Edited by: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Last update: December 2023
More infoThis study aimed to investigate chlorhexidine’s efficacy in preventing ventilator-associated pneumonia (VAP).
DesignA systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
SettingsThe data were obtained from Pubmed, Cochrane Library, and EMBASE.
Patients or participantsOnly mechanically ventilated patients for at least 48h were included.
InterventionsRandomized clinical trials applying any dosage form of chlorhexidine were eligible.
Main variables of interestThe relative risk (RR) of the VAP incidence and all-cause mortality was assessed using the random-effects model. The mean difference in days of mechanical ventilation duration and intensive care unit (ICU) length of stay were also appraised.
ResultsTen studies involving 1233 patients were included in the meta-analysis. The oral application of CHX reduced the incidence of VAP (RR, 0.73 [95% CI, 0.55, 0.97]) and did not show an increase in all-cause mortality (RR, 1.13 [95% CI, 0.96, 1.32]).
ConclusionsCHX proved effective to prevent VAP. However, a conclusion on mortality rates could not be drawn because the quality of the evidence was very low for this outcome.
Este estudio tuvo como objetivo investigar la eficacia de la clorhexidina en la prevención de la neumonía asociada al ventilador (NAV).
Diseñose realizó una revisión sistemática y un metanálisis siguiendo los elementos de informe (PRISMA) Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
ÁmbitoLos datos se obtuvieron de Pubmed, Cochrane Library y EMBASE.
Pacientes o participantessolo se incluyeron pacientes con ventilación mecánica durante al menos 48 horas.
IntervencionesFueron elegibles los ensayos clínicos aleatorios que aplicaban cualquier forma de dosificación de clorhexidina.
Variables de interés principalesSe evaluó el riesgo relativo (RR) de incidencia de NAVM y mortalidad por todas las causas mediante el modelo de efectos aleatorios. También se evaluó la diferencia media en los días de duración de la ventilación mecánica y la duración de la estancia en la unidad de cuidados intensivos (UCI).
ResultadosDiez estudios con 1233 pacientes se incluyeron en el metanálisis. La aplicación oral de CHX redujo la incidencia de VAP (RR, 0,73 [IC 95%, 0,55, 0,97]) y no mostró un aumento en la mortalidad por todas las causas (RR, 1,13 [IC 95%, 0,96, 1,32].
ConclusionesCHX demostró ser eficaz para prevenir la VAP. Sin embargo, no se pudo establecer una conclusión sobre las tasas de mortalidad porque la calidad de la evidencia fue muy baja para este resultado.
Ventilator-associated pneumonia (VAP) is defined as a type of pneumonia established 48h after endotracheal intubation.1 VAP is a frequent complication found in intensive care patients receiving mechanical ventilation. About 15% of patients exposed to intubation show pneumonia symptoms2; however, the incidence of pneumonia can vary widely between studies. A recent multicenter observational study conducted in Italy revealed that the incidence of VAP in hospitalized COVID-19 patients was estimated at 29%.3 Therefore, the high frequency and severity of the disease make VAP one of the most important and common causes of death related to hospital-acquired conditions.2,4
Respiratory assistance in intensive care units (ICUs) is necessary because of the individual's inability to breathe without mechanical assistance. In this context, the hospitalized patients' oral microenvironment can function as a reservoir of opportunistic microorganisms,5 propagating bacteria from the oral cavity toward the lungs.6 Also, poor oral hygiene, reduced natural cleaning mechanism of the mouth, and low salivary flow can contribute to the imbalance of the oral microflora.7
Reducing the oral colonization of microorganisms might be a potential mechanism to control VAP. Many clinical trials reported that routine oral care with chlorhexidine (CHX) substantially reduced the number of VAP episodes in ICU patients.8,9 CHX is a broad-spectrum antiseptic agent with bacteriostatic and bactericidal activity and a low potential for inducing dermatological reactions.10 CHX has been widely used in clinical practice for preventing VAP, either as a gel or as an aqueous solution.6
However, a recent systematic review by Klompas et al.11 revealed no significant difference in ventilator-associated pneumonia risk in double-blind studies of non-cardiac surgery patients. Rabello et al.12 also showed inconsistency in a recent overview evaluating 14 systematic reviews and meta-analyses with high-quality evidence. The authors showed that CHX effectively prevented nosocomial pneumonia among adults in cardiothoracic ICU but not in other clinical-surgical units.
This systematic review evaluated randomized clinical trials of mechanically ventilated patients in ICU (patients), being applied any dosage form of chlorhexidine (intervention) in comparison to patients that did not receive any drug (comparison). VAP incidence, all-cause mortality, ICU length of stay, and mechanical ventilation duration were the variables investigated (outcomes). Recent RCTs (randomized clinical trials) were included, and the proposed eligibility criteria and stratification substantially reduced the sources of heterogeneity.
