To describe mechanical ventilation (MV) practices in Argentina, and to explore factors associated with ICU mortality in this population.
DesignA prospective, multicenter, observational study was carried out.
SettingIntensive Care.
PatientsWe enrolled patients above 18 years old admitted to any of the participating ICUs requiring invasive MV for at least 12 h since the admission to the healthcare institution, including MV initiation in emergency department, operating room or other hospitals.
InterventionsNone.
VariablesAll variables were classified into three categories: variables related to demographic and clinical factors before the MV, factors related to the first day on MV, and factors related to events happening during the MV (complications and weaning from MV). Mechanical ventilation weaning and mortality were classified according to WIND.
ResultsThe primary analysis included 950 patients. The main indication for MV was acute respiratory failure (58% of patients). Initial ventilation mode was volume control-continuous mandatory ventilation in 75% of cases. ICU and hospital mortality were 44.6% and 47.9% respectively. The variables identified as independent predictors of mortality in ICU were age (OR 3.48 IC 95% 1.22–11.66; p = 0.028), failure to implement NIV before MV (OR 2.76 IC 95% 1.02–7.10; p = 0.038), diagnosis of sepsis (OR 2.46 IC 95% 1.09–5.47; p = 0.027) and extubation failure (OR 4.50 IC 95% 2.05–9.90; p < 0.001).
ConclusionsThe present study allowed us to describe the characteristics and clinical course of the patients who received mechanical ventilation in Argentina, finding as the main result that mortality was higher than that reported in international studies.
Describir las prácticas relacionadas a ventilación mecánica (VM) en Argentina y explorar los factores asociados a la mortalidad en UCI en esta población.
DiseñoSe realizó un estudio observacional, prospectivo, multicéntrico.
ÁmbitoUnidad de Cuidados Intensivos.
PacientesIncluimos pacientes mayores de 18 años ingresados en las UCI participantes que requirieron VM invasiva durante al menos 12 horas desde el ingreso a la institución de salud.
IntervencionesNinguna.
VariablesTodas las variables se clasificaron en tres categorías: variables relacionadas con factores demográficos y clínicos antes de la VM, factores relacionados con el primer día de VM y factores relacionados con los eventos ocurridos durante la VM (complicaciones y destete de la VM). El destete de la ventilación mecánica y la mortalidad se clasificaron según WIND (Weaningaccording to a New Definition).
ResultadosEl análisis primario incluyó a 950 pacientes. La principal indicación de VM fue insuficiencia respiratoria aguda (58% de los pacientes). El modo de ventilación inicial fue ventilación mandatoria continua con control de volumen en el 75% de los casos. La mortalidad en UCI y hospitalaria fue del 44,6% y 47,9% respectivamente. Las variables identificadas como predictoras independientes de mortalidad en UCI fueron edad (OR 3,48 IC 95% 1,22–11,66; p = 0,028), fracaso en la implementación de VNI antes de VM (OR 2,76 IC 95% 1,02–7,10; p = 0,038), diagnóstico de sepsis (OR 2,46 IC 95% 1,09–5,47; p = 0,027) y fracaso de la extubación (OR 4,50 IC 95% 2,05–9,90; p < 0,001).
ConclusionesEl presente estudio permitió describir las características y evolución clínica de los pacientes que recibieron ventilación mecánica en Argentina, encontrando como principal resultado que la mortalidad fue mayor a la reportada en estudios internacionales.
Invasive mechanical ventilation (MV) is a fundamental tool for the management of patients with acute respiratory failure (ARF). The existing evidence shows that a number of interventions designed to prevent ventilator-associated lung injury and optimize weaning have a strong impact upon the duration of mechanical ventilation and mortality.1–5 Several epidemiological studies have investigated the different tendencies in the use of mechanical ventilation throughout the world.6–8 In addition, these studies have afforded useful information leading to updates in routine clinical practice referred to patients subjected to MV.
In Latin America, the epidemiological data on the implementation patterns referred to MV are limited. To our knowledge, no studies to date have provided epidemiological information on a general population of patients subjected to invasive mechanical ventilation in Argentina.9,10 To address this issue, we have described the mechanical ventilation practices in this country and explored the factors associated with mortality in the Intensive Care Unit (ICU) in patients of this kind.
Material and methodsA prospective, multicenter observational study was carried out between 1 September 2019 and 31 December 2019 in different ICUs in Argentina. The original study protocol was approved by the Ethics Committee of the Sociedad Argentina de Terapia Intensiva (ref. no. 1 2019) and was registered with clinicaltrials.gov (no. NCT04107467). Each participating center in turn received the corresponding approval from its own local Ethics Committee. The study was carried out following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational cohort studies.11
We included patients over 18 years of age admitted to any of the participating ICUs and who required invasive MV during at least 12 h from the time of admission to the participating institution; patients having started mechanical ventilation outside the ICU in a different Department (emergency or operating room); and patients admitted from a different institution and transferred to the ICU of one of the centers participating in the study. We excluded patients admitted to pediatric ICUs, anesthesia recovery or resuscitation wards, or coronary units. The patients were only enrolled in the study on the occasion of their first admission to the ICU. Patients with more than 10% of missing data referred to key study parameters used to generate the predictive model were excluded from the analysis.
Data collection and processingThe data were collected on a daily basis between 8:00 a.m. and 11:00 a.m. by the co-investigator of the study assigned to each participating Unit, or by any member of the study team, using customized case report forms (CRFs). The information obtained was entered into an online database using the REDCap application (Research Electronic Data Capture, Vanderbilt University, TN, USA) at Centro del Parque (Buenos Aires, Argentina) to guarantee data protection and confidentiality according to the recommendations of the Declaration of Helsinki.12,13 All those in charge at the participating centers had access to the website containing all the study documentation, including the operational definition of the variables, the operations manual, and the corresponding links to facilitate calculation of the patient severity scores.
In order to minimize missing information, a mobile offline data loading application was habilitated. The patients were followed-up until day 28 from enrollment or until hospital discharge or death – whichever occurred first.
All the principal investigators of the study (GAP, EG, MA, EN and JHD) provided support and feedback via e-mail to the local co-investigators of the participating centers, and a telephone line was made available for consultations and immediate support.
The local co-investigators were in charge of training their teams in data compilation and quality control. Data consistency was assessed on a daily basis (missing information, atypical values, data entry error) by one of the principal investigators (EN). Whenever further information was needed, the rest of the members of the research team (GAP, EG, MA, EN and JHD) contacted the investigators of the participating centers as required.
Statistical analysisThe primary endpoint was mortality in the ICU. All the variables were classified into three categories: variables related to demographic and clinical factors before MV; variables related to the first day of MV; and variables related to the events occurring during MV (complications and weaning from MV). Weaning from mechanical ventilation and mortality were classified according to the definition of the WIND study.14 Continuous variables were reported as the mean and standard deviation (SD), or as the median and interquartile range (IQR 25–75), as required. Normal data distribution was assessed with the Shapiro-Wilk test. Categorical variables in turn were reported as absolute values and percentages. The Student t-test or Mann–Whitney U-test was used to compare continuous variables as required. The chi-square test or Fisher exact test was used to compare categorical variables. In order to identify mortality predictors, we adjusted a logistic regression model with key predictors as independent variables and mortality in the ICU as dependent variable. Relationships between outcome and exposure were initially explored by univariate analysis. The variables selected for the multivariate analysis were those considered to be relevant by the authors and/or which obtained p < 0.1 in the univariate analysis. Linearity between the numerical covariables and the dependent variable was evaluated.15 In the absence of linearity, stratification was carried out as described in the literature.6,9,10 In the case of multilevel categorical variables, the level of reference was selected according to the least probability related to the dependent variable. Sensitivity testing was performed using different criteria to select the final model (best-subset, backward, recursive feature elimination).15,16 The model with the smallest value of the Akaike information criterion (AIC) was selected. All the selected variables were reported with their corresponding odds ratio (OR) and 95% confidence interval (95%CI). The goodness of fit of the final model was analyzed using the Hosmer-Lemeshow test, and its discriminating capacity was assessed based on the area under the curve (AUC) and 95%CI. Lastly, the predictive capacity of the model was evaluated through K-fold cross-validation (10-fold).16 As this was an observational study, we decided to include as many patients as possible, with no pre-established sample size. The data were analyzed using the R version 3.6.2 package.17
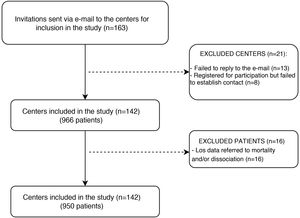
ResultsParticipating Units and included patientsA total of 142 Units in 22 provinces and the city of Buenos Aires (Fig. 1) participated in the study. This represented 14% of all the ICUs in the country. Of the participating Units, 48.5% belonged to public hospitals (n = 461) and 51.5% to private institutions (n = 489).18 The primary analysis comprised 950 patients, of which 41.1% (n = 390) were females; the mean age was 58.1 ± 18.5 years, and the mean SAPS II score was 46.8 ± 16.7. The main indication of MV was acute respiratory failure (58%; n = 555). The baseline characteristics are described in Table 1.
Characteristics of the patients admitted to the ICU. Demographic parameters and variables referred to the start of mechanical ventilation.
| Variables | Total (n = 950) | Live (n = 526) | Deceased (n = 424) | OR (95%CI) | p-Value | Missing data |
|---|---|---|---|---|---|---|
| n (%) | ||||||
| Female gender, n (%) | 390 (41.1) | 221 (42.0) | 169 (39.9) | 0.91 (0.70–1.19) | 0.502 | 0 (0) |
| Age, mean (SD) | 58.1 (18.5) | 55.0 (19.3) | 62.1 (16.6) | 1.02 (1.01–1.03) | <0.001 | 0 (0) |
| BMI, mean (SD), kg | 27.8 (6.8) | 27.9 (6.9) | 27.7 (6.6) | 1.00 (0.97–1.02) | 0.681 | 0 (0) |
| Charlson index, median [IQR] (n = 892) | 4 [1–6] | 3 [1–5] | 4 [2–6] | 1.19 (1.13–1.25) | <0.001 | 58 (6.1) |
| SAPS II, mean (SD) (n = 879) | 46.8 (16.7) | 44 (17.2) | 50 (17.6) | 1.02 (1.01–1.03) | <0.001 | 71 (7.5) |
| Geographical region* | - | - | - | - | - | |
| CABA, n (%) | 268 (28.2) | 167 (31.7) | 101 (23.8) | 1 | N/A | |
| PBA, n (%) | 289 (30.4) | 150 (28.5) | 139 (32.8) | 1.53 (1.09–2.15) | 0.013 | |
| Center, n (%) | 186 (19.6) | 93 (17.7) | 93 (21.9) | 1.65 (1.13–2.42) | 0.009 | 0 (0) |
| Cuyo, n (%) | 57 (6.0) | 37 (7.0) | 20 (4.7) | 0.89 (0.48–1.61) | 0.713 | |
| North, n (%) | 105 (11.1) | 57 (10.8) | 48 (11.3) | 1.39 (0.88–2.20) | 0.155 | |
| Patagonia, n (%) | 45 (4.7) | 22 (4.2) | 23 (5.4) | 1.73 (0.91–3.28) | 0.091 | |
| Type of institution | - | - | - | - | - | |
| Public | 461 (48.5) | 254 (54.7) | 210 (45.3) | 1 | N/A | 0 (0) |
| Private | 489 (51.5) | 273 (55.8) | 216 (44.2) | 0.96 (0.74–0.24) | 0.769 | |
| Time to admission to the ICU from hospital admission, days, median [IQR] | 0 [0-2] | 0 [0-1] | 0 [0-3] | 1.04 (1.02–1.07) | <0.001 | 0(0) |
| NIV failure before MV, n (%) | 102 (10.7) | 43 (8.2) | 59 (13.9) | 1.82 (1.20–2.77) | 0.005 | 0 (0) |
| Reasons for MV, n (%) | - | - | - | - | - | |
| ARF | 555 (58.4) | 296 (31.1) | 260 (27.3) | 1 | NA | 0 (0) |
| ARF in CLD | 79 (8.3) | 39 (4.1) | 41 (4.3) | 1.17 (0.73–1.88) | 0.509 | |
| Coma | 301 (1.7) | 182 (19.1) | 120 (12.6) | 0.76 (0.57–1.01) | 0.056 | |
| NMD | 15 (1.6) | 10 (1) | 5 (0.5) | 0.57 (0.18–1.63) | 0.313 | |
| PaO2/FIO2 prior to MV, mean (SD) (n = 312) | 236.7 (136.0) | 265.0 (142.0) | 208.0 (123.6) | 0.99 (0.98–0.99) | <0.001 | 638 (67.1) |
BMI: body mass index; SAPS II: Simplified Acute Physiology Score; ICU: Intensive Care Unit; NIV: noninvasive mechanical ventilation; MV: mechanical ventilation; ARF: acute respiratory failure; CLD: chronic lung disease; NMD: neuromuscular disease.
Geographical regions: CABA: city of Buenos Aires; PBA: province of Buenos Aires; Center: Cordoba, Santa Fe and Entre Rio; Cuyo: Mendoza, San Luis and San Juan; North: Tucuman, Salta, Misiones, Chaco, Corrientes, Santiago del Estero, Jujuy, Formosa, Catamarca and La Rioja; Patagonia: Rio Negro, Neuquen, Chubut, La Pampa, Santa Cruz and Tierra del Fuego.
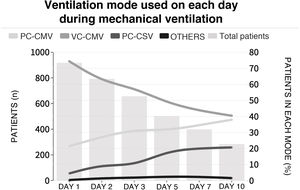
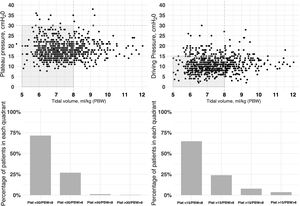
The initial ventilation mode was continuous demand ventilation with volume control (VC-CMV) in 75% of the cases (n = 677) (Fig. 2). Independently of the ventilation mode used, the tidal volume (Vt) was <8 ml/kg of predicted body weight in 75.1% of the cases (n = 663), with a positive end-expiratory pressure (PEEP) of <8 cmH2O in 64.8% of the cases (n = 585). The plateau pressure was measured in over 80% of the patients (n = 781). The mean plateau pressure (SD) and the driving pressure were 18 ± 4.6 and 11.2 ± 3.9 cmH2O, respectively. On the first day of mechanical ventilation, the peak pressure, the plateau pressure and the driving pressure were significantly lower among the survivors (p < 0.001) (Fig. 3 and Appendix B Electronic Supplementary material A).
Proportion of patients subjected to mechanical ventilation according to selected mode during admission to the ICU.
Each line represents the proportion of patients in each mechanical ventilation mode over follow-up. The height of the bars represents the number of patients each day in the ICU. PC-CMV: Pressure control-Continuous mandatory ventilation; VC-CMV: Volume control-Continuous mandatory ventilation; PC-CSV: Pressure control-Continuous spontaneous ventilation.
Relationship between plateau pressure, driving pressure and tidal volume (ml/kg) expressed according to predicted body weight (PBW), during the first day of ventilatory support.
The figure represents the distribution of tidal volume corresponding to day 1 versus the plateau pressure (left: figure at top and bottom) and tidal volume versus driving pressure (right:) for each patient. Most of the patients were within the limits of protective ventilation, defined as plateau pressure ≤30 cmH2O, driving pressure ≤15 cmH2O and tidal volume ≤8 ml/kg predicted body weight. The data are referred to the first day of mechanical ventilation.
Sepsis was recorded in 29.7% of the cases (n = 281), ventilator-associated pneumonia (VAP) in 17.6% (n = 166) and acute respiratory distress syndrome (ARDS) in 12.9% (n = 122). Sedation was administered as a continuous infusion in 86% of the patients (n = 815), and neuromuscular block (NMB) was used in 11.4% (n = 108). Fifty-seven percent of the patients (n = 540) presented the failure of at least one organ, with cardiovascular failure being the most frequent presentation (37.8%; n = 359) (Table 2).
Variables referred to complications during mechanical ventilation.
| Variables | Total (n = 950) | Live (n = 526) | Deceased (n = 424) | OR (95%CI) | p-Value | Missing data |
|---|---|---|---|---|---|---|
| n (%) | ||||||
| VAP, n (%) (n = 945) | 166 (17.6) | 87 (16.6) | 79 (18.7) | 1.15 (0.82–1.61) | 0.402 | 5 (0.5) |
| Sepsis, n (%) (n = 945) | 281 (29.7) | 108 (20.7) | 173 (41.0) | 2.67 (2.01–3.57) | <0.001 | 5 (0.5) |
| ARDS, n (%) (n = 945) | 122 (12.9) | 39 (7.5) | 83 (19.7) | 3.04 (2.04–4.60) | <0.001 | 5 (0.5) |
| Prone, n (%) (n = 945) | 41 (4.3) | 13 (2.5) | 28 (6.6) | 2.79 (1.45–5.63) | 0.003 | 5 (0.5) |
| Sedation in CIP, n (%) (n = 945) | 815 (86.2) | 434 (83.0) | 381 (90.3) | 1.91 (1.29–2.85) | 0.001 | 5 (0.5) |
| Use of NMB, n (%) | 108 (11.4) | 45 (8.6) | 63 (14.9) | 1.86 (1.25–2.81) | 0.003 | 0 (0) |
| WAICU (n = 946) | – | – | – | – | – | |
| Yes, n (%) | 142 (15.0) | 93 (17.8) | 49 (11.6) | 1 | N/A | 4 (0.4) |
| No, n (%) | 361 (38.2) | 276 (52.8) | 85 (20.1) | 0.58 (0.38–0.89) | 0.013 | |
| Delirium (n = 945) | – | – | – | – | – | |
| Yes, n (%) | 168 (17.8) | 126 (24.1) | 42 (10.0) | 1 | N/A | |
| No, n (%) | 360 (38.1) | 266 (50.9) | 94 (22.3) | 1.06 (0.70–0.63) | 0.786 | 5 (0.5) |
| Without organ failure, n (%) (n = 950) | 410 (43.2) | 323 (61.4) | 87 (20.5) | 0.16 (0.12–0.22) | <0.001 | 10 (1) |
| Cardiovascular failure, n (%) (n = 950) | 359 (37.8) | 124 (23.6) | 235 (55.4) | 4.03 (3.06–5.33) | <0.001 | 10 (1) |
| Liver failure, n (%) (n = 950) | 105 (11.1) | 33 (6.3) | 72 (17.0) | 3.06 (2.00–4.77) | 0.005 | 10 (1) |
| Renal failure, n (%) (n = 950) | 302 (31.8) | 86 (16.3) | 216 (50.9) | 5.31 (3.95–7.20) | <0.001 | 10 (1) |
| Hematological failure, n (%) (n = 950) | 119 (12.5) | 31 (5.9) | 88 (20.8) | 4.18 (2.74–6.,) | 0.003 | 10 (1) |
| Neurological failure, n (%) (n = 950) | 139 (14.6) | 48 (9.1) | 91 (21.5) | 2.72 (1.88–3.99) | <0.001 | 10 (1) |
VAP: ventilator-associated pneumonia; ARDS: acute respiratory distress syndrome; CIP: continuous infusion pump; NMB: neuromuscular blockers; WAICU: weakness acquired in the ICU (See electronic Supplement C).
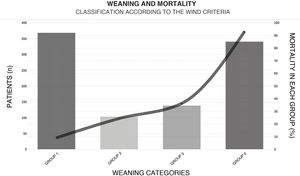
With regard to weaning from MV, a total of 38.5% (n = 365) of the patients were weaned within the first 24 h after their first spontaneous breathing test (SBT) (Fig. 4). The median [IQR] number of days of MV was 6 [2–13]. The T-tube test was used as SBT in 72.5% (n = 419) of the cases. Extubation failed in 16% (n = 75) of the patients. Noninvasive ventilation (NIV) was used to prevent extubation failure in 6.8% of the cases (n = 65). The proportion of patients subjected to tracheostomy was 22.4% (n = 213) (Appendix B Electronic Supplementary material B).
Weaning according to the WIND classification14 and mortality within each group.
GROUP 0: Never entered weaning; GROUP 1: Weaning ended within 24 h after the first spontaneous breathing test (SBT); GROUP 2: Weaning ended between the second day and the first week after the first SBT; GROUP 3: No successful weaning 7 days after the first SBT.
This figure shows the number of patients (height of the bars) for the corresponding weaning group according to the WIND classification, and mortality (solid line).
The mortality rate in the ICU and in hospital was 44.6% (n = 424) and 47.9% (n = 455), respectively, with a median (IQR) of 10 [5–20] and 17 [9–30] days of stay in each case (Appendix B Electronic Supplementary material B). Among the patients with ARF as the main indication of MV, the highest mortality rates corresponded to sepsis (62.6%, n = 62), ARDS (60%, n = 15) and pneumonia (58%, n = 52). Of all the variables considered in the univariate model and entered in the multivariate model, those identified as independent predictors of mortality in the ICU were age (over 70 years), the implementation of NIV before MV, the diagnosis of sepsis, and extubation failure before 72 h (Table 3).
Multiple logistic regression analysis for variables associated with mortality.
| Mortality | |||
|---|---|---|---|
| Predictors | OR | 95%CI | p-Value |
| Age | |||
| Age <40 years | Ref | Ref | Ref |
| Age 40–70 years | 0.98 | 0.33–3.33 | 0.972 |
| Age >70 years | 3.48 | 1.22–11.66 | 0.028 |
| Renal failure | 2.08 | 0.90–4.68 | 0.079 |
| Use of NIV before MV | 2.76 | 1.02–7.10 | 0.038 |
| Sepsis | 2.46 | 1.09–5.47 | 0.027 |
| Liver failure | 2.76 | 0.90–7.86 | 0.064 |
| Extubation failure (72 h) | 4.50 | 2.05–9.90 | <0.001 |
NIV: noninvasive ventilation; MV: mechanical ventilation; CI: confidence interval; OR: odds ratio; area under the receiver operating characteristic (ROC) curve for predicting mortality (95%CI): 0.79 (0.71–0.87).
Hosmer–Lemeshow X2: 8.654 (df = 8); p = 0.3804.
This multicenter study provides new epidemiological data on mechanical ventilation in Argentina. The key findings can be summarized as follows: mortality in the ICU and in hospital among patients subjected to MV in this country is higher than reported at international level.6,8,10 Both the days of MV and the need for reintubation were high. With regard to the potential predictors, patient age, the diagnosis of sepsis, the use of NIV before MV, and extubation failure were correlated to increased mortality.
In coincidence with the observations of international studies, the most frequent reasons for starting MV were acute respiratory failure and coma.6,7,19
In their epidemiological study, Esteban et al.8 found VC-CMV to be the most widely used ventilation mode at the start of MV. Our own results are consistent with this; however, the percentage use of VC-CMV doubled that reported in the aforementioned study (EpVAr 2019: 74.9%; Esteban 2013: 38%).
Based on the results of our study, it can be concluded that the mortality rate among patients on MV for over 12 h is high in Argentina. Although the severity of the patients at the time of admission could explain this high percentage, other multicenter studies have revealed a mortality rate up to 15% lower in patient cohorts with similar characteristics in terms of variables such as age, SAPS II score upon admission and the reasons for MV.6,8,10 In a population of similar characteristics, Peñuelas et al. attributed mortality in the ICU to changes in clinical practice associated with the adoption of a protective ventilation strategy allowing the end-expiratory airway pressure to be maintained below the level considered to be harmful for the lungs.20 However, ventilator adjustment during the first day does not appear to be associated with mortality. In the same way as in other international reports, we found variables such as Vt and PEEP to be within ranges that could be regarded as acceptable in terms of lung protection.6,7,10 We also found that the values recorded for monitoring parameters such as driving pressure and plateau pressure were low.21–23 A high plateau pressure recording rate was observed (90%); this percentage was far higher than that recorded in the LUNG SAFE study (42%), and could reflect the interest in lung protection.23 This could be explained in terms of increased adherence to the use of protective mechanical ventilation protocols.24
We found four variables to be independently associated with increased mortality: age (over 70 years), the diagnosis of sepsis, the use of NIV before MV, and extubation failure. In coincidence with the study published by Estenssoro et al. in 2018,25 the need for mechanical ventilation in patients diagnosed with sepsis was an independent predictor of mortality. Unfortunately, we were unable to obtain information on the implementation of NIV as the first ventilatory support strategy, since the patients with successful initial NIV were not documented. Furthermore, in relation to extubation failure, the patients did not seem to have been prematurely extubated, since our median days on MV were similar to that reported by Esteban in 2013.8 However, the percentage of patients that were extubated in the first 24 h of the first SBT was lower than the figure reported in the WIND study (38.5% versus 57%).14 The T-tube SBT was used in a large proportion of patients (72.5%), despite the latest publications in relation to the process of weaning from MV, which point to this strategy as being more demanding than others for patients subjected to MV.26–29 It is known that sedation can have a negative impact upon ventilation-free days and on the days of ICU stay.30,31 According to the variables recorded by our investigators, many of the patients were subjected to deep sedation at some time during MV, even though almost half of the subjects had no organ failure. This may indicate an excessive use of sedation in our population – a fact that could have influenced the outcomes. Furthermore, another aspect that could indicate excessive use of sedatives is the fact that in our study the VC-CMV mode was the most frequently used ventilation mode, even on the tenth day of MV - while other studies report the use of spontaneous modes in preference to other modes before the end of the first week of MV.7–9
Although a confirmatory study specifically designed for the purpose would be needed, we believe that the high percentage of mortality recorded in our study could reflect the obstacles found throughout Argentina regarding the routine implementation of protocols referred to general patient care, such as those described in the PADIS guides.32 However, these results in relation to mortality could also be explained by differences in human and technological resources that were not considered in our study, or may reflect the findings of the LUNG SAFE and ICON studies, in which medium to low-income countries showed greater mortality due to sepsis and ARDS than high-income countries.33,34 Complex organizational and economic factors in medium to low-income countries account for the poorer outcomes in patients admitted to the ICU. Profound inequities, defined as systematic, unfair and avoidable differences in health determinants such as socioeconomic level, demographics and geography, can generate differences in access to healthcare services among different population groups – a situation which in turn has an impact upon health-related outcomes.35 Furthermore, in medium to low-income countries, the health systems tend to be divided into public and private sectors, and this sustains the differences based on socioeconomic level, affecting healthcare – particularly in the critical care setting.36 Of note in our study is the fact that we did not observe differences in mortality between these two healthcare sectors. This is possibly because we also did not observe structural differences in terms of human resources – with a similar distribution in the proportions physician/patient, nurse/patient and ventilatory assist specialist/patient in the two the mentioned sectors (unpublished data).
Our study has limitations. Firstly, this is an analysis of prospectively compiled clinical data corresponding to a large range of ICUs in our country, with different conditions regarding the patients and clinical practices – some of which could have influenced the results obtained. However, our analysis took this into account in the model to minimize possible bias related to the variables. Nevertheless, although use was made of multivariate models, the presence of unmeasured confounding factors cannot be discarded. Secondly, missing data are a problem in studies of this kind, since they may lead to misinterpretation of the results. To reduce this impact, we eliminated those records with over 10% missing data referred to the relevant study variables. Lastly, the data compiled in Argentina might not be representative of other medium to low income countries or other regions.
ConclusionsThe present study describes the characteristics and clinical evolution of patients subjected to mechanical ventilation in Argentina. The main finding was a mortality rate higher than that reported in the international literature. Patient age, the diagnosis of sepsis, the use of noninvasive mechanical ventilation as an initial support measure, and extubation failure, were identified as independent mortality risk factors – some of them being potentially modifiable. The information obtained in this study is essential for our specialty and constitutes the basis for the development of care protocols seeking to optimize the management of patients on MV and thus improve the care outcomes in our country.
Conflicts of interestGAP has received funding for teaching programs from Medtronic Argentina and for teaching programs from Vapotherm Inc., USA.
EG is currently employed by Medtronic Argentina.
The mentioned sponsors had no role in the designing of the study, data collection and analysis, or in preparation of the manuscript.
Contributions of the authorsGAP conceived and designed the study; collected, interpreted and analyzed the data; conducted the literature search; and drafted the manuscript. EG, MA and JHD designed the study; collected the data; and conducted a critical review of the manuscript. EN designed the study, collected and analyzed the data; and conducted a critical review of the manuscript.
All the authors approved the final version of the manuscript and assumed responsibility for all aspects of the study in order to guarantee that the issues referred to the accuracy and integrity of any part of the study were reviewed and adequately resolved.
Judith Sagardía (Hospital Nacional Dr. Alejandro Posadas, Buenos Aires), Gretel R. Báez (Hospital de Trauma y Emergencia Dr. Federico Abete, Buenos Aires), Silvia Zidarich (Hospital Italiano de Córdoba, Córdoba), José Robles (Sanatorio Parque, Santa Fe), María del Carmen Gorostegui (Hospital Dr. Oscar Alende, Buenos Aires), Raúl A. Gómez (Sanatorio de los Arcos, CABA), Marcela Ducrey (Hospital Italiano de Buenos Aires, CABA), Norberto Tiribelli (Complejo Médico PFA Churruca Visca, CABA), Lorena Krzisnik (Hospital el Cruce Dr. Néstor Kirchner, Buenos Aires), Evangelina Pereira Zamora (Hospital E. Vera Barros, La Rioja), Belen Spath (Clínica la Sagrada Familia, CABA), Marcela Gil (Hospital San Bernardo, Salta), Lorena Impagliazzo (Hospital del Carmen, Mendoza), Federico Iglesias (Hospital Italiano de La Plata, Buenos Aires), Ignacio Castro (Hospital Central, Mendoza), Sofia Iriarte (Sanatorio Nuestra Señora del Rosario, Jujuy), Carlos G. Sosa (Hospital de Clínicas José de San Martín, CABA), Santiago Izza (Hospital Cullen, Santa Fe), María R. Marteau González (Hospital Español de Rosario, Santa Fe), Antonella Teves (Hospital Escuela Gral. José de San Martín, Corrientes), Damián Zarza Benítez (Sanatorio Trinidad Quilmes, Buenos Aires), Valeria Rienzi (Hospital General de Agudos Dr. Cosme Argerich, CABA), Leonardo Montelar (Hospital Provincial del Centenario, Santa Fe), Estela C. Cañete (Hospital Central de Formosa, Formosa), Alejandra Barrientos (Hospital Naval Pedro Mallo, CABA), Gastón Schmidt (Instituto Médico de Alta Complejidad, CABA), Alfredo Kamegawa (Clínica y Maternidad Suizo Argentina, CABA), Santiago Saavedra (Hospital Alemán, CABA), María L. Feijoo (Hospital Regional Dr. Ramon Carrillo, Sgo. del Estero), Daniel Varela (Hospital Universitario Fundación Favaloro -UTI 2, CABA), Manuel Ferreyra (Hospital Córdoba, Córdoba), Guillermo Chiappero (Hospital Fernandez, CABA), Silvia Bagnolo (Clínica Modelo de Lanús, Buenos Aires), Federico Puzzo (Clínica Monte Grande, Buenos Aires), Pablo Saul (Hospital Francisco Javier Muñiz, CABA), Hernán Nunia (Sanatorio Allende Nueva Córdoba, Córdoba), Mariana Piatti (Hospital Público Provincial de la Ciudad de Córdoba San Roque - UTI 1, Córdoba), Georgina Leites (Sanatorio San José, CABA), Daniela I Gilgado (Sanatorio Anchorena de San Martín, Buenos Aires), Gustavo Bongiorni (Sanatorio Allende Cerro, Córdoba), Romina Pratto (Sanatorio Anchorena Recoleta, CABA), Guillermina García (Sanatorio Colegiales, CABA), Janet Vallejos (Hospital Eva Perón, Buenos Aires), Vanina E. Perri (HIGA Luisa Cravenna de Gandulfo, Buenos Aires), Sabrina Cagide (Hospital Houssay, Buenos Aires), Verónica Galende (Hospital Público Provincial de la Ciudad de Córdoba San Roque - UTI 2, Córdoba), Laura Bergallo (Sanatorio Mapaci, Santa Fe), Ariel A. Zacco (Hospital Nodal Dr. Alejandro Gutiérrez, Santa Fe), Betsabe Calvet (Clínica Santa María, Mendoza), Mariana Aguirre (Hospital Santojanni-SR, CABA), Federico Camelli (Sanatorio Finochietto, CABA), Ricardo González (Hospital Fiorito, Buenos Aires), Gustavo Chaparro (Instituto Médico Platense, Buenos Aires), Jose Gelmetti (HIGA San Martín de La Plata, Buenos Aires y Hospital Español, Buenos Aires), Cinthia Ferreyra (Hospital Escuela de Agudos Dr. Ramon Madariaga, Misiones), Claudia Aramayo (Hospital del Señor del Milagro, Salta), Alejandro Risso (Sanatorio Otamendi, CABA), Marcelo G Alonso (Clínica Pasteur, Neuquén), Mariela Mogadouro (Sanatorio Trinidad de Palermo, CABA), Lucas Semorile (Clínica de los Virreyes, CABA), Ayelén Baqueiro (Hospital Artemides Zatti, Río Negro), Florencia Di Vruno (Hospital Área Programa Bariloche Ramón Carrillo, Neuquén), Mariano L. Braccini (Instituto de Cardiología Juana Francisca Cabral, Corrientes), Eliana Roggero (Hospital Eva Perón, Santa Fe), Guillermo Pardal (HIGA San Roque, Buenos Aires), Roberto Teira (Instituto Argentino de Diagnóstico y Tratamiento, CABA), Ariel Chena (Hospital Luis C. Lagomaggiore, Mendoza), Sebastián Fredes (Sanatorio Mitre, CABA), María J. Sakugawa (HZGA Mariano y Luciano de la Vega, Buenos Aires), Adrian Gallardo (Sanatorio Clínica Modelo de Morón, Buenos Aires), Mariana Greca (Policlínico PAMI 1, Santa Fe), Anabella Reboredo (Clínica la Pequeña Familia, Buenos Aires), Mercedes N. Ruffo (Hospital Dr. Guillermo Rawson, San Juan), Mauricio Petre (Hospital de Alta Complejidad Cuenca Alta SAMIC, Buenos Aires), Marta Di María (HIGA Petrona V. de Cordero de San Fernando, Buenos Aires), Marcelino Díaz (Sanatorio Santa Fe, Santa Fe), Marco Bezzi (Hospital Santojanni UCI, CABA), Cecilia Ruffo (Clínica Sociedad Española de Socorros Mutuos, Mendoza), Pablo Lovazzano (CEMIC H.U. Saavedra, CABA), Mariano Setten (CEMIC H.U. POMBO, CABA), María C Villafañe (Hospital Carrillo de Ciudadela, Buenos Aires), Silvina Picón Fuster (Hospital Italiano de San Justo, Buenos Aires), Patricia Cordeiro (Sanatorio Juncal, Buenos Aires), María A García (Hospital Misericordia Nuevo Siglo, Córdoba), Marina Busico (Clínica Olivos, Buenos Aires), Luciana Ayala Nogueira (Hospital Militar Regional de Paraná, Entre Ríos), Daniela V. Morales (Clínica Universitaria Reina Fabiola, Córdoba), Luis D. Fedes (Clínica San Agustín, Neuquén), Roberto Stratta (Sanatorio Santa Fe, Santa Fe), Iona García (Sanatorio de la Ciudad S.R.L, Chubut), Mónica Stefe (Trinidad Ramos Mejía, Buenos Aires), Mónica Conde (Hospital Universitario UAI, CABA), Antonio Abdala (Hospital Alvarez, CABA), Tatiana Ruiz Jalil (Clínica Bazterrica, CABA), Alberto Quereda (Hospital Municipal del Carmen de Chacabuco, Buenos Aires), Darío S. Villalba (Hospital de Chivilcoy, Buenos Aires), Lucrecia García Iriarte (Policlínico Modelo Cipolletti, Río Negro), María L. Quiles (Clínica del Valle, Chubut), Martin C. Lugaro (Hospital Profesor Dr. Luis Guemes, Buenos Aires), Marcelo Camargo (Hospital San Luis, San Luis), Pablo Staffolani (Hospital Santa Isabel de Hungría, Mendoza), Verónica N. Kosaka (Hospital de Autogestión SAMIC Iguazú, Misiones), Ana Mazzola (Hospital San Felipe, Buenos Aires), María V. Vilaseca (Trinidad San Isidro, Buenos Aires), