appearance of enzymes that inactivate the antibiotic.
Adaptive resistance increases the capacity of the bacterium to survive in response to an environmental stimulus and which is reversible once the stimulus ceases. In the case of PA, adaptive resistance involves the formation of biofilm7,8.
A study published in 2019 analyzed a sample of 1445 PA isolates from healthcare-related infections in 51 Spanish hospitals. The study detected incidences of 26.2% for PA-MDR, 17.3% for PA-XDR and 0.1% for PA-PDR, with higher percentages in strains isolated in the ICU (41.4%, 28.4% and 1.6%, respectively). In 3.1% of the analyzed PA strains (16.7% of the PA-XDR strains), the underlying resistance mechanism was ESBL/carbapenemase, including VIM (1.9%), IMP (0.3%), GES (0.6%), PER (0.2%) and OXA (0.1%), and up to 65% of the XDR strains exhibited a resistance profile suggestive of AmpC hyperproduction. Other non-enzymatic resistance mechanisms were also identified: over 60% of the strains presented alterations in OprD, and mutations of the efflux pumps were also frequent (56 strains). In addition to resistance to betalactams, 56% of the PA-XDR strains had acquired aminoglycoside-modifying enzymes implicated in resistance to tobramycin and other aminoglycosides, and 75% presented a mutation of GyrA, responsible for resistance to ciprofloxacin. This study highlighted the importance of knowing the local microbiology, since it detected significant geographical variations9.
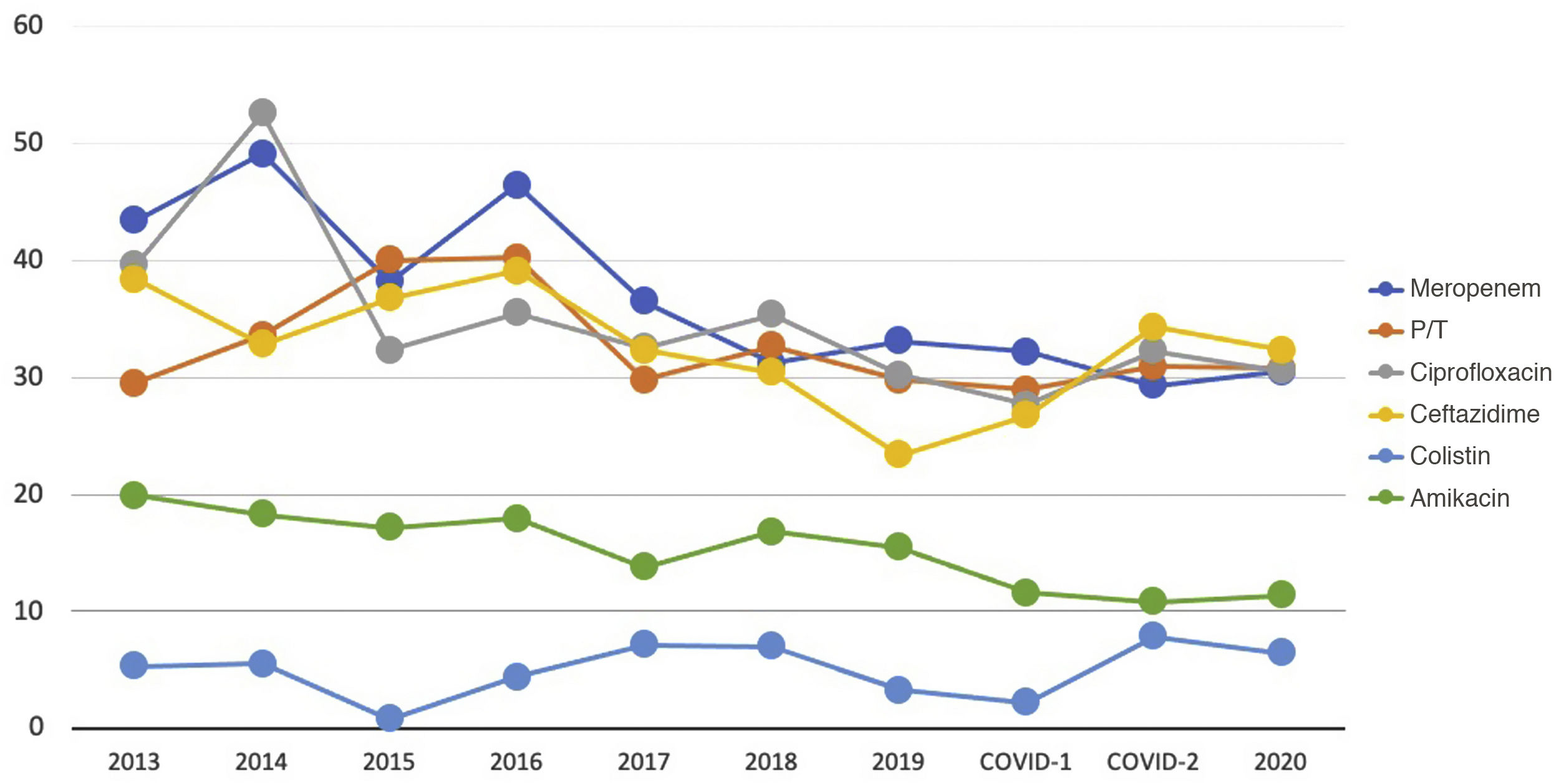
Considering the infections acquired in Spanish ICUs, the latest ENVIN reports indicate that approximately 30% of all PA strains are resistant to carbapenems, piperacillin-tazobactam, ceftazidime and quinolones, and the only antibiotics with a lower resistance rate (close to 10%) are the aminoglycosides and colistin (Fig. 1)2.
The International Nosocomial Infection Control Consortium (INICC) has reported the data on PA resistance to the main antibiotics in medical device-related infections. In VAP, the resistance rates referred to carbapenems, quinolones, piperacillin-tazobactam and cefepime were found to be 39.4%, 34.6%, 39.2% and 40.5%, respectively; in BC-UTI the figures were 39.3%, 40.2%, 38.2% and 48.1% (in the same order); and in central venous catheter-associated bacteremia (CAB) the figures were 43.5%, 20%, 33% and 41.7%. Only amikacin maintained resistance rates of under 30%: 24.7% in VAP, 26.8% in BC-UTI and 21.4% in CAB10.
It should be mentioned that to date, neither the aforementioned study nor the ENVIN reports include data referred to the new antibiotics.
A European study has reported that 99.5% of all PA strains are sensitive to colistin, 95.5% to ceftazidime/avibactam, 94.3% to imipenem-relebactam, 93.3% to ceftolozane-tazobactam, and 88.7% to meropenem/vaborbactam11.
In the same line, it has been seen that ceftolozane-tazobactam and ceftazidime-avibactam maintain sensitivity rates of 73.4% and 71.0%, respectively, in PA-XDR, and of 84.3% and 83.3% in PA not sensitive to carbapenems. Only 3.9% of the analyzed PA strains were resistant to both antibiotics, generally due to the presence of carbapenemases, but also to the presence of mutant AmpC9,12–15.
Other publications have reported somewhat different results. A study of strains isolated between 2013 and 2018 in 24 European countries found 99.7% of the PA strains to be sensitive to cefiderocol - including 97.5% of those resistant to carbapenems16. The results of the analysis of the strains from Spain included in this study showed 99.2% of the PA strains to be sensitive to cefiderocol – the figure decreasing to 96% in the case of PA resistant to carbapenems. In this series, only 40% and 50% of the PA strains resistant to carbapenems were sensitive to ceftazidime-avibactam and ceftolozane-tazobactam, respectively, and only colistin came close to the results of cefiderocol, with 94% of the PA strains being sensitive17. Similar data were obtained in another study that analyzed PA resistant to carbapenems detected in 44 Spanish hospitals: up to 30.6% were carbapenemase producers - fundamentally type VIM metallobetalactamases - and 26.7% were sensitive only to colistin18.
It has been seen that mutations in the AmpC-AmpR region, which are associated with the development of resistances to ceftolozane-tazobactam and ceftazidime-avibactam during treatment, also reduce the activity of cefiderocol and, in contrast can increase sensitivity to imipenem-relebactam19.
In addition to the above, the in vitro activity of imipenem-relebactam against PA without carbapenemases is up to 5-fold greater than that of imipenem alone, with high activity against the presence of an AmpC type betalactamases associated with impermeability and also against KPC-producing strains20,21. On adding relebactam, the data change from 2% of the PA strains resistant to carbapenems (non-carbapenemase producers) and sensitive to imipenem, to 63% of the strains sensitive to imipenem-relebactam. It should be noted that 98% and 80% of this PA group are moreover sensitive to colistin and ceftolozane-tazobactam, respectively22. A Spanish study described imipenem-relebactam as the antibiotic with the highest percentage of sensitive PA strains (97.3%)23.
Regarding the other carbapenem with betalactamase inhibitor currently available, meropenem-vaborbactam is active against 82.1% of all PA strains (versus 67.3% with meropenem alone). Even more relevantly, it is active against 41.0% of all PA-MDR strains (versus 13.0% in the case of meropenem alone) recovered from pneumonias (including VAP) in Europe24.
Sader et al.11,25 have published two studies on the in vitro activity of the new betalactams associated with new betalactamase inhibitors (ceftazidime-avibactam, ceftolozane-tazobactam, imipenem-relebactam and meropenem-vaborbactam) against PA strains recovered from patients with pneumonia and skin and soft tissue infections. Of note is the observation that in isolates resistant to some antibiotic without a betalactamase inhibitor, the addition of an inhibitor increased the percentage of sensitive strains: 78.7–87.5% of the isolates resistant to imipenem were sensitive to the combination imipenem-relebactam, and 79.8-87.5% of the cases resistant to ceftazidime became sensitive on adding avibactam. In the case of meropenem, the addition of vaborbactam caused 61.1% of the strains to become sensitive with a minimum inhibitory concentration (MIC)>2mg/l and 5.7% with MIC>8mg/l.
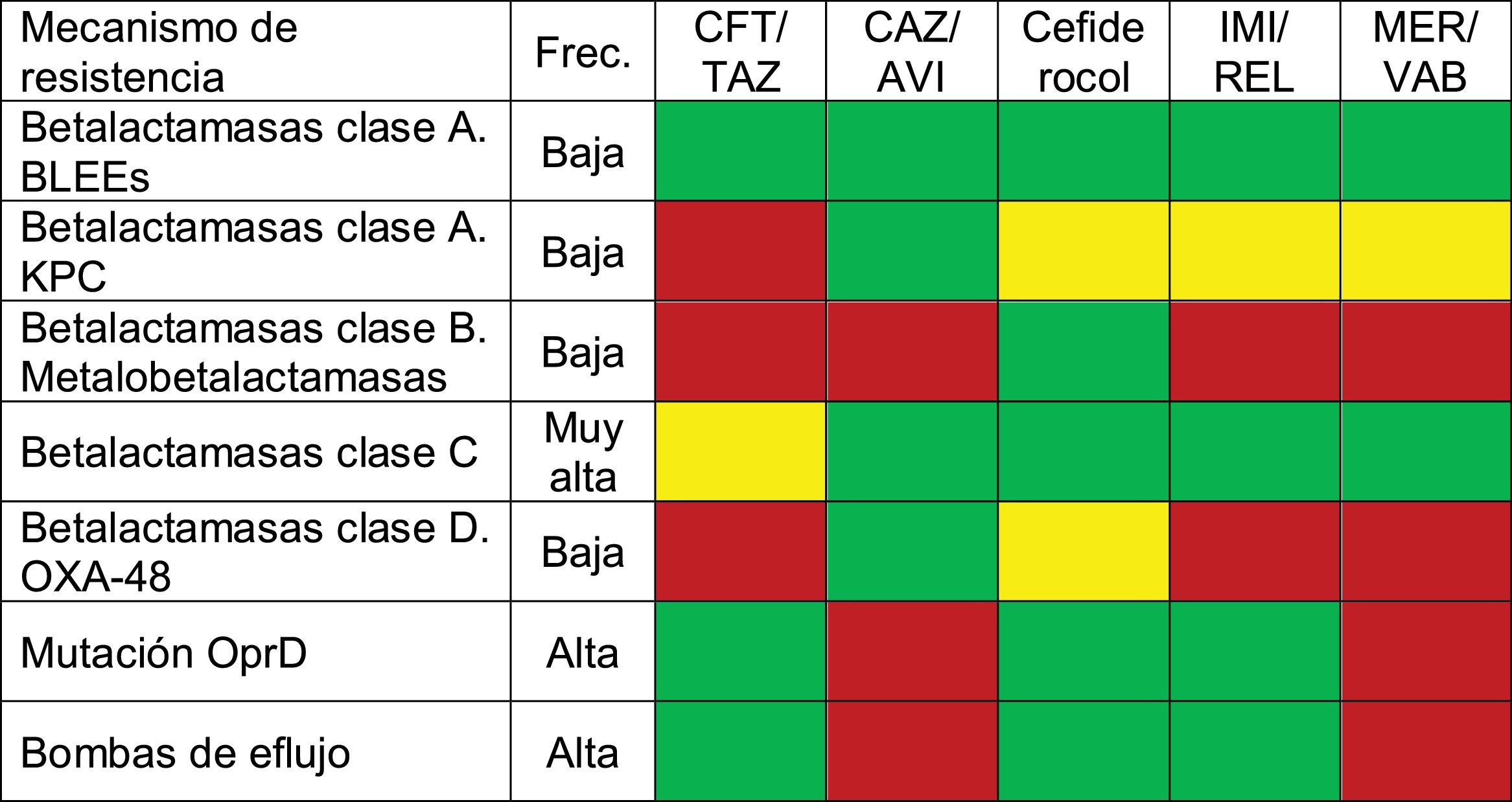
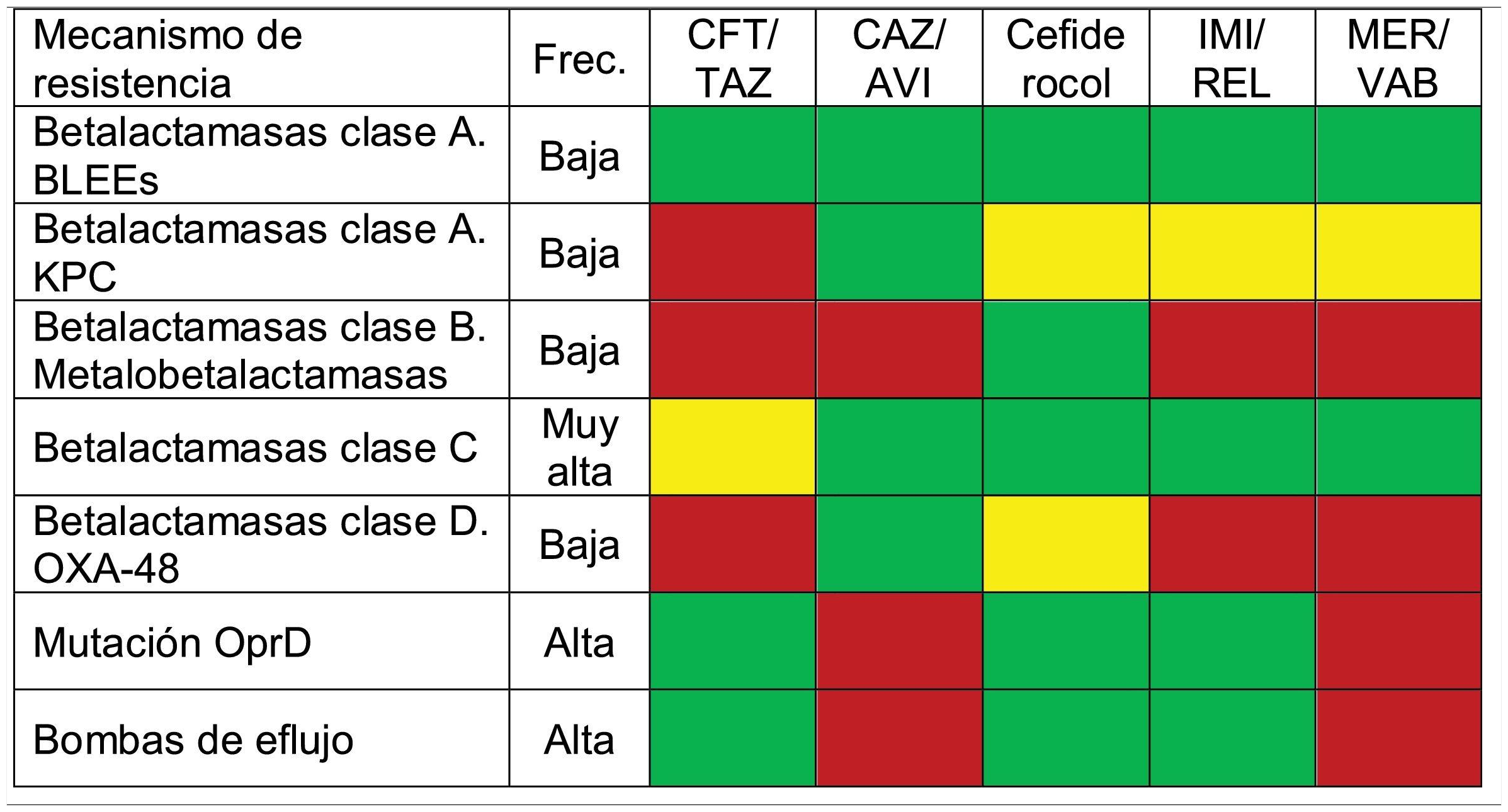
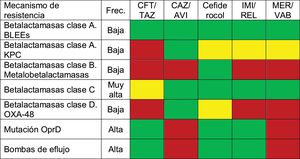
Table 1 summarizes the activity of the new antibiotics against the different mechanisms of resistance of PA.
Activity of new antibiotics against the different resistance mechanisms of PA.
ESBL: Extended-spectrum betalactamase-producing Enterobacteriaceae; CAZ/AVI: ceftazidime-avibactam; CFT/TAZ: ceftolozane-tazobactam; Freq: frequency; IMI/REL: imipenem-relebactam; MER/VAB: meropenem-vaborbactam. Color codes: red indicates no activity against that resistance mechanism; green corresponds to antibiotics with activity not exceeded by any other drug against that resistance mechanism (various antibiotics may be involved, which would be regarded as equivalent); and yellow indicates those antibiotics with activity against the resistance mechanism, but with the availability of other drugs of greater activity.
The classical risk factors for PA infection are structural lung disease (cystic fibrosis, bronchiectasis), hematological neoplasms (especially in the presence of neutropenia), solid organ transplants, major burns, antibiotic treatment in the previous 90 days, the presence of a venous catheter or bladder catheter, prolonged hospitalization and mechanical ventilation26.
It seems to be of greater interest (and difficulty) to know those situations in which PA may be PA-MDR. In this regard, a score has been proposed for predicting infection due to carbapenem-resistant PA that includes the following variables: patient residency in a sociosanitary care center, tracheostomy carrier status, infection due to carbapenem-resistant PA in the 30 days prior to admission, hospitalization in the last 6 months, and treatment with carbapenems, cephalosporins or quinolones in the last 30 days. The area under the receiver operating characteristic (ROC) curve of this score is 0.81. The same authors have proposed another score to identify infections produced by PA strains resistant to carbapenems, ceftazidime and piperacillin-tazobactam, involving the following variables: tracheostomy or central venous catheter carrier status, infection due to carbapenem-resistant PA in the 30 days prior to admission, hospitalization in the last 6 months, and treatment with carbapenems in the 30 days before the date of infection - with an area under the ROC curve of 0.8227.
Other identified risk factors are: age, presence of a bladder catheter, parenteral nutrition, previous antibiotherapy, corticosteroid treatment, severity of the episode, comorbidities (Charlson index ≥ 3), nosocomial origin or hematological neoplasm28.
A systematic review identified up to 38 different risk factors for infection due to carbapenem-resistant PA. These factors were subsequently classified by a group of experts according to their importance and relevance in our setting. Those classified as important are related to previous contact with the hospital (prior hospitalization or days of stay until infection) and with previous antibiotherapy (carbapenems, aminoglycosides, cephalosporins, quinolones or aminoglycosides). Admission to the ICU, severity measured by the SAPS II or APACHE scores, and the presence of invasive devices were regarded as moderately important risk factors29.
In general, the risk factors or scores offer high sensitivity but low specificity (and consequently high negative predictive value but low positive predictive value). It is therefore essential to know the local microbiology and resistance profile.
Infections due to PAPseudomonas aeruginosa causes acute infections in hospitalized patients and in immune-depressed individuals, as well as chronic infections in patients with structural lung damage such as cystic fibrosis or bronchiectasis26.
Considering hospital infections, an international study has reported PA to cause 23% of all infections acquired in the ICU30.
In Spain, and in accordance with the successive ENVIN reports, PA is the leading cause of VAP, and its impact has moreover increased as a result of the COVID-19 pandemic. On the other hand, PA is one of the most frequent causes of bladder catheter-related urinary tract infection (accounting for 10–15% of all cases) and of CAB (approximately 5%)2. This situation is similar to that found in other countries in our setting. According to data from the United States National Healthcare Safety Network (NHSN), PA was the second most frequent causal agent of VAP (16.5%), exceeded only by Staphylococcus aureus31, and caused 10.3% of all nosocomial BC-UTI (surpassed only by Escherichia coli and Candida albicans) and 4% of the global cases of CAB31.
The relevance of nosocomial pneumonia in patients admitted to the ICU but not subjected to ventilation (ICU-NVAP) has recently been demonstrated. Giuliano et al.32 described an incidence of ICU-NVAP of 1.6% (3.63 episodes per 1000 patient-days), and PA may constitute the first pathogen in order of frequency – causing up to one-third of all cases6.
Furthermore, PA is the gram-negative bacterium most often implicated in burn patient wound infections33.
Regarding community-acquired infections, PA plays an important role in lung infections among patients with chronic structural disease such as cystic fibrosis and bronchiectasis. In Europe, the prevalence of PA in patients with cystic fibrosis over 18 years of age is estimated to be between 40–53%34. Likewise, PA often colonizes the lungs of individuals with bronchiectasis not related to cystic fibrosis, and moreover increases the risk of exacerbation35.
Another special risk group is represented by immune-depressed patients, particularly those with neutropenia: up to 11–13% of all cases of sepsis in cancer patients are caused by PA36, and the pathogen is also frequently found in solid organ transplant recipients.
Prognosis of infections due to PA-MDRCompared with infections produced by other bacteria, PA is known to cause greater mortality (odds ratio [OR]: 1.435; 95% confidence interval [95%CI]: 1.043–1.933)37 in both the general population and more markedly in neutropenic patients with hematological disorders (OR: 36.07; 95%CI: 9.36–138.9)38.
One of the most influencing factors is inappropriate antibiotic treatment39, particularly in infections produced by multi-drug resistant pathogens40. A study involving 78hospitals in the United States evaluated the excess mortality and costs41 related to PA infections in which PA-MDR was the causal pathogen. The excess mortality was found to be 20%, and the excess cost totaled $ 20,000. In turn, a meta-analysis published in 2016 determined an OR of 3.07 (95%CI: 1.60–5.89) for mortality due to carbapenem-resistant PA42. However, Tumbarello et al.43 reported that the presence of PA-MDR in both pneumonia and in bacteremia is not associated with increased mortality. These authors found inappropriate antibiotic treatment to be an independent predictor of mortality.
Treatment of infections due to PA-MDRTable 2 shows the main antimicrobials with activity against PA.
Dosing specifications of antibiotics active against Pseudomonas aeruginosa.
| Antibiotic | Dosage |
|---|---|
| Amikacin | 20mg/kg iv and adjustment according to levels |
| Cefiderocol | 2g/8h iv, infused in 3h |
| Ceftazidime/avibactam | 2.5g/8h iv, infused in 3h |
| Ceftolozane/tazobactam | 3g/8h iv, infused in 3h |
| Ciprofloxacin | 400mg/8h iv |
| Colistin | Starting dose: 9 MU colistimethate (300mg of colistin base) iv, infused in 0.5−1h• Normal renal function: 4.5 MU iv/12h, infused in 0.5−1 h |
| Imipenem-cilastatin | 500mg/6h iv, infused in 30 minutes |
| Imipenem-cilastatin-relebactam | 1.25g/6h iv, infused in 30 minutes |
| Levofloxacin | 750mg iv every 24h |
| Meropenem | 2g/8h iv, infused in 3h |
| Meropenem-vaborbactam | 4 g/8h iv, infused in 3h |
| Plazomicin | 15mg/kg and adjustment according to levels |
| Tobramycin | 7 mg/kg iv and adjustment according to levels |
In the event of PA infection, it is of great help to know the sensitivity profile of the pathogen, and in some cases the classical antibiotics can be used. The situation is more complicated when it comes to starting treatment in a patient who has already received previous antibiotic therapy. It must be taken into account that when a PA strain is resistant to a given anti-Pseudomonas drug, it is normally also resistant to some additional drugs. For example, patients who have received ceftazidime will more often present cross-resistance to piperacillin-tazobactam, cefepime or aztreonam, and the use of imipenem or ceftolozane-tazobactam a priori would be a better option44. Lob et al.5 have reported that among the isolates resistant to piperacillin-tazobactam, barely 20% are sensitive to ceftazidime, and only about 40% are sensitive to imipenem or meropenem.
Several drugs have been developed in recent years. Some are new molecules, while others are combinations of existing drugs with betalactamase inhibitors, such as cefepime-tazobactam, cefepime-enmetazobactam, cefepime-zidebactam, aztreonam-avibactam, meropenem-nacubactam, cefepime-taniborbactam or plazomicin45. Some of these drugs will soon become available, though we will focus this review on some of the currently existing options for treating serious infections due to PA-MDR.
Ceftolozane-tazobactamThis drug combines a new cephalosporin with an already known betalactamase inhibitor. It exhibits activity against extended-spectrum betalactamase (ESBL) producing Enterobacteriaceae, PA (including extremely resistant strains), and some species of Streptococcus46. Ceftolozane-tazobactam has been approved for the treatment of complicated intraabdominal infections46 and complicated urinary infections, with a dosage of 1.5g/8h47, and for the treatment of nosocomial pneumonia - including VAP - with a dosage of 3g/8h48.
A randomized clinical trial (RCT) on abdominal infection isolated PA in 72 patients (8.9% of the cases), with 9 isolates that were not sensitive to three or more classes of anti-Pseudomonas drugs. The sensitivity of the PA isolates was 98.6% for ceftolozane-tazobactam and 89.9% for meropenem46. In complicated urinary tract infection (UTI), the PA eradication rate was greater with ceftolozane-tazobactam than with levofloxacin47. In the RCT published by Kollef et al.48, which assessed the non-inferiority of ceftolozane-tazobactam versus meropenem in nosocomial pneumonia, the presence of PA was detected in 128 (17.6%) of the episodes, and in 50 (39%) of them the strains corresponded to MDR or XDR. Los results of ceftolozane-tazobactam against PA in the RCT and in the main observational studies are described in Table 3.
Results of ceftolozane-tazobactam against Pseudomonas aeruginosa.
| Study | Infection | C | Microbiological eradication | Clinical cure | Mortality | |||
|---|---|---|---|---|---|---|---|---|
| CFT/TAZ | C | CFTA/TAZ | C | CFT/TAZ | C | |||
| ASPECT-cUTI47 (RCT) | Complicated UTI | Levofloxacin | 85.7% | 58.3% | ||||
| ASPECT-CIAI46 (RCT) | Complicated abdominal infection | Meropenem | 100% | 93.1% | ||||
| ASPECT-NP48 (RCT) | Nosocomial pneumonia | Meropenem | 57.1% | 60.0% | 25.4% | 18.5% | ||
| Pogue84 (Obs) | PA-MDR or PA-XDR | Regimens based on aminoglycoside or colistin | 81% | 61% | 20% | 25% | ||
| Almangour85 (Obs) | PA-MDR | Colistin | 77% | 57% | 39% | 49% | ||
| Pinilla-Rello86 (Obs) | PA-MDR and PA-XDR | Aminoglycosides/colistin | 67.4% | 68% | 67.4% | 68.0% | 32.6% | 26.6% |
| Balandin87 (Obs) | PA in ICU. 48.4% MDR, 84.2% R to carbapenem | 42.1% | 71.6% | 36.5% | ||||
| Gallagher88 (Obs) | PA-MDR | 70.7% | 73.7% | 19% | ||||
| Haidar89 (Obs) | PA-MDR | 71% | 10% | |||||
| Munita90 (Obs) | PA resistant to carbapenem | 74% | ||||||
| Bassetti91 (Obs) | 83.2% | |||||||
| Díaz-Cañestro92 (Obs) | 63.8% | 27.6% | ||||||
C: comparator; CFT/TAZ: ceftolozane-tazobactam; RCT: randomized clinical trial; UTI: urinary tract infection; Obs: observational study; PA-MDR: multi-drug resistant Pseudomonas aeruginosa; PA-XDR: extensively drug-resistant Pseudomonas aeruginosa.
Treatment with 2g of ceftolozane and 1g of tazobactam would reach the target pK/pD (time during which the concentration of the free fraction of ceftolozane exceeds MIC [fT>MIC], which should be 100% in the critical patient) in over 90% of the PA strains sensitive to the drug (MIC≤4mg/l). On optimizing administration through extended infusion (4h), a probability of target attainment (PTA)>90% would be reached in strains with MIC up to 6mg/l, and administration as a continuous infusion would allow PTA>90% even in strains with MIC 8mg/l and for a target of 100% fT > 4 x MIC (this at least in theory allowing the avoidance of resistant strain selection)49.
A meta-analysis comparing ceftolozane-tazobactam in monotherapy versus combination treatment (with a great variety of antibiotics, including quinolones, aminoglycosides, colistin, carbapenems and other betalactams) detected an association between combination treatment and a decrease in mortality (OR: 0.31; 95%CI: 0.10–0.97)50.
Ceftazidime-avibactamIn this case, the combination involves a known cephalosporin with avibactam, which is a non-betalactam betalactamase inhibitor. Avibactam inactivates class A and class C betalactamases, and some class D betalactamases (such as OXA-48), but not the metallobetalactamases.
Ceftazidime-avibactam is indicated for the treatment of complicated intraabdominal infections51, complicated UTI, including pyelonephritis52 and nosocomial pneumonia - including VAP53. In all three scenarios, the studies establishing the indication of ceftazidime-avibactam were non-inferiority trials with a comparison against a carbapenem. Los results referred to ceftazidime-avibactam against PA in the RCT and in the main observational studies are described in Table 4.
Results of ceftazidime-avibactam against Pseudomonas aeruginosa.
| Study | Infection | C | Microbiological eradication | Clinical cure | Mortality | |||
|---|---|---|---|---|---|---|---|---|
| CAZ/AVI | C | CAZ/AVI | C | CAZ/AVI | C | |||
| Mazuski51 (RCT) | Complicated abdominal infection | Meropenem | 90.6% | 94.4% | ||||
| RECAPTURE52 (RCT) | Complicated UTI | Doripenem | 66.7% | 75.0% | ||||
| REPRISE93 (RCT) | Complicated UTI and AI | Best available therapy | 79% | 60% | 86% | 100% | ||
| REPROVE53 (RCT) | Nosocomial pneumonia | Meropenem | 42.0% | 40.0% | 64.3% | 77.1% | ||
| Corbella60 (Obs) | PA-MDR and PA-XDR | 54.1% | 13.1 | |||||
| Vena59 (Obs) | PA resistant to carbapenem | 87.8% | ||||||
| Jorgensen94 (Obs) | PA-MDR | 69.8% | 17.5% | |||||
| Rodríguez-Núñez95 (Obs) | PA-MDR and PA-XDR | 50% | 13 and 38% at 30 and 90 days | |||||
C: comparator; CAZ/AVI: ceftazidime-avibactam; RCT: randomized clinical trial; AI: abdominal infection; UTI: urinary tract infection; Obs: observational study; PA-MDR: multi-drug resistant Pseudomonas aeruginosa; PA-XDR: extensively drug-resistant Pseudomonas aeruginosa.
The data published to date regarding optimization according to pK/pD parameters of ceftazidime-avibactam in application to PA are limited54. The standard dose (2g of ceftazidime, 0.5g of avibactam, every 8h) makes it possible to reach PTA>90%, taking as target 100% fT>MIC, in PA sensitive to the antibiotic (MIC≤4mg/l), though it would be necessary to increase the dose in the case of higher MIC values, with practically no benefit from extended infusion (beyond two hours) or continuous infusion55.
Despite the in vitro evidence of synergy with antibiotics such as meropenem, aztreonam, amikacin, colistin56,57 and fosfomycin58, the available clinical data indicate no benefit of combination treatment for PA6,59°. Nevertheless, in general, most of the patients included in real-life studies on the treatment of PA with ceftazidime-avibactam have received combination treatment61,62.
CefiderocolCefiderocol is a new siderophore cephalosporin with wide activity against gram-negative aerobic pathogens, including MDR strains. Cefiderocol takes advantage of an ion transport mechanism and, after binding to free iron, penetrates the bacteria. The drug is active against enterobacteria, including ESBL producers, PA, Acinetobacter baumannii, Stenotrophomonas maltophilia and other gram-negative aerobic bacteria, including strains sensitive to and resistant to carbapenems.
Cefiderocol is indicated for the treatment of complicated UTI63, serious infections due to gram-negative aerobic bacteria resistant to carbapenem64, and nosocomial pneumonia - including VAP and healthcare-related pneumonia65. Studies have been made comparing the drug with carbapenem63,65 or with the best available treatment at the time of the study in the case of infections due to pathogens resistant to carbapenems64. The results of cefiderocol against PA in these RCTs and in an observational study are shown in Table 5.
Results of cefiderocol, imipenem-relebactam and meropenem-vaborbactam against Pseudomonas aeruginosa.
| Study | Infection | C | Microbiological eradication | Clinical cure | Mortality | |||
|---|---|---|---|---|---|---|---|---|
| Cefiderocol | C | Cefiderocol | C | Cefiderocol | C | |||
| Portsmouth63 (RCT) | Complicated UTI | Imipenem-cilastatin | Clinical and microbiological resolution | |||||
| Cefiderocol 47% | ||||||||
| C: 50% | ||||||||
| APEKS-NP65 (RCT) | Nosocomial pneumonia | Meropenem | 38% | 46% | 67% | 71% | 8% | 13% |
| CREDIBLE64 (RCT) | PA resistant to carbapenem | Best possible treatment | 8% | 20% | 58% | 50% | 35%Without A: 18% | 17%Without A: 18% |
| Meschiari67 | [0.2-3]PA-DTR | [0.4-5]76.5% | [0.6-7]70.6% | [0.8-9]35.3% | ||||
| Imipenem-relebactam | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infection | C | Microbiological eradication | Clinical cure | Mortality | |||
| IMP/REL | C | IMP/REL | C | IMP/REL | C | |||
| RESTORE-IMI 170 (RCT) | PA resistant to imipenem | Imipenem+colistin | 81% | 63% | ||||
| RESTORE-IMI 271 (RCT) | Nosocomial pneumonia | Piperacillin/tazobactam | 46.7% | 68% | 33.3% | 12.0% | ||
| Rebold72 (Obs) | 16 infections due to PA (94% PA-MDR) | 68.7% | 18.7% | |||||
| Meropenem-vaborbactam | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infection | C | Microbiological eradication | Clinical cure | Mortality | |||
| MER/VAB | C | MER/VAB | C | MER/VAB | C | |||
| Alosaimy76 (Obs) | 8 infections due to PA | 12.5% | ||||||
C: comparator; IMP/REL: imipenem-relebactam; MER/VAB: meropenem-vaborbactam; RCT: randomized clinical trial; UTI: urinary tract infection; Obs: observational study; PA: Pseudomonas aeruginosa; PA-MDR: multi-drug resistant Pseudomonas aeruginosa; PA-DTR: difficult to treat resistance Pseudomonas aeruginosa.
The administration of cefiderocol in a three-hour extended infusion is advised, based on studies in animal models, where this treatment regimen has been seen to afford a greater decrease in bacterial burden66.
It is common to combine cefiderocol with other antibiotics, though no studies on the efficacy of this strategy are available67.
Imipenem-cilastatin-relebactamRelebactam is a new betalactamase inhibitor with activity against class A and class C betalactamases68. In an enzyme study, Young et al.68 found that the addition of relebactam to imipenem-cilastatin allows the recovery of activity against PA resistant to imipenem, with an up to an 8-fold decrease in MIC. Lob et al.69 showed that the addition of relebactam restored sensitivity in 80% of the isolates of PA not sensitive to imipenem. The combination of imipenem-cilastatin with relebactam also restores activity against KPC and other carbapenemase-producing enterobacteria and PA, including those that produce or over-express betalactamases, loss of porins and efflux pumps70. Imipenem-cilastatin-relebactam is not active against class B and class D carbapenemases68.
The indications of imipenem-cilastatin-relebactam approved by the European Medicines Agency (EMA) comprise nosocomial pneumonia (including VAP), bacteremia occurring in association or suspected association with nosocomial pneumonia, and in the treatment of infections produced by gram-negative microorganisms in adults with few treatment options70,71. Table 4 summarizes the results of cefiderocol against PA in RCTs and observational studies.
An observational study has reported the use of imipenem-relebactam in combination in approximately 30% of the cases72.
Meropenem-vaborbactamMeropenem-vaborbactam is indicated in UTI (including pyelonephritis), complicated intraabdominal infections and nosocomial pneumonia - including VAP. The addition of vaborbactam restores activity against some betalactamase-producing gram-negative bacteria, particularly carbapenemase-producing K. pneumoniae isolates73, though as commented above, it also increases the percentage sensitivity of PA. Neither of the two pivotal RCTs on meropenem-vaborbactam include infections due to PA74,75, and the published experience is very limited76 (Table 4).
Treatment recommendationsReputed scientific societies and recognized experts have published recommendations on the treatment of infections caused by multi-drug resistant gram-negative bacteria, including PA4,77,78.
It should be taken into account that few studies have established comparisons among the antibiotics with activity against PA-MDR. Furthermore, none have directly compared the new antimicrobials, and caution is required when comparing the results of different studies79.
The Infectious Disease Society of America (IDSA) has established different recommendations according to the disease focus involved, proposing ceftolozane-tazobactam, ceftazidime-avibactam and imipenem-cilastatin-relebactam as first-line treatment in monotherapy for infections due to difficult to treat resistance PA (PA-DTR) outside the urinary tract, with cefiderocol as an alternative. The recommendations contemplate combination treatment in situations where none of the three first-line antibiotics prove active in vitro, and suggest the use of an aminoglycoside if active, or colistin if not active, combined with the betalactam affording the MIC closest to the sensitivity threshold4.
In turn, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), in guides endorsed by the European Society of Intensive Care Medicine (ESICM), describes ceftolozane-tazobactam as the treatment of choice for serious infections caused by carbapenem-resistant PA (conditional recommendation, with a very low level of evidence). With the same grading, combination treatment is recommended in serious infections where it proves necessary to use colistin, aminoglycosides or fosfomycin, without establishing recommendations on combination treatment in the case of prescribing ceftolozane-tazobactam, ceftazidime-avibactam or cefiderocol, due to a lack of evidence77.
Bassetti and Garau78 proposed a treatment scheme for carbapenem-resistant PA starting with ceftolozane-tazobactam, ceftazidime-avibactam and cefiderocol combined with colistin/aminoglycosides / fosfomycin as first options and, in the case of resistance to avibactam, relebactam, vaborbactam or ceftolozane-tazobactam, they proposed a regimen based on colistin or aminoglycosides combined with a carbapenem and/or fosfomycin and/or rifampicin.
Treatment in combination regimens or monotherapy is a controversial issue in which the available evidence is too limited to allow the definition of firm recommendations. From the theoretical perspective, based on in vitro synergy studies against carbapenem-resistant PA, the following combinations possibly could offer benefits: imipenem+amikacin, ceftolozane-tazobactam+colistin, colistin+imipenem/meropenem and meropenem+amikacin. In contrast, ceftolozane-tazobactam+aztreonam/amikacin or ceftazidime+amikacin are very unlikely to be useful80. Likewise, in vitro synergy has been observed for imipenem-relebactam with colistin, but not with amikacin, against PA-MDR81.
When recommending combination treatment or monotherapy, two different scenarios must be considered: empirical treatment or targeted treatment.
In the context of a patient with suspected PA-MDR infection and with severity criteria (sepsis), we recommend empirical combination treatment with the aim of increasing the chances that the antimicrobial regimen will be active against the causal microorganism82. The choice of antibiotic agents will depend on the local microbiology, taking into account the most frequent resistance mechanisms in each setting (Table 1) for both PA and for other gram-negative bacteria, according to the infection focus involved.
Once the antibiogram becomes available, we consider that targeted treatment may involve monotherapy if PA is sensitive to a first-line antibiotic – classical or new betalactam (with or without betalactamase inhibitor) – chosen according to the antibiogram, and with adjusted doses to secure maximum pK/pD performance (Table 2). In this situation, there is no evidence that combination treatment improves the prognosis, and it might possibly add side effects/toxicity. In those cases where it is not possible to use a first-line antibiotic, and the regimen must be based on colistin or an aminoglycoside, we advise combination treatment, in coincidence with the guides4,77.
ConclusionsTwo antibiotics are available, ceftolozane-tazobactam and ceftazidime-avibactam, that exhibit high in vitro activity even against PA-MDR, PA-XDR and carbapenem-resistant PA, and which have demonstrated good clinical results in highly complex infections. Two new antibiotics, cefiderocol and imipenem-relebactam, offer promising results in vitro, though the supporting evidence is more limited. These antimicrobials moreover offer a better safety profile than other classical antibiotics with comparable in vitro activity (colistin, aminoglycosides).
It is essential to know not only the resistance/activity percentages of each antibiotic for PA in our setting, but also the resistance mechanisms involved, in order to be able to select the most appropriate treatment.
Conflicts of interestPablo Vidal-Cortés has received fees from MSD, Pfizer and Shionogi. The rest of the authors declare that they have no conflicts of interest.
Please cite this article as: Díaz Santos E, Mora Jiménez C, del Río-Carbajo L, Vidal-Cortés P. Tratamiento de las infecciones graves por Pseudomonas aeruginosa multirresistente. Med Intensiva. 2022;46:508–520.