MethodsThis systematic review followed the Cochrane13 and PRISMA14 criteria for systematic reviews and meta-analyses. Registration in the PROSPERO database was accomplished under submission: CRD42020209760 and title: "Does oral care with chlorhexidine reduce the Incidence of ventilator-associated pneumonia and all-cause mortality in intensive care unit patients? A systematic review and meta-analysis".
Data sources and searches methodsThe appraised databases were PubMed, Cochrane Library, and EMBASE. An additional investigation was conducted by searching the included articles’ references, previous meta-analyses, Google scholar, and ClinicalTrials.gov. The selection of the terms was based on satellite articles. The complete search strategy is presented in the supplementary material (Table S1).
Two independent reviewers (JCC and CKM) screened the titles and abstracts extracted from the databases. Any disagreement was addressed to a third reviewer (JEVP). The searches started in February 2019 and were updated on January 1st, 2022. Endnote software was used to manage citations and identify duplicates.
Study selectionThe review was based on the following PICO strategy: 1. Population: intensive care unit patients receiving mechanical ventilation; 2. Intervention: oral application of chlorhexidine. 3. Comparison: intubated intensive care unit patients that did not receive any pharmaceutical formulation containing antiseptic agents with proven activity; 4. Outcomes: all-cause mortality, the incidence of VAP, ICU length of stay, and days of mechanical ventilation.
The studies were selected according to the search strategy with the following inclusion criteria: 1) randomized clinical trials applying any dosage form of chlorhexidine; 2) daily administration of CHX; 3) no restriction of date or location; 4) mechanically ventilated patients for at least 48h; 5) publication period analysis until January 1st, 2022. The exclusion criteria included: 1) studies related to in vitro methodology; 2) studies containing chlorhexidine preparations in both control and intervention arms; 3) studies presented only in the abstract form; 4) Studies conducted in pediatric ICU; 5) studies containing only pre-operatively administration of CHX; 6) studies using substances with known antiseptic activity in the control arm.
Data extraction and quality assessmentThe extracted data were analyzed individually in an orderly fashion, and the collected information was obtained in a standardized design. The following data were gathered from the articles: authorship, location of study, number of patients, intervention, and control characteristics (pharmaceutical form and concentrations, additional teeth brushing). The data collection was entered in an Excel spreadsheet (Excel, Microsoft, Washington, USA) by one author (JCC), and all information was verified by another reviewer (JEVP). No efforts were made to contact the authors regarding incomplete data.
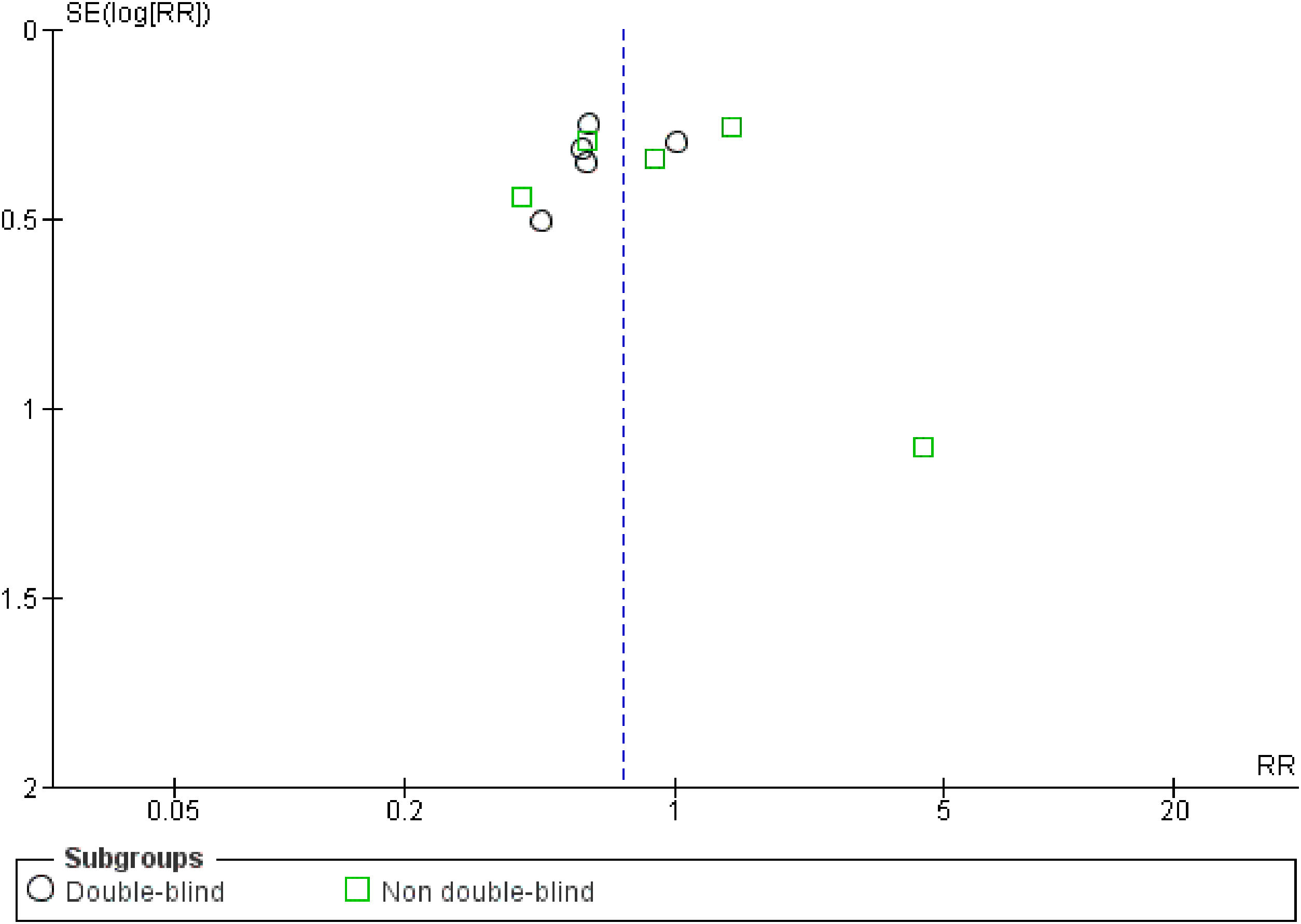
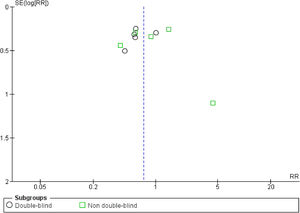
The quality of the included trials was appraised by the Cochrane criteria13 to assess the risk of bias in 6 domains (i.e., sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias). Review Manager 5.2.7 software (Cochrane Collaboration, Oxford, United Kingdom) was used to generate the risk of bias summary and graph. The funnel plot was also created to suggest reporting bias. Based on the information extracted from each trial, the studies were classified as high, low, or unclear risk of bias (when not enough details were reported). Two independent reviewers made these classifications (JCC and CKM), and any disagreements were solved by consensus.
Data synthesis and analysisThe incidence of ventilator-associated pneumonia was set as the primary outcome. All-cause mortality, duration of mechanical ventilation, and ICU length of stay were selected as the secondary outcome. Subgroup analyses were performed to avoid heterogeneity. The influence of the CHX concentration was also appraised by conducting an additional analysis with the studies stratified according to the antiseptic dosage. The tests for subgroup differences were also appraised for this setup. These further analyses aimed to facilitate the comparison with other studies and investigate the influence of clinical parameters on the VAP outcomes.
Review Manager 5.2.7 software (Cochrane Collaboration, Oxford, United Kingdom) was used to perform the quantitative synthesis. The weighted relative risk (for the incidence of VAP and mortality) and mean difference (for ICU length of stay and mechanical ventilation duration) were calculated using the generic inverse variance random-effects model. The I², tau² and chi² statistical tests were used to estimate heterogeneity. Forest plots were constructed for each outcome and all data were calculated considering a confidence interval (CI) of 95% (p<0.05). Stratification was performed based on the study’s blindness for the VAP incidence and all-cause mortality. Sub-group analysis was not assessed for ICU length of stay and mechanical ventilation duration due to a lack of available data.
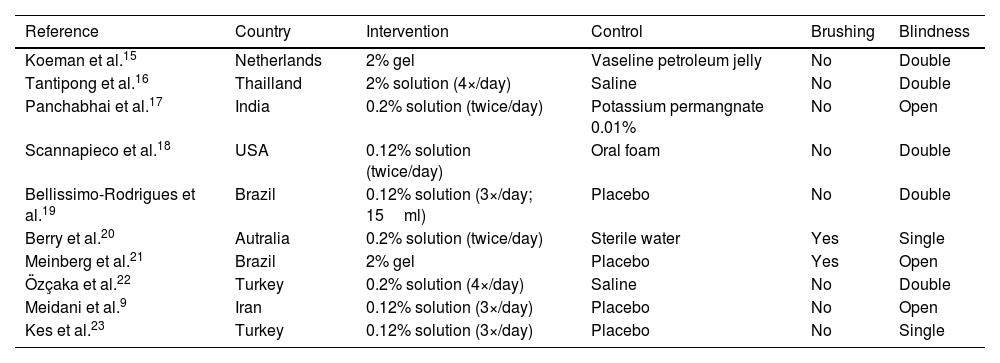
ResultsDescription of included studiesThe search strategy identified 2661 studies, of which 1005 were duplicates. After assessing the eligibility criteria, ten studies were included in the meta-analysis (including 1233 patients). Fig. S1 shows the PRISMA flowchart for the summarized results. The main characteristics of each study are summarized in Table 1. Table S2 shows detailed quantitative data of the studies included in this review, and Table S3 presents the information about trials not included in the meta-analysis, including the reasons for exclusion.
Characteristics of the included studies.
| Reference | Country | Intervention | Control | Brushing | Blindness |
|---|---|---|---|---|---|
| Koeman et al.15 | Netherlands | 2% gel | Vaseline petroleum jelly | No | Double |
| Tantipong et al.16 | Thailland | 2% solution (4×/day) | Saline | No | Double |
| Panchabhai et al.17 | India | 0.2% solution (twice/day) | Potassium permangnate 0.01% | No | Open |
| Scannapieco et al.18 | USA | 0.12% solution (twice/day) | Oral foam | No | Double |
| Bellissimo-Rodrigues et al.19 | Brazil | 0.12% solution (3×/day; 15ml) | Placebo | No | Double |
| Berry et al.20 | Autralia | 0.2% solution (twice/day) | Sterile water | Yes | Single |
| Meinberg et al.21 | Brazil | 2% gel | Placebo | Yes | Open |
| Özçaka et al.22 | Turkey | 0.2% solution (4×/day) | Saline | No | Double |
| Meidani et al.9 | Iran | 0.12% solution (3×/day) | Placebo | No | Open |
| Kes et al.23 | Turkey | 0.12% solution (3×/day) | Placebo | No | Single |
Chlorhexidine concentration, control arm, location of studies, and brushing
Four studies applied 0.12% CHX solution,9,18,19,23 three trials employed CHX at a concentration of 0.2%,17,20,22 and three studies used CHX at a concentration of 2%.15,16,21
Besides the placebo (considered when the control maintained the same organoleptic conditions as the control),9,19,21,23 saline,16,22 sterile water,20 and potassium permanganate17 solutions were also used in the control group. Some studies described the control arm as "usual care" or oral foam.18 Petroleum jelly15 was also used as the control arm when CHX gel was administered as the intervention. Two studies20,21 described brushing the patients' teeth with a toothbrush.
Studies were carried out on almost all continents. America: Brazil,19,21 US,18 Europe: Turkey,22,23 Netherlands15; Asia: India,17 Thailand,16 Iran,16 and Oceania: Australia.20 Among them, five (Brazil, India, Thailand, Iran, and Turkey) are low and middle-income countries, and three (US, Netherlands, and Australia) are considered high-income countries
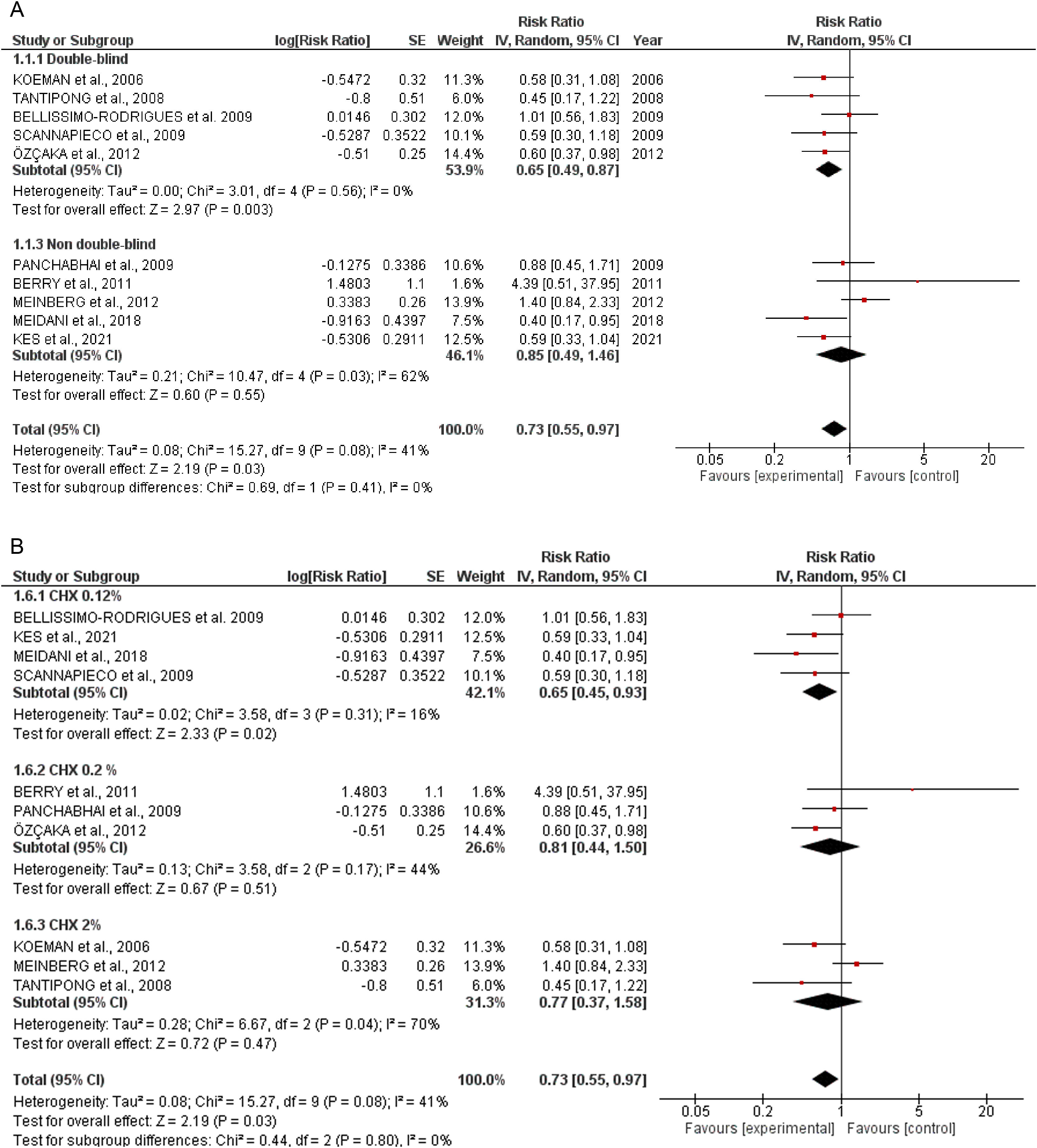
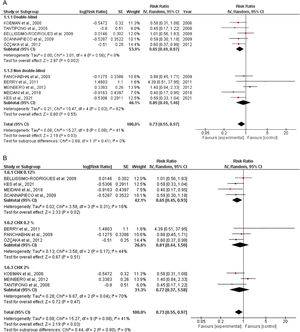
Quantitative dataIncidence of VAPTen studies containing 1233 patients receiving oral application of CHX to prevent VAP were identified. The meta-analysis showed that CHX was effective to reduce VAP (0.73 [0.55, 0.97] p=0.03) (Fig. 1A). The heterogeneity for the analysis was: Tau²=0.08; Chi²=15.27, df=9 (p=0.08); I²=41%. The RR for VAP incidence assessing only double-blind studies resulted in lower RR: 0.65 [0.49, 0.87] p=0.0005. The hetoregeneity for this assay was considered negligible: Tau²=0.00; Chi²=3.11, df=5 (p=0.68); I2=0%. The RR appraised for the incidence of VAP including only studies that was not fully blinded was 0.85 [0.49, 1.46] (p=0.55). The heterogeneity in this test was considered moderate: Tau²=0.21; Chi²=10.47, df=4 (p= 0.03); I²=62%.
The CHX concentration did not result in differences in VAP incidence according to the tests for subgroup differences (p=0.80) (Fig. 1B).
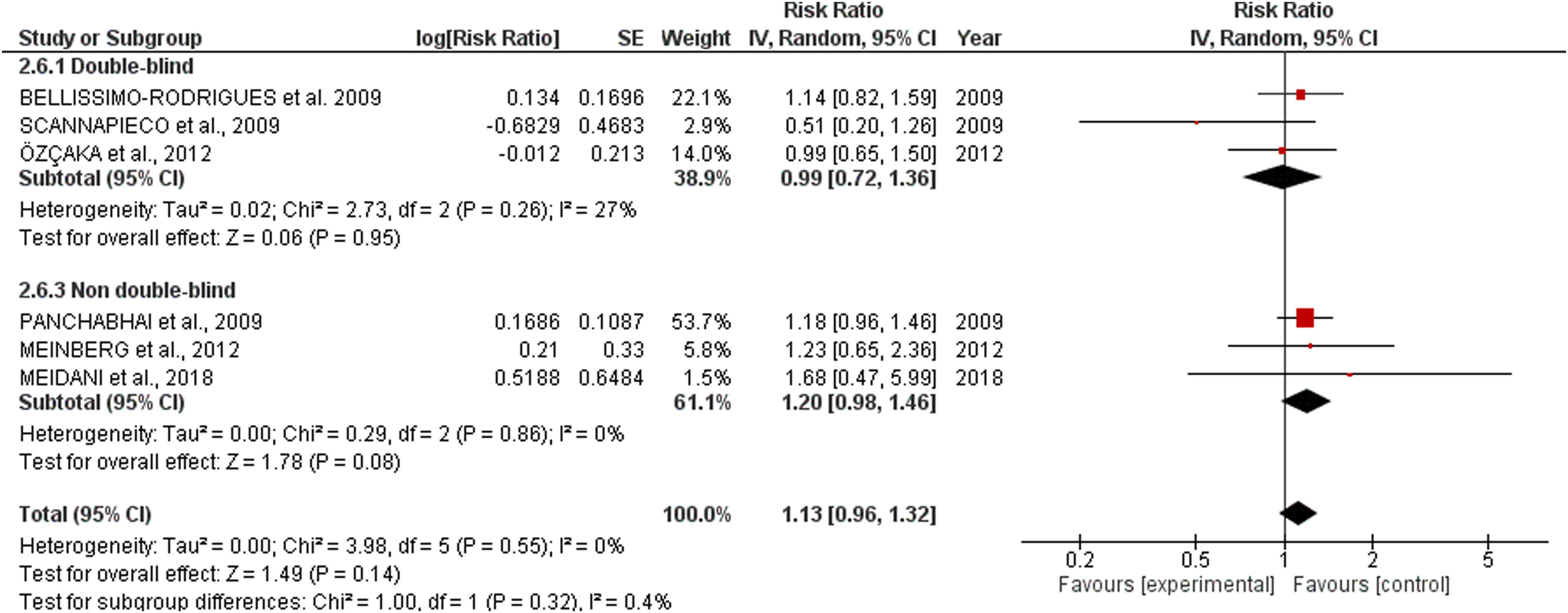
All-cause mortalitySix studies involving 718 patients receiving oral application of CHX were identified. CHX did not influence mortality rates in critically ill patients. The RR risk of all-cause mortality for CHX were: 1.13 [0.96, 1.32] p=0.14. The heterogeneity was considered neglible: Tau²=0.00; Chi²=3.98, df=5 (p=0.55); I²=0%). The RR for the Incidence of VAP assessing only double-blind studies were: 0.99 [0.72, 1.36] p=0.95 (Heterogeneity: Tau²=0.02; Chi²=2.73, df=2 (p=0.26); I²=27%). The RR appraised for the Incidence of VAP including only studies that was not fully blinded were: 1.20 [0.98, 1.46] p=0.08 (Heterogeneity: Tau²=0.00; Chi²=0.29, df=2 (p=0.86); I²=0%) (Fig. 2).
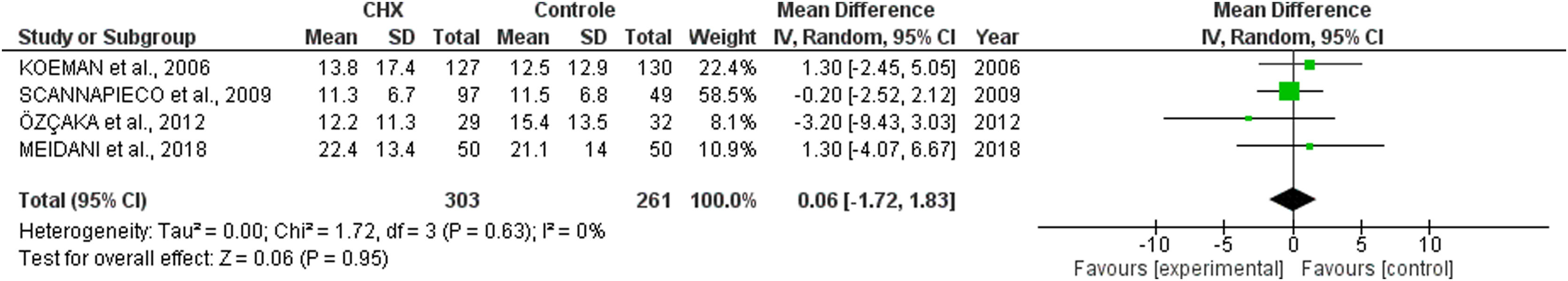
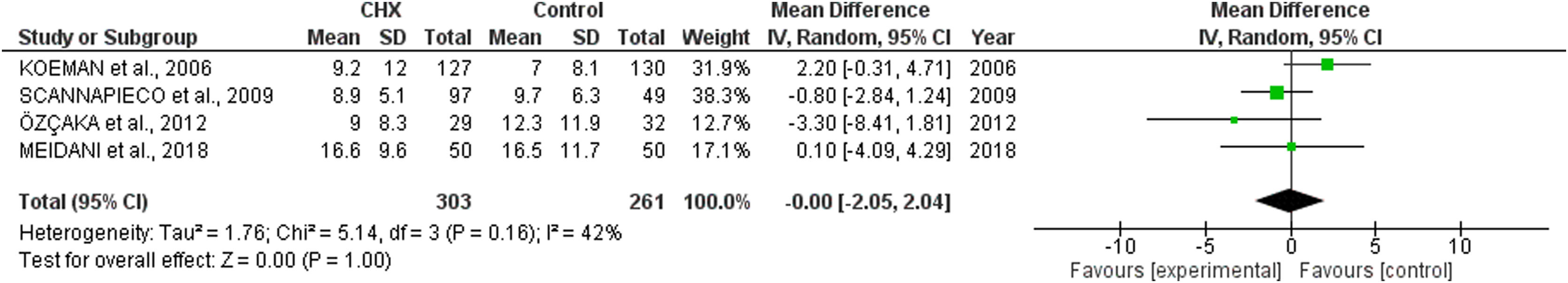
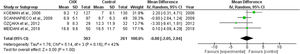
ICU length of stay and days of mechanical ventilationCHX administration had no effect in the in the ICU length of stay (mean difference 0.06 [1.72, 1.68; p= 0.63]) (Fig. 3) and mechanical ventilation duration (mean difference 0.00 [-2.05, 2.04; p=1.00]) (Fig. 4) in patients receiving endotracheal intubation. No subgroup analysis was performed due to the reduced number of available data.
Risk of biasOf the total trials included, five were double-blinded,15,16,18,19,22 two were single-blinded,20,23 and three were open-label.9,17,21 Less studies reported mortality (n=6) than VAP incidence (n=10), suggesting reporting bias. At least half of the included trials presented some concerns regarding missing outcome data. 60% of the studies showed some drawbacks in the randomization process. Only 50% of the RCTs presented low risk of bias (overall). Fig. S2 shows the risk of bias summary and graph of all included studies. No publication bias was observed (Fig. S3).
DiscussionThis systematic review and meta-analysis assumed that oral care with CHX in ICU patients could be beneficial in preventing VAP. Based on the available literature and the eligibility criteria, our data revealed that the daily application of oral CHX resulted in a reduction of 27% in the number of VAP cases. These results are in accordance with other meta-analyses that showed similar RR.6,24,25 The heterogeneity was considered negligible when only double-blinded trials were included (I²=0) and moderate for the overall analysis (I²=41%). Differences in the severity of the disease, patient's baseline characteristics, study design, hospital structure, frequency of CHX administration and other factors could be a source of heterogeneity between studies.
To evaluate the influence of the CHX concentration on the VAP outcomes, an additional investigation was also conducted. However, the percentage of CHX in the formulations did not affect the overall analysis (p=0.80). In this context, even the lower concentrations of CHX (0.12%) afforded a significant reduction in the number of VAP episodes. Although CHX in the concentration of 0.2 and 2% did not show a statistically significant reduction in VAP incidence, it might be related to the number of enrolled studies. Therefore, a definitive statement about the best percentage of CHX was not claimed (Fig. 1B).
Concerning mortality, the safety of routine oral care with CHX to reduce VAP incidence must be interpreted with awareness. Although the proximity of the lower limit of the confidence interval observed in the overall analysis (1.13 [0.96, 1.32]) revealed a sign that CHX could negatively affect mortality, the double-blinded studies indicated no effect in the mortality ratios (0.99 [0.72, 1.36]). However, our analysis revealed that some studies did not report mortality rates. While VAP is complex to assess due to subjectivity, high inter-observer variability, and false positives; mortality is an objective outcome and is simpler and more patient-centered. Therefore, the discrepancy in the number of articles reporting both effects might suggest a reporting bias.
Moreover, some authors state that the antiseptic activity of CHX might select resistant bacteria towards the lungs.26,27 Kampf28 showed that exposure to sub-lethal CHX concentrations might enhance resistance in Acinetobacter spp., K. pneumoniae, and Pseudomonas spp. (known species for emerging antibiotic resistance and high mortality rate). Zhang et al.29 also showed increased resistance to chlorhexidine and colistin in Klebsiella strains. The cepA gene expression was upregulated in the adapted strains, suggesting a cross-sectional adaptive resistance to chlorhexidine. Another potential explanation for the CHX harm is that some patients may aspirate small amounts of chlorhexidine, leading to acute lung injury and acute respiratory distress syndrome.11
The results related to mortality could be conflicting because the evaluated patients in the trials involved in this review were critically ill by trauma, surgery, or other condition. In this context, the mortality ratios could be more related to the severity of the disease rather than the development of a respiratory infection, especially in non-blinded and small studies. Therefore, we propose that new trials focus on the safety of CHX in further studies.
No significant difference regarding the mean duration of mechanical ventilation and ICU length of stay was observed. These results might be underpowered due to the lack of available evidence. Some studies reported these outcomes as the median and the interquartile ranges.17,21,23
Our review showed similar results compared to a recent meta-analysis24 evaluating the impact of CHX on preventing VAP. Differences in effects could be related to the eligibility criteria. We decided not to include patients in pediatric ICU and receiving a pre-operatively application of CHX as it could lead to a potential source of heterogeneity. Although the review by Klompas et al.11 showed no benefit of using CHX to prevent VAP, this article included only a few trials due to the restricted inclusion criteria (double-blind studies with non-cardiac patients), giving wide confidence intervals.
Also, other reviews included patients that were expected (but not mandatory) to be mechanically ventilated for at least 48h (which is the minimum time required for a VAP diagnostic).6,25 Therefore, some patients (especially those who underwent cardiac surgery) might have been extubated within one day, which could interfere with the number of patients able to be diagnosed with VAP, compromising the meta-analysis.6,25,30–32
Although the analysis showed clear benefits of oral care with CHX to prevent ventilator-associated pneumonia in critically ill patients, our study presented several limitations. First, some papers could be missed as they were not available in English. In addition, the studies included in this meta-analysis reported different subjective diagnostic criteria to characterize VAP. This lack of pattern could cause significant variations across trials.
Currently, the CDC (Centers for diseases control) has developed a more objective criterion, namely VAE (ventilator-associated events), to diagnose worsening oxygenation in mechanically ventilated patients. This new outcome has been suggested to replace VAP for monitoring intubated patients.33
Different protocols were also used concerning the CHX application. While some articles described the application of CHX (either in solution or as a gel) in gauzes or swabs, other studies brushed the patient's teeth several times. In this context, some studies have stratified their data according to brushing.24 However, recent meta-analyses revealed that brushing does not affect the incidence of VAP or all-cause mortality.6,24,34 Even though brushing is an important issue, our study focused only on whether adding any concentration of CHX was beneficial compared to oral care alone. Therefore, our review did not compare oral regimens and brushing frequency differences.
Besides study blinding, incomplete data was also a major limiting factor. Regarding the duration of mechanical ventilation and ICU length of stay, only a fraction of the articles reported these outcomes. Some studies did not report mortality ratios,15,16,20,23 which is more accessible and objective than VAP incidence, suggesting reporting bias. Also, we identified that the randomization process was also a source of limitation. In most trials, information about sequence generation and allocation concealment was not properly reported.
As only a few studies presented high risk of bias, we believe that the quality of the evidence remained moderate concerning the application of CHX to reduce the VAP incidence. For all-cause mortality, we support that this outcome had a very low quality of evidence. First, we identified that some articles did not declare mortality ratios. Also, the confidence interval of this outcome was very large and the number of events was low, suggesting significant imprecision. For the duration of mechanical ventilation and ICU length of stay, we state that the quality of evidence was deficient as few articles were included, and the information regarding the patient's discharge was unclear in most studies.
Future research should adhere to the Consolidated Standards of Reporting Trials (CONSORT) statement to improve study quality and report mortality and incidence ratios related to VAE instead of VAP.
Concluding remarksThis systematic review and meta-analysis showed that CHX was beneficial in preventing VAP in critically ill patients. However, no conclusion could be made on mortality rates because the quality of the evidence was very low.
Authors contributionConceptualization: Jonas Cruz, Carolina Martins, Jonathas Piassi, Idelmo júnior, Joel Júnior, Leonardo Faverani
Methodology: Jonas Cruz, Carolina Martins, Jonathas Piassi,
Formal analysis: Jonas Cruz, Carolina Martins, Jonathas Piassi, Idelmo júnior, Joel Júnior, Leonardo Faverani
Investigation: Jonas Cruz, Carolina Martins, Jonathas Piassi, Idelmo júnior, Joel Júnior, Leonardo Faverani
Writing original: Jonas Cruz, Carolina Martins, Jonathas Piassi, Idelmo júnior, Joel Júnior, Leonardo Faverani
Writing review: Jonas Cruz, Joel Júnior, Leonardo Faverani
Project administration: Jonas Cruz, Joel Júnior, Leonardo Faverani
Role of funding sourceThis work was supported financially by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, SãoPaulo, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, Brazil). The funding sources had no impact in the design, conduct, or reporting of the article or the decision to publish the study.
Conflict of interestThe authors declare no conflict of interest.
None.