Comparison of different diagnostic criteria for early liver allograft dysfunction (EAD) and their capability to predict mortality.
DesignSingle-center, prospective, cohort study.
SettingsICU in a Regional Hospital with a liver transplant program since 1997.
Patients253 consecutive patients admitted to our ICU immediately after liver transplantation between 2009 and 2015.
Variables of interestDifferences in the incidence of EAD and its relation with ICU, Hospital and 2-year mortality depending on the definition applied using as comparator the UNOS (United Network for Organ Sharing) primary non-function criterion.
ResultsThe incidence of early liver allograft dysfunction according to UNOS was 13.8%, to Makowka 6.3%, to Ardite 10.7%, to Nanashima 20.6%, to Dhillon 30.8% and to MEAF 13.4%. Kappa test did not show a good correlation among these criteria. EAD was related with ICU mortality for all diagnostic criteria except Dhillon but only UNOS, Makowka and MEAF were associated with 2-year mortality. Hospital mortality was poorly predicted by all criteria except for the MEAF score.
ConclusionWe found a poor agreement between different criteria analyzed for the diagnosis of EAD. In our population, the MEAF score showed the best relationship with short- and long-term mortality.
Comparar diferentes criterios diagnósticos de disfunción temprana del aloinjerto hepático y su capacidad para predecir mortalidad.
DiseñoEstudio de cohortes prospectivo, unicéntrico.
ÁmbitoUnidad de Cuidados Intensivos de un Hospital Regional con programa de trasplante hepático desde 1997.
Pacientes253 pacientes consecutivos ingresados en nuestra UCI inmediatamente después del trasplante entre 2009–2015.
Variables de interésIncidencia de disfunción temprana del aloinjerto hepático según cada criterio diagnóstico, relación entre disfunción grave acorde a cada criterio y mortalidad en UCI, mortalidad hospitalaria y a los 2 años utilizando como comparador el criterio para fallo primario de la UNOS (United Network for Organ Sharing).
ResultadosLa incidencia de disfunción temprana según UNOS fue 13.8%, Makowka 6.3%, Ardite 10.7%, Nanashima 20.6%, Dhillon 30.8% y MEAF 13.4%. El coeficiente kappa mostró una pobre correlación entre ellos. Todos los criterios, excepto el de Dhillon, mostraron relación con la mortalidad en la UCI, pero solo los criterios de UNOS, Makowka y MEAF se asociaron con la mortalidad a 2 años. Finalmente, la capacidad predictiva de la mortalidad hospitalaria fue baja para todos, excepto para MEAF.
ConclusiónExiste una pobre correlación entre diferentes criterios diagnósticos de disfunción temprana del injerto hepático. El MEAF muestra la mejor relación con el pronóstico a corto y largo plazo en nuestra población.
Orthotopic liver transplant (OLT) is the main therapeutic approach for patients with a severe liver disease but insofar as clinical results of transplant have steadily improved, a shortage of donors has emerged as a serious problem, evidencing the necessity for the best possible donor-recipient matching. With a rising demand for organs and a steady improvement in immunosuppression strategies, more and more marginal donors and recipients will be transplanted,1 thus the number of dysfunctional allografts might increase; therefore clear criteria to set limits of reasonable acceptance of marginal donors and recipients is paramount to achieve the best performance in OLT policies. In this scenario, identifying those risk factors that signal early liver allograft dysfunction (EAD) can give us some indications for the best donor-recipient matching according to their outcome, but would also allow us a prompt diagnosis and apply early measures that could diminish the intensity or even arrest the development of EAD.2–6
Defining EAD after OLT is a difficult task due lack of consensus among researchers.6–9 Almost all criteria proposed to date rely on the determination of widely used markers of hepatic function, such as transaminases, coagulation factors, bilirubin, ammonia and/or lactate,10 but there is a lack of agreement about which ones should be applied. Moreover, some criteria also include less common parameters, such as the rate of elimination of molecules cleared by the liver (e.g., indocyanine green or C-Methacetin).11–13 Different classifications define diverse grades of dysfunction (some as dichotomous,12,14–25 some as progressively graded4,26–30 and finally a few as a numeric value31–33) based on different intervals of these parameters.
