There is controversy regarding the influence of humidification systems upon the incidence of respiratory infections associated to invasive mechanical ventilation (IMV). An evaluation was made of the differences in the incidence of pneumonia and tracheobronchitis associated to mechanical ventilation (VAP and VAT, respectively) with passive and active humidification.
DesignA retrospective pre–post quasi-experimental study was carried out.
SettingA polyvalent ICU with 14 beds.
PatientsAll patients connected to IMV for >48h during 2014 and 2016 were included.
InterventionsDuring 2014, passive humidification with an hygroscopic heat and moisture exchanger (HME) was used, while during 2016 active humidification with a heated humidifier (HH) and an inspiratory heated wire was used. Identical measures for the prevention of VAP were established (Zero Pneumonia Project).
Main outcome measuresThe incidence of VAP and VAT was estimated for 1000 days of IMV in both groups, and statistically significant differences were assessed using Poisson regression analysis.
ResultsA total of 287 patients were included (116 with HME and 171 with HH). The incidence density of VAP per 1000 days of IMV was 5.68 in the HME group and 5.80 in the HH group (p=ns). The incidence density of VAT was 3.41 and 3.26 cases per 1000 days of VMI with HME and HH respectively (p=ns). The duration of IMV was identified as a risk factor for VAP.
ConclusionsIn our population, active humidification in patients ventilated for >48h was not associated to an increase in respiratory infectious complications.
Existen controversias sobre la influencia del sistema de humidificación en la incidencia de infecciones respiratorias asociadas a la ventilación mecánica invasiva (VMI). Nuestro objetivo fue evaluar las diferencias en la incidencia de neumonía y traqueobronquitis asociadas a la ventilación mecánica (NAV y TAV respectivamente) con humidificación pasiva y activa.
DiseñoEstudio retrospectivo cuasi-experimental de tipo pre-postintervención.
ÁmbitoUCI polivalente de 14 camas.
PacientesSe incluyeron todos los pacientes conectados a la VMI durante>48horas durante los años 2014 y 2016.
IntervencionesDurante el año 2014 se empleaba humidificación pasiva con un intercambiador calor-humedad (HME) y, durante 2016, humidificación activa (HH) con calentamiento de la tubuladura inspiratoria. Se establecieron medidas idénticas para la prevención de NAV (proyecto Neumonía Zero).
Variables de interés principalesSe estimaron tasas de incidencia NAV y TAV por 1.000 días de VMI en ambos grupos y se valoraron diferencias estadísticamente significativas mediante regresión Poisson.
ResultadosSe incluyeron 287 pacientes (116 con HME y 171 con HH). La densidad de incidencia de NAV por 1.000 días de VMI fue de 5,68 en el grupo de HME y 5,80 en el grupo de HH (p=ns). La densidad de incidencia de TAV fue 3,41 y 3,26 casos por 1.000 días de VMI con HME y HH respectivamente (p=ns). Se identificó como factor de riesgo de NAV la duración de la VMI.
ConclusionesEn nuestro estudio la humidificación activa en pacientes ventilados durante>48horas no se asoció con un aumento de las complicaciones infecciosas respiratorias.
The need to ensure that the gases administered in mechanical ventilation (MV) reach the patient under adequate conditions of warmth and humidity through the respirator circuit is undeniable. Many studies have shown that deficient humidification of such gases, when sustained over time, results in alterations such as mucociliary dysfunction, inflammation, ulceration and necrosis of the respiratory epithelium, as well as increased viscosity of the respiratory secretions. This in turn leads to an increased incidence of endotracheal tube obstruction, greater airflow resistance, and more respiratory infections (pneumonia and tracheobronchitis) associated to MV1.
Orotracheal intubation implies short circuiting to the nasal-oropharyngeal space, with this anatomical zone being in charge of administering 75% of the warmth and humidity of the gases during breathing. Furthermore, it must be taken into account that medicinal gases are commonly found under conditions of low temperature (10–15°C) and anhidrosis (absolute humidity [AH] 0–0.5mg H2O/l and relative humidity [RH] 2%). As a result, different methods have been developed to warm and humidify the gases administered during MV: passive heat and moisture exchangers (HMEs) and active heat humidifiers (HHs)1,2.
Heat and moisture exchangers act passively, trapping warmth and humidity from the exhaled air of the patient and returning them with the next inspiration. The main advantages of these systems are their simplicity and low cost. However, they are contraindicated in cases of abundant respiratory secretions and in situations of ventilation with low tidal volumes (as in protective ventilation), since they increase the dead space, which may increment PaCO2. Active humidifiers are equipped with a reservoir containing sterile water heated by a plate. The cold and anhydrous medicinal gases pass through the chamber, acquiring warmth and humidity to the point of optimum conditioning (37°C, AH 44mg H2O/l and RH 100%), and are then transported through the inspiratory tubing to the patient. Use is generally made of tubing with a heating wire, which prevents water vapor condensation secondary to the lowering of temperature over the trajectory from heater to patient3.
On starting MV, the habitual defense mechanisms such as cough and mucociliary clearance are diminished, and this considerably increases the risk of infection. Ventilator associated pneumonia (VAP) is the main cause of mortality due to hospital care-related infections, particularly when such infections are attributable to multiresistant microorganisms. The diagnosis is sometimes imprecise, however, and this leads to antibiotic overtreatment4–7.
There is controversy regarding which humidification system is best and its influence upon the incidence of MV associated respiratory infection. The main objective of the present study was to evaluate the differences in the incidence of MV associated infection (VAP and ventilator associated tracheobronchitis [VAT]) between patients ventilated with passive and active humidification systems, following adoption of all the ventilator associated infection preventive measures contemplated by the Zero Pneumonia Project.
Patients and methodsA retrospective, pre–post intervention quasi-experimental single-center cohort study was carried out from January to December 2014, and from January to December 2016, in the Department of Intensive Care Medicine of a third-level university hospital. The Department has 14 beds and is dedicated to the care of medical, trauma and surgical patients, with 52% of all patients on MV and with a mean APACHE II score of 17.5 points. We consecutively enrolled all patients subjected to invasive mechanical ventilation (IMV) for more than 48h. Patients under 18 years of age were excluded. Humidification was provided from the time of intubation.
During the year 2014, use was made of passive humidification with hygroscopic heat-humidity exchangers equipped with antibacterial filters (EdithFlex, AirlifeTM®) in all ventilated patients. According to the manufacturer, these systems afford an AH of between 32.5 and 33.5cm H2O/l, adding a dead space volume of 90ml. The HME devices were replaced every 72h according to protocol, but could be replace earlier if the filter was found to be contaminated with respiratory secretions or minimal condensation causing increased airway resistance.
During the year 2016, all patients received active humidification using an automatic filling chamber (Fisher & Paykel® MR850) equipped with a heating wire in the inspiratory tubing at a temperature of 37°C (AH 44mg H2O/l, RH 100%). The circuit was replaced every 21 days in the case of prolonged IMV.
The patients subjected to MV during 2015 were not included in the study, since this was a period in which the humidification strategy was modified due to the introduction of active humidification in the patients subjected to IMV.
The same VAP measures were adopted in both periods of the study in the context of participation in the Spanish national Zero Pneumonia Project: education and training of the healthcare staff in airway management (aspiration of bronchial secretions), strict hand hygiene using alcoholic solutions when manipulating the airway, oral hygiene with 0.12% chlorhexidine solution, control and maintenance of endotracheal tube cuff pressure, semi-raised patient position, promotion of procedures and protocols destined to reduce ventilation time (weaning and sedation protocols), avoidance of programmed changing of the ventilator circuit, humidifiers and endotracheal tubes, selective decontamination of the oropharynx (tobramycin, colistin and nystatin solutions), and the use of endotracheal tubes with a continuous subglottic secretions aspiration system8.
The following variables were recorded: patient age and gender, VAP risk factors (human immunodeficiency virus [HIV] infection, chronic kidney disease [CKD], chronic obstructive pulmonary disease [COPD], diabetes mellitus [DM], transplantation, active neoplastic disease, neutropenia, liver cirrhosis), APACHE II score upon admission, days of IMV, type of humidification used, previous antibiotherapy (defined as the utilization of antibiotics via the parenteral route in the 24h prior to the start of IMV), incidence of VAP and VAT, days to development of pneumonia, type of microbiological sample obtained (bronchoaspirate, bronchoalveolar lavage [BAL], telescoping catheter sampling), result of the quantitative cultures, days of stay in the Intensive Care Unit (ICU), and patient condition at discharge from the ICU and from hospital (alive, deceased). The data were compiled on a retrospective basis from the electronic clinical management system of our center (IMASIS). Two clinicians independently reviewed all the patients subjected to MV during the study period, and jointly re-evaluated the diagnosis of VAP and VAT. In the case of disagreement, a third clinician was consulted to reach consensus.
The diagnosis of VAP was established based on the criteria of the European Center for Disease Prevention and Control (ECDC), while the diagnosis of VAT was based on the criteria of the Centers for Disease Control (CDC)9. Clinical (fever, leukocytosis or leucopenia, purulent secretion, respiratory auscultation, cough, dyspnea or tachypnea and progressive worsening of gas exchange), radiological (new images suggestive of pneumonia on the chest X-rays or thoracic CAT scan) and microbiological criteria (quantitative cultures of bronchoaspirate, bronchoalveolar lavage or telescoping catheter samples) were used. Early VAP was defined as pneumonia manifesting within ≤7 days after the start of IMV.
Statistical analysisQuantitative variables were expressed as the median and quartiles (p25 and p75), while categorical variables were reported as frequencies and percentages.
Possible differences between the active and passive humidification groups were explored using the chi-squared test for categorical variables (or the Fisher exact test, as applicable), while the Mann–Whitney U-test was used for continuous variables. These same tests were applied in the bivariate analysis for the assessment of possible factors associated to VAP and VAT. Likewise, multivariate analysis was performed of the VAP and VAT outcomes, taking the humidification group as principal factor and adjusting for possible confounding factors, with the selection of those variables seen to yield statistically significant differences in the comparison between the active and passive humidification groups.
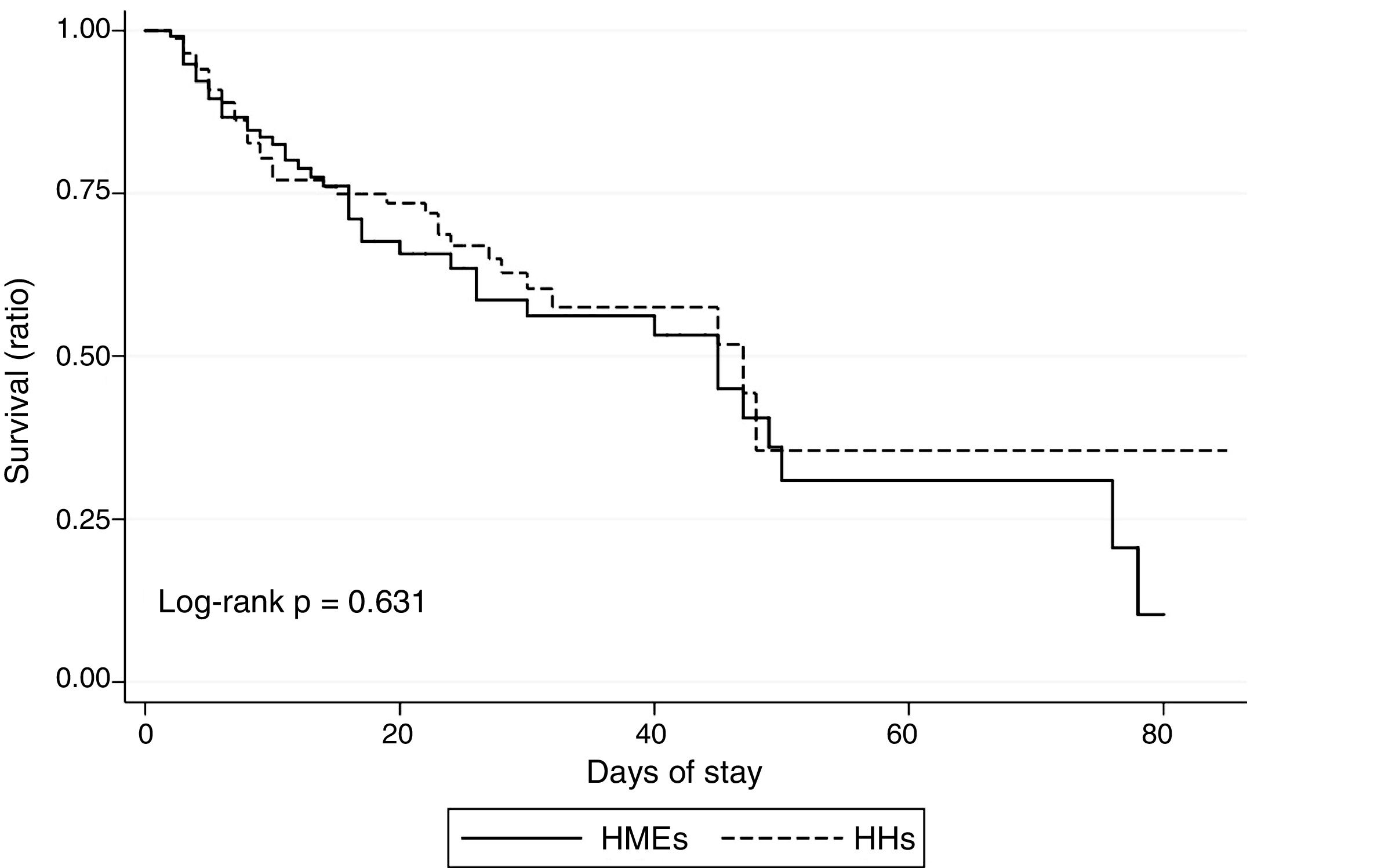
Estimations were made of VAP and VAT incidence per 1000 days of IMV in both groups, as well as of early and late VAP, and statistically significant differences were assessed via Poisson regression analysis, contrasting the rate ratio=1 hypothesis. Lastly, we evaluated the possible existence of differences in ICU mortality between the groups, based on the plotting of survival curves, with application of the log-rank test.
The statistical analysis was performed using the STATA version 15.1 package (StataCorp, College Station, TX, USA). The study was approved by the Clinical Research Ethics Committee (CREC) (2018) of Hospital del Mar (Barcelona, Spain).
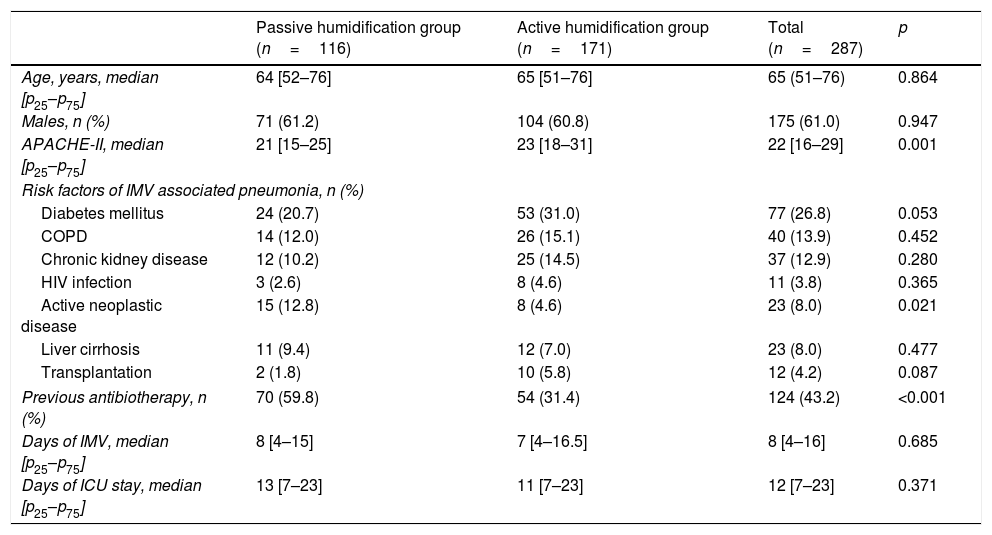
ResultsA total of 287 patients were analyzed, of which 116 received passive humidification and 171 active humidification. The baseline characteristics of the patients are shown in Table 1. Both groups were similar in terms of age and gender distribution, APACHE II score, a history of diabetes mellitus, COPD, chronic kidney disease, HIV infection, transplantation or active neoplastic disease, days of MV and days of ICU stay. Significant differences were observed in relation to the prevalence of a diagnosis of active neoplastic disease and prior antibiotherapy (defined as the introduction of antibiotic treatment in the 24 hours before starting IMV) – both parameters being greater in the HME group.
Baseline characteristics of the study population.
| Passive humidification group (n=116) | Active humidification group (n=171) | Total (n=287) | p | |
|---|---|---|---|---|
| Age, years, median [p25–p75] | 64 [52–76] | 65 [51–76] | 65 (51–76) | 0.864 |
| Males, n (%) | 71 (61.2) | 104 (60.8) | 175 (61.0) | 0.947 |
| APACHE-II, median [p25–p75] | 21 [15–25] | 23 [18–31] | 22 [16–29] | 0.001 |
| Risk factors of IMV associated pneumonia, n (%) | ||||
| Diabetes mellitus | 24 (20.7) | 53 (31.0) | 77 (26.8) | 0.053 |
| COPD | 14 (12.0) | 26 (15.1) | 40 (13.9) | 0.452 |
| Chronic kidney disease | 12 (10.2) | 25 (14.5) | 37 (12.9) | 0.280 |
| HIV infection | 3 (2.6) | 8 (4.6) | 11 (3.8) | 0.365 |
| Active neoplastic disease | 15 (12.8) | 8 (4.6) | 23 (8.0) | 0.021 |
| Liver cirrhosis | 11 (9.4) | 12 (7.0) | 23 (8.0) | 0.477 |
| Transplantation | 2 (1.8) | 10 (5.8) | 12 (4.2) | 0.087 |
| Previous antibiotherapy, n (%) | 70 (59.8) | 54 (31.4) | 124 (43.2) | <0.001 |
| Days of IMV, median [p25–p75] | 8 [4–15] | 7 [4–16.5] | 8 [4–16] | 0.685 |
| Days of ICU stay, median [p25–p75] | 13 [7–23] | 11 [7–23] | 12 [7–23] | 0.371 |
APACHE-II: Acute Physiology and Chronic Health Evaluation II; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; IMV: invasive mechanical ventilation.
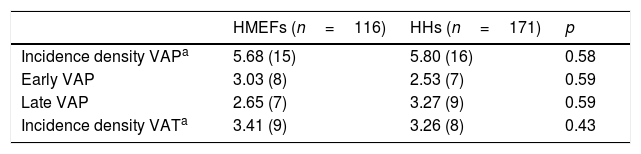
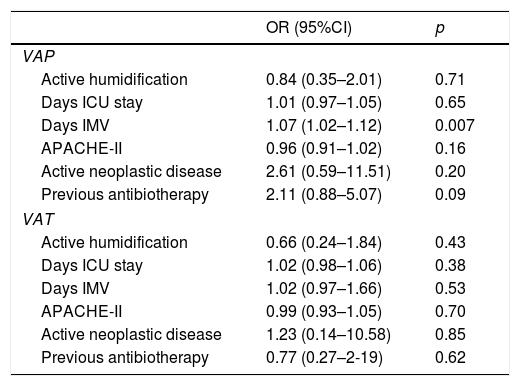
Table 2 shows the incidence density of VAP and VAT according to the type of humidifying system used. The logistic regression analysis identified the duration of IMV as the only risk factor for the development of MV associated infection (odds ratio [OR] 1.07 [95%CI: 1.02–1.1]; p=0.007). While statistical significance was not reached, prior antibiotherapy tended to increase the frequency of VAP. No risk factor associated to the development of VAT was identified (Table 3).
Incidence density of invasive mechanical ventilation associated respiratory infection.
| HMEFs (n=116) | HHs (n=171) | p | |
|---|---|---|---|
| Incidence density VAPa | 5.68 (15) | 5.80 (16) | 0.58 |
| Early VAP | 3.03 (8) | 2.53 (7) | 0.59 |
| Late VAP | 2.65 (7) | 3.27 (9) | 0.59 |
| Incidence density VATa | 3.41 (9) | 3.26 (8) | 0.43 |
HHs: heated humidifiers; HMEFs: heat and moisture exchangers; VAP: ventilator associated pneumonia; VAT: ventilator associated tracheobronchitis; IMV: invasive mechanical ventilation.
Logistic regression analysis of risk factors of VAP and VAT.
| OR (95%CI) | p | |
|---|---|---|
| VAP | ||
| Active humidification | 0.84 (0.35–2.01) | 0.71 |
| Days ICU stay | 1.01 (0.97–1.05) | 0.65 |
| Days IMV | 1.07 (1.02–1.12) | 0.007 |
| APACHE-II | 0.96 (0.91–1.02) | 0.16 |
| Active neoplastic disease | 2.61 (0.59–11.51) | 0.20 |
| Previous antibiotherapy | 2.11 (0.88–5.07) | 0.09 |
| VAT | ||
| Active humidification | 0.66 (0.24–1.84) | 0.43 |
| Days ICU stay | 1.02 (0.98–1.06) | 0.38 |
| Days IMV | 1.02 (0.97–1.66) | 0.53 |
| APACHE-II | 0.99 (0.93–1.05) | 0.70 |
| Active neoplastic disease | 1.23 (0.14–10.58) | 0.85 |
| Previous antibiotherapy | 0.77 (0.27–2-19) | 0.62 |
HHs: heated humidifiers; 95%CI: 95% confidence interval; VAP: ventilator associated pneumonia; OR: odds ratio; VAT: ventilator associated tracheobronchitis; ICU: Intensive Care Unit; IMV: invasive mechanical ventilation.
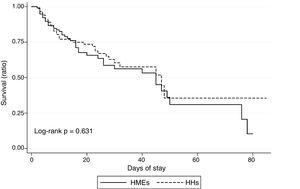
The in-ICU mortality rate in our sample was 31.6%, with no significant differences between the cohort subjected to passive humidification and the active humidification group (36.2% and 28.2%, respectively; p=0.628). Fig. 1 shows the results of the in-ICU survival analysis (Kaplan–Meier curves).
At microbiological level, the most frequent microorganisms in both groups were gram-negative bacilli followed by gram-positive bacteria, viruses and fungal species, in both the VAP and VAT cases. In three cases of ventilation with HME (20%) and in 5 cases of VAP (31.2%), we isolated Candida albicans from the respiratory samples – this circumstance being interpreted as indicating colonization. Table 4 describes the isolated microorganisms.
Respiratory sample isolates in VAP.
| HMEs | HHs | |
|---|---|---|
| VAP | ||
| Gramnegative, n (%) | 12 (48) | 13 (52) |
| Escherichia coli | 1 (4) | 1 (4) |
| Klebsiella pneumoniae | 3 (12) | 2 (8) |
| Enterobacter spp. | 1 (4) | 1 (4) |
| Haemophilus influenzae | 1 (4) | 1 (4) |
| Stenotrophomonas maltophilia | 3 (12) | 1 (4) |
| Serratia marcescens | 1 (4) | 1 (4) |
| Providencia stuartii | 0 (0) | 1 (4) |
| Citrobacter freundii | 1 (4) | 0 (0) |
| Acinetobacter baumannii | 0 (0) | 1 (4) |
| Pseudomonas aeruginosa | 1 (4) | 2 (8) |
| Achromobacter xylosoxidans | 0 (0) | 2 (8) |
| Grampositive, n (%) | 7 (28) | 5 (20) |
| Methicillin-sensitive Staphylococcus aureus | 3 (12) | 2 (8) |
| Methicillin-resistant Staphylococcus aureus | 1 (4) | 1 (4) |
| Streptococcus pneumoniae | 1 (4) | 0 (0) |
| Streptococcus constellatus | 1 (4) | 0 (0) |
| Enterococcus faecalis | 1 (4) | 1 (4) |
| Enterococcus faecium | 0 (0) | 1 (4) |
| Virus, n (%) | 5 (20) | 3 (12) |
| Herpes simplex virus type 1 | 3 (12) | 3 (12) |
| Cytomegalovirus | 2 (8) | 0 (0) |
| Fungi, n (%) | 1 (4) | 4 (16) |
| Aspergillus fumigatus | 1 (4) | 1 (4) |
| Aspergillus flavus | 0 (0) | 2 (8) |
| Aspergillus niger | 0 (0) | 1 (4) |
| VAT | ||
| Gramnegative, n (%) | 7 (70) | 9 (69.2) |
| Escherichia coli | 0 (0) | 3 (23.1) |
| Enterobacter spp. | 0 (0) | 1 (7.8) |
| Haemophilus influenzae | 2 (20) | 0 (0) |
| Serratia marcescens | 0 (0) | 2 (15.4) |
| Stenotrophomonas maltophilia | 1 (10) | 0 (0) |
| Pseudomonas aeruginosa | 3 (30) | 3 (23.1) |
| Proteus mirabilis | 1 (10) | 0 (0) |
| Grampositive, n (%) | 2 (20) | 4 (30.7) |
| Methicillin-sensitive Staphylococcus aureus | 1 (10) | 2 (15.4) |
| Streptococcus pneumoniae | 1 (10) | 0 (0) |
| Streptococcus agalactiae | 0 (0) | 1 (7.8) |
| Enterococcus faecium | 0 (0) | 1 (7.8) |
| Virus, n (%) | 1 (10) | 0 (0) |
| Herpes simplex virus type 1 | 1 (10) | 0 (0) |
HHs: heated-humidifiers; HMEs: heat and moisture exchangers; VAP: ventilator associated pneumonia; VAT: ventilator associated tracheobronchitis.
The present study has examined the impact of the type of humidification used upon the incidence of VAP and VAT following adoption of all the measures contemplated by the Spanish national Zero Pneumonia Project. Most of the existing studies were published before the year 2006, with few later articles, and none of them have been carried out in the context of a prevention program. The most current evidence is based on published meta-analyses that document studies that are over 10 years old. As a result, the data might not reflect the current situation following the improvements that have been introduced in this field. Furthermore, the studies involved are of low quality and are very heterogeneous in terms of the criteria used for defining pneumonia10–14.
Ventilator associated pneumonia was the most frequent cause of nosocomial pneumonia in Spanish ICUs up until implementation of the Zero Pneumonia Project15. Its strong impact upon patient morbidity-mortality, with an associated prolongation of hospital stay of 7–9 days on average per patient and an imputable mortality rate of 33–50%16, justifies the ongoing interest in defining factors capable of influencing its physiopathogenesis and in establishing appropriate preventive strategies. Ventilator associated tracheobronchitis in turn is an intermediate condition between tracheobronchial colonization and VAP, and it remains to be established whether VAT can be regarded as a step in the evolution toward VAP17.
Due to the lack of agreement among the published studies, the guides and recommendations of the different scientific societies – based on the available evidence – do not establish recommendations regarding the type of humidification to be preferred in order to prevent VAP8,18. Passive humidification has poorer performance in terms of warmth and humidity versus active humidification18. One of the main disadvantages of HME is an increase in dead space dependent mainly upon the internal volume of the filter, and which could prove difficult to tolerate by patients with a low ventilatory reserve – this aspect being of particular relevance when the underlying disease condition requires the adoption of protective ventilation strategies19–24. The greater resistance that may be associated with HMEs, though sometimes regarded as negligible, might not be a minor issue in the event of fluid accumulation due to condensation or the impaction of secretions. On the other hand, the use of active heat humidifiers (HHs) has been associated to a risk of airway damage due to excessive temperature levels, the limitation of airflow as a consequence of condensation within the tubing, the appearance of asynchrony, and possible microbial colonization of the circuit3,25,26.
The Zero Pneumonia Project was launched in 2011 in 181 ICUs in Spain. As a result of the adoption of the Project measures, a decrease of over 50% was observed in the national incidence density of VAP (from 9.83 to 4.34/1000 days of IMV) in the 19–21 months following introduction of the Project. These results remained constant over the subsequent years, thus indicating that the recommendations have been incorporated to routine practice in Spanish ICUs27. According to the annual report of the ENVIN-HELICS registry (a national study on nosocomial infections in the ICU), the incidence density of VAP was 6.31/1000 days of IMV in 2014 and 6.26/1000 days of IMV in 201628. In our study, the incidence density of VAP during the same period of time was lower than the above figures, and was more in line with the results of the ENVIN-HELICS registry at national level in 2018 (5.87/1000 days of IMV)28. Likewise, our figure is lower than the standard of 7 episodes per 1000 days of IMV recommended in the year 2017 among the quality indicators of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC])29. This suggests that our group is strongly sensitized and has broad experience in the control of nosocomial infections in the critically ill. Accordingly, in this context we sought to evaluate the possible impact of two humidification systems upon the incidence not only of VAP but also of VAT. No differences in incidence density were observed – thus confirming the safety of active humidification in this scenario.
Of note in our study is the observation of an increased number of Aspergillus isolates as a cause of pneumonia in the HH group: four isolates in three patients of the HH group and a single isolate in one patient of the HME group. No conclusions can be drawn in this regard, however, in view of the small sample size involved. Likewise, C. albicans was isolated in three patients of the HME group and in 5 patients of the HH group – all these cases being taken to represent colonization. This increase in fungal isolates among patients with HH in our sample must be interpreted with caution, and quality studies are needed to confirm the data obtained.
The present study has a number of limitations. Firstly, its retrospective, quasi-experimental single-center design logically implies external validity problems. Furthermore, the sample size was limited, though it is similar to those reported in previous studies. Secondly, the prevalence of active neoplastic disease and of parenteral antibiotherapy before intubation was significantly greater in the HME group – a fact that may have influenced our results. Nevertheless, neither parameter was identified as a risk factor for VAP and VAT in the logistic regression analysis. A third limitation is the fact that the diagnosis of VAP, although based on the criteria of the CDC, was not carried out with an invasive procedure in all cases. However, use was made of quantitative cultures of samples such as bronchoaspirate, BAL or telescoping catheter samples in all cases. Lastly, of note was the low incidence density of VAT recorded in our series. This was probably related to difficulties in establishing the clinical diagnosis of VAT, which in some cases may go unnoticed and can be confused with VAP in the presence of radiologically established infiltrates attributable to other causes. Nevertheless, we attempted to reduce this source of bias through the separate review of each case by two different clinicians, with the inclusion of evaluation by a third clinician in order to reach consensus in the event of discordances in data compilation.
Our results suggest that there are no differences between HME and HH with regard to the incidence of VAP or VAT following implementation of the bundle of VAP preventive measures. However, quality prospective studies are needed to compare the different types of HME and HH in order to draw more solid conclusions of use in routine clinical practice.
Authorship- •
Lucía Picazo-Moreno: study design, data acquisition and drafting of the manuscript.
- •
M. Pilar Gracia-Arnillas: study design, data interpretation, critical review of the intellectual content and approval of the definitive version of the article.
- •
Rosana Muñoz-Bermúdez: data analysis, critical review of the intellectual content and approval of the definitive version of the article.
- •
Xavier Durán: data analysis, critical review of the intellectual content and approval of the definitive version of the article.
- •
Francisco Álvarez-Lerma: data interpretation, critical review of the intellectual content and approval of the definitive version of the article.
- •
Joan-Ramón Masclans: study conception and design, data analysis and interpretation, critical review of the intellectual content and approval of the definitive version of the article.
The research group at Instituto Hospital del Mar de Investigaciones Médicas (IMIM) pertaining to the Department of Intensive Care Medicine of Hospital del Mar receives research support from Fisher & Paykel.
Please cite this article as: Picazo L, Gracia Arnillas MP, Muñoz-Bermúdez R, Durán X, Álvarez Lerma F, Masclans JR. La humidificación activa en ventilación mecánica no se asocia con un aumento de complicaciones infecciosas respiratorias en un estudio cuasi-experimental pre-postintervención. Med Intensiva. 2021;45:354–361.