In a search of the literature using PubMed regarding liver allograft dysfunction criteria, we retrieved 38 definitions since 1985, most of them including some but not all of the aforementioned parameters, besides the presence of overt clinical evidence of primary non-function. This lack of agreement explains why, depending on the criterion selected, EAD after OLT is reported to appear with a broad range (8–29%), and the same applies to primary non-function (1–7%).7
Our aim was to evaluate the capability of some of these criteria, specifically those developed by Makowka et al.,26 Ardite et al.,27 Nanashima et al.,23 Dhillon et al.29 and Pareja et al.,33 for detecting EAD using as comparator the United Network for Organ Sharing (UNOS) criterion for primary non-function34 and their relationship with outcome (hospital mortality and 2-year survival) in our population.
Patients and methodsStudy designWe conducted an observational, prospective, longitudinal, single center study registering a cohort of all OLT patients admitted to our Unit during the period of study. For the design of the study and the preparation of the manuscript, we adhered to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.
SettingsIntensive Care Unit (ICU) of a Regional University Hospital in Spain. Ours is a polyvalent ICU that has been caring for the early postoperative course after OLT since 1997 (when the program was started in our center). During the period covered by this study (2009–2015) patients were managed according to a hospital management protocol developed by a multidisciplinary team that included all stages of the process (preoperative, operative and postoperative) and this protocol has been maintained without substantial changes along the period of the study, as well as the professionals of the team.
Our protocol includes the majority of cases the use of the piggy-back technique and end to end anastomosis of the common bile duct; immediate postoperative care in our ICU and any of three possible immunosuppressive strategies: calcineurin inhibitors plus steroids (the most frequently used), mammalian target of rapamycin inhibitors plus steroids, or interleukin 2 receptor antibodies plus steroids, according to patient's characteristics and past medical history. Graft-recipient match was allocated for every case in accordance with the Spanish National Transplant Organization (ONT).
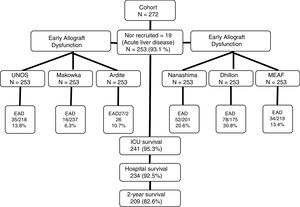
Patient information and data collectionRecruitment was conducted between February 2009 and February 2015. Two years after hospital discharge, survival status of the patient was checked by consultation of their clinical records (all the follow-up consultations after OLT are centralized in our center), there were no losses in the follow-up, as can be seen in the study recruitment flow-chart (Fig. 1). All patients admitted to our ICU for postoperative care after OLT that did not present exclusion criteria were enrolled in the study.
Exclusion criteria were: age less than 18 years for recipients, refusal from patient or her/his representative to participate in the study, and emergent OLT after acute liver failure. This last exclusion criteria was because that kind of patients usually has different liver function test profiles than rest of recipients.35
Clinical and laboratory data were collected and/or registered prospectively in an electronic health record that has not changed over this period and for which all the ICU team has been trained. As per hospital protocol, blood samples were obtained at least on admission in the ICU and then every 12h until ICU discharge.
DefinitionsWe made a pragmatic selection of criteria for comparison, choosing those which contains variables already measured our hospital protocol for the monitoring of graft function and were easily calculated in most of the clinical scenarios during the first 72h. Those criteria used for the diagnosis of EAD in our study are described in Table 1.
Definitions of graft dysfunction criteria analyzed in the study.
| Criterion | Variables | Grade | Criteria |
|---|---|---|---|
| UNOS | ALT/INR/pH/lactate | Primary non-function | ALT≥3000U/L and: INR≥2.5 and/or arterial pH≤7.3 and/or venous pH≤7.25 and/or lactate≥4 |
| Makowka (1987) | ALT/AST/PT | Mild | ALT between 1000 and 2500U/L, AST between 1500 and 3500U/L, PT>25 |
| Severe | ALT≥2500U/L, AST≥3500U/L | ||
| Ardite (1999) | ALT | Mild | ALT<2500U/L |
| Severe | ALT>2500U/L | ||
| Nanashima (2002) | ALT/AST | Dysfunction | AST or ALT>1500U/L |
| Dhillon (2010) | AST+ALT/2 | Mild | 285–986U/L |
| Severe | >986U/L | ||
| MEAF (2015) | ALTmax.3POD+scoreINRmax.3POD+scorebilirubin3POD | Dysfunction | ≥8 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; INR: international normalized ratio; PT: protrombin time; TBil: total bilirubin; UNOS: United Network for Organ Sharing; MEAF: model for early allograft function scoring; max.3POD: maximum value in the first 3 postoperative days; 3POD: third postoperative day.
In their original description, UNOS criterion is evaluated in the first week; Dhillon and Nanashima criteria at 48h and Ardite and MEAF criteria on the third day after OLT. For the purpose of the study, we have analyzed the performance of all these criteria in the first 72h after OLT, splitting our sample in two: those patients having their most severe grade and those with mild-moderate or nil grade of dysfunction.
The MEAF criterion was developed when our study was closing, but insofar as all the comprising variables were registered prospectively in our database, we opted for its inclusion in the study.
Statistical analysisWe used UNOS as a reference for comparison between criteria. For the analysis of their relation to outcome, ICU, Hospital and 2-year follow-up mortality were registered. As secondary outcome variables, ICU and hospital length of stay were included.
Continuous variables are presented as median and interquartile range. Categorical variables are presented as percentages. Normality was tested with the Kolmogorov–Smirnov equation. Chi-square, U-Mann–Whitney and Kruskal–Wallis tests where used. A p<0.05 threshold was used for all tests.
Cohen's Kappa statistics and its 95% confidence interval were used to evaluate concordance between criteria. The criteria for its application was: poor concordance for a K value <0.20, mild for K between 0.21 and 0.40, fair 0.41 and 0.60, good 0.61 and 0.80 and very good >0.80.
In order to test the behavior of the EAD criteria as predictors of outcome, a model of logistic regression was computed for each criterion by backward conditional stepwise method, including said criterion plus all variables that showed a statistical relationship below 0.1 in the univariate analysis and ICU and in-hospital mortality as dependent variables; results are presented as Odds ratio (95% confidence interval). For the analysis of 2-year survival, a Kaplan–Meier curve was computed, and comparisons made by Log-rank test; a model of Cox regression was computed for each criterion by backward conditional stepwise method, including said criterion plus all variables that showed a statistical relationship below 0.1 in the univariate analysis; results are shown as Hazard ratio (95% confidence interval).
Finally, a receiver operating characteristics (ROC) curve was drawn for each criterion against mortality and its correspondent area under the curve (95% confidence interval) was calculated.
For statistical analysis and creation of figures, we used the statistical package R 3.1.2 for Os X and Prism 6 for Mac Os X (GraphPad Software Inc®).
Ethical issuesThe study protocol was in full compliance with the ethical principles of the Declaration of Helsinki and with the Good Clinical Practice guidelines. The study was approved by Committee for Ethics in Research of the Regional University Hospital of Málaga.
Patients or closest relatives signed at admission in the ICU an agreement for the use of the registered clinical data of the patients. All the laboratory data collected were already included in the usual hospital management protocol for these patients in our center.
Patient's data were registered in a disaggregated database, and identification data were erased once the integrity of the registered data had been evaluated.
ResultsDuring the study, 272 patients were admitted to our unit after OLT, of them, 19 showed exclusion criteria leaving us 253 patients for analysis. Main characteristics are presented in Table 2.
Profile of patients included in the study (N=253).
| Baseline | |
|---|---|
| Main indication for OLT (%) | |
| Alcohol | 116 (45.9) |
| Virus | 78 (30.8) |
| Biliary | 22 (8.7) |
| Cryptogenic | 14 (5.5) |
| Other | 23 (9.1) |
| Previous morbidity (%) | |
| Chronic renal disease | 30 (11.9) |
| Arterial hypertension | 70 (27.7) |
| Diabetes | 63 (24.9) |
| Hepatocarcinoma | 90 (35.6) |
| Females (%) | 60 (23.5) |
| Previous OLT | 19 (7.5) |
| Age | 56 (48.5–63) |
| Creatinine base (mgr/dL) | 0.9 (0.7–1.05) |
| MELD | 16 (11–21) |
| CHILD-PUGH C | 97 (41.3) |
| Ascites>2L | 66 (55.3%) |
| Admission | |
|---|---|
| APACHE II | 14.1 (13.6–14.6) |
| SOFA | 6.5 (6.2–6.9) |
| AST (IU/L) | 1133 (611–2005) |
| ALT (IU/L) | 639 (357.5–1176.5) |
| INR | 1.91 (1.66–2.26) |
| Factor V (%) | 26 (17.2–36.8) |
| Bilirubin (mg/dL) | 3.5 (2.4–5.7) |
| Lactate (mmol/L) | 1.8 (1.1–2.8) |
| ICU | |
|---|---|
| Higher AST (IU/L) | 1216 (659–2413) |
| Higher ALT (IU/L) | 730 (398.5–1513) |
| Higher INR | 1.95 (1.71–2.35) |
| Higher bilirubin (mg/dL) | 3.9 (2.6–6.3) |
| Higher lactate (mmol/L) | 39 (15.4) |
| CRRT | 26 (10.3) |
| MARS albumin dialysis | 5 (2) |
| Stay (days) | 3 (3–5) |
| ICU mortality | 14 (4.7) |
| Outcome | |
|---|---|
| Hospital Stay (days) | 12 (8–19.5) |
| In-hospital mortality | 19 (7.5) |
| 3-month mortality | 22 (9.4) |
| 6-month mortality | 30 (12.8) |
| 2-year mortality | 44 (17.4) |
Data as n (%) and median (interquartile range); ALT: alanine aminotransferase; AST: aspartate aminotransferase; INR: international normalized ratio; CRRT: continuous renal replacement therapy; AKIN: acute kidney injury network.
Mortality in our series was 17.4% (44 patients): 12 (4.7%) in the early postoperative course at the ICU, 7 (2.8%) in the hospital after ICU discharge and 25 (9.9%) during 2-year follow-up after hospital discharge. Cause for postoperative (in-hospital) mortality was acute rejection in 1 patient, primary non-function in 4, vascular complications in 4 and multi-organ failure after a complicated ICU course in 10 (one secondary to infection). During the two-year follow-up period, causes of mortality were motivated by graft problems in 14 cases: rejection in 2 (8%) patients, vascular complications in 5 (20%), a recurring disease in 6 (4%) and biliary complication in 1 (4%). None of the patients with past medical history of hepatocellular carcinoma died.
According to UNOS criterion, we diagnosed 35 cases of severe EAD (13.8%), but by Makowka they would have been 16 (6.3%), by Ardite 27 (10.7%), by Nanashima 52 (20.6%), by Dhillon 78 (30.8%), and by MEAF criterion 34 cases (13.4%). In order to assess the concordance between those criteria and UNOS, we calculated Cohen's Kappa statistic, that showed only fair concordance for all of them: Nanashima 0.55 (0.39–0.68), Ardite 0.49 (0.47–0.50), Makowka 0.46 (04.29–0.6), Dhillon 0.44 (0.32–0.56) and MEAF 0.41 (0.25–0.58).
Regarding the univariate analysis, the relationship between EAD diagnosed by each criterion and ICU length of stay, hospital length of stay and mortality are depicted in Table 3.
Relation between diagnostic criteria for early graft dysfunction and outcome. Univariate analysis.
| Score | No/mild dysfunction | Severe dysfunction | |
|---|---|---|---|
| ICU length of stay | |||
| UNOS | 3 (3–4) | 5 (3–10) | p<0.01 |
| Makowka | 3 (3–4) | 7 (4–20.75) | p<0.01 |
| Ardite | 3 (3–4) | 5 (3–10) | p<0.01 |
| Nanashima | 3 (3–4) | 4 (3–8) | p<0.01 |
| Dhillon | 3 (3–4) | 4 (3–6) | p<0.01 |
| MEAF | 3 (3–4) | 5 (3–9.25) | p<0.01 |
| Hospital length of stay | |||
| UNOS | 11 (8–18) | 18 (10–26) | p<0.01 |
| Makowka | 11 (8–19) | 19 (9.5–23.75) | p=ns |
| Ardite | 11 (8–19) | 12 (9–23) | p=ns |
| Nanashima | 11 (8–18) | 16 (10–23.75) | p=ns |
| Dhillon | 11 (8–18) | 13.5 (9–24.5) | p=ns |
| MEAF | 11 (8–18) | 19 (11–26.25) | p<0.01 |
| ICU mortality | |||
| UNOS | 4/218 (1.8%) | 8/35 (22.9%) | p<0.01 |
| Makowka | 8/237 (3.4%) | 4/16 (25%) | p<0.01 |
| Ardite | 7/226 (3.1%) | 5/27 (18.5%) | p<0.01 |
| Nanashima | 4/201 (2%) | 8/52 (15.4%) | p<0.01 |
| Dhillon | 4/175 (2.3%) | 8/78 (10.3%) | p=0.01 |
| MEAF | 5/219 (2.3%) | 7/34 (20.6%) | p<0.01 |
| Hospital mortality | |||
| UNOS | 10/218 (4.6%) | 9/35 (25.7%) | p<0.01 |
| Makowka | 14/237 (5.9%) | 5/16 (31.3%) | p<0.01 |
| Ardite | 13/226 (5.8%) | 6/27 (22.2%) | p<0.01 |
| Nanashima | 9/201 (4.5%) | 10/52 (19.2%) | p=0.01 |
| Dhillon | 9/175 (5.1%) | 10/78 (12.8%) | p<0.05 |
| MEAF | 9/219 (4.1%) | 10/34 (29.4%) | p<0.01 |
Length of stay in days, median (interquartile range).
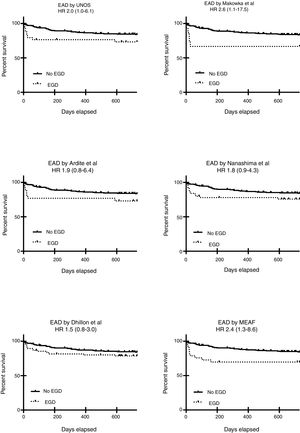
We next performed a multivariate logistic regression and a ROC curve analysis for each one of the EAD diagnostic criteria looking for an independent relationship between these and ICU or Hospital mortality, and the results are presented in Table 4. This table shows only the OR for each criterion analyzed but in the final step of all the models age, gender, comorbidities, etiology of end-stage liver disease and MELD were excluded and only chronic kidney disease, APACHE II at admission; acute kidney injury during the postoperative period and previous OLT remained in he model. Goodness-of-fit was tested with the Hosmer–Lemeshow test (p 0.43). Finally, we analyzed 2-year survival, and the results are presented in Table 4 and Fig. 2. Table 4 shows only the HR for each criterion analyzed but in the final step of the Cox regression analysis for each model, only etiology of end-stage liver disease and acute kidney injury during the postoperative period remained.
Performance of diagnostic criteria on prediction of mortality.
| Criterion | ICU | Hospital | 2-year follow-up |
|---|---|---|---|
| OR | HR | ||
| UNOS | *10.4 (1.1–31.7) | 3.0 (0.9–10.0) | *2.0 (1.0–6.1) |
| Makowka | *12.9 (2.6–63) | 2.0 (0.5–8.3) | *2.6 (1.1–17.5) |
| Ardite | *7.1 (1.7–29.3) | 1.5 (0.4–5.5) | 1.9 (0.8–6.4) |
| Nanashima | *8.8 (2.3–34.1) | 2.6 (0.9–7.9) | 1.8 (0.9–4.3) |
| Dhillon | 3.3 (0.6–17.3) | 1.4 (0.5–4.3) | 1.5 (0.8–3.0) |
| MEAF | *6.5 (1.7–24.4) | *5 (1.6–15.5) | *2.4 (1.3–8.6) |
| AUC | |||
| UNOS | 0.61 (0.49–0.72) | 0.61 (0.49–0.72) | 0.57 (0.46–0.67) |
| Makowka | 0.61 (0.45–0.77) | 0.63 (0.47–0.79) | 0.58 (0.42–0.73) |
| Ardite | 0.58 (0.45–0.70) | 0.58 (0.46–0.71) | 0.55 (0.43–0.67) |
| Nanashima | 0.57 (0.47–0.66) | 0.57 (0.48–0.67) | 0.55 (0.46–0.64) |
| Dhillon | 0.54 (0.46–0.62) | 0.54 (0.46–0.62) | 0.53 (0.45–0.61) |
| MEAF | 0.59 (0.48–0.70) | 0.63 (0.51–0.74) | 0.59 (0.48–0.70) |
We designed the present study to assess the performance of various criteria to diagnose EAD and their relationship with outcome. Diagnosing EAD is a challenge because of a large number of criteria proposed, as a consequence, its epidemiology is not well characterized yet. To further complicate this scenario, studies comparing EAD criteria are scarce and whilst most of them intend to validate new sets of variables, almost none has established comparisons among existing criteria, and no study has encompassed them all.
Incidence of EAD reported by several research groups differs from ours: primary non-function by UNOS criterion was 13.8% in our population; Makowka26 reported poor function in 17.5% against 6.3% in our population, and Ardite27 reported a 19% of severe initial graft injury, whereas in our series just 10.7% were detected. Closer results were found for Nanashima23 (reporting 18.3% “initial poor graft function” against ours 20.6%) or Dhillon29 (26% of “initial poor graft function” against ours 30.8%). Our results disclose a huge variability in EAD diagnosis depending on the criteria used, as it is also pointed out in a recent review by Chen.7
Several factors could account for these differences: the fact that they were single-center studies or with a retrospective design, or perhaps the advances in the care for OLT, as the incidence of EAD and other complications seems to be decreasing despite marginal donor use.37
In our current settings of a limited number of donors, the appearance of EAD after OLT implies a double problem: a complicated postoperative course with risk for the receptor, and the possibility that in a different recipient this complication would not have developed.38 Thus, it is necessary to accurately define risk factors for EAD but this is difficult due to lack of consensus among researchers about how to define it.6–8 Obviously, this lack of consensus is detrimental to the design of research strategies that could help clinicians to decide and treat these patients, hampering, in the end, our capability to improve the transplant process.
Our main challenge when comparing these diagnostic sets was how to measure their performance. As definite criteria – either analytical or pathological – are lacking, we decided to analyze their capability to predict early and late outcome (e.g., length of stay or mortality) insofar as there is a clear relationship between EAD and poor outcome. This relationship has been confirmed in our population, where we found an increased length of stay and mortality for those patients developing EAD, irrespective of the diagnostic set used (Table 3).
According to our results, the UNOS, MEAF, Makowka, Ardite, and Nanashima criteria showed a good performance in predicting ICU mortality and all of them performed well predicting ICU length of stay. This was expected as most of these criteria were developed for the first postoperative week, despite the poor concordance shown by weak Kappa values, which points to a different set of patients detected by each criterion. Nevertheless, the capability to discriminate ICU mortality was poor according to ROC curve for all the criteria.
When addressing in-hospital mortality, all the criteria except MEAF showed a poor relationship. In our cohorts, MEAF performed well for in-hospital mortality, in agreement with the results reported in the original publication and in an external validation cohort.33,36
Among all the criteria analyzed in this study, the UNOS, Makowka, and MEAF criteria showed the better relationship with an early and late outcome and among them the MEAF score outperformed. Similar results have been reported from one study comparing the MEAF score with the widely used criteria proposed by Olthoff et al.39
We must acknowledge some limitations of our study. As in all previous studies addressing EAD, criteria were based on clinical and laboratory parameters that, in some way or another, mark a group of patients with a poorer outcome, but corresponding pathological findings (that could definitely ascertain that EAD is responsible for the clinical picture) are lacking. Despite this, taking into account different criteria already used in clinical practice and comparing their performance, even when lacking definitive confirmation of EAD, can be of aid in defining which of these tools behave better in our population to predict morbidity and mortality.
In addition, a limitation of this study is the single-center setting, which restricts the external validity of our results. Being a study about diagnostic criteria this aspect is critical, but the OLT patient and the transplant process are well characterized, and our team has large experience in its management. Criteria for graft allocation, patient selection, surgical approach and immunosuppressive regimes are widely agreed and there is scarce variability among centers, so our data can be reasonably extrapolated to other groups. Moreover, comparison of these criteria in different populations helps to precise the validity of these tools.
Part of the problem when studying EAD is the relatively low incidence, making desirable multicenter studies with larger and more varied populations. Also, in order to make sound comparisons, we included as EAD only the severe cases for Makowka, Ardite, Dhillon, and MEAF criteria; if we had also included patients with mild dysfunction, comparisons would not have been clinically consistent, and results would have been even poorer with those criteria. Taken these facts into account, we believe that our results are fair and mark the path for new studies on this topic.
Another possible drawback is that one group of patients was excluded from the study, namely acute liver failure. Our decision was motivated by the possibility of a different behavior of markers of cytolysis. Taking into account that all the criteria included in the study rely heavily on transaminases levels, inclusion of those patients would have potentially biased the results as they present with a serious derangement of liver function parameters before surgery and a different profile of cytolysis markers in the first hours after the transplant can be expected.35 Thus, we believe that the exclusion of these group assured a more homogeneous profile within our population and strengthened the results.
Still other aspect that can be challenged in our protocol is the inclusion of a score that was published in the last stages of patient's recruitment, namely the MEAF criterion. Even when this decision breaks the integrity of the “prospective” condition of the study, we opted for this strategy because, based on the first reports published, this was a promising diagnostic tool that needed external validation, and the set of parameters that compound the criterion had been prospectively registered in all our patients. Moreover, all the variables included in this criterion are routinely used and the laboratory procedures are standardized and have not suffered significant variations during the period of study. Thus, we assumed that this approach did not compromise the validity of our conclusion and could even add usefulness to the study, as it helped to evaluate this new diagnostic tool.
In summary, when comparing different severe EAD criteria against UNOS criterion we found that all show poor in-between concordance. MEAF criterion showed in our population the best performance to predict short and long term mortality and if these results are confirmed in wider population studies, this could be a useful tool for the characterization of EAD after OLT.
AuthorshipAll author participated in writing and revising the paper. H.G.M.E, S.P.G, and B.F.J.E contributed to design, the performance of research, data collection, and analysis. A.V.M.D and L.S.R contributed to the performance of research and data collection.
FundingThis work has not received external funding.
Conflict of interestNone of the authors declare any kind of conflict with the contents of the present manuscript.
We thank to Angel García-Forcada MD and Elisa López-Dolado PhD for their help with the manuscript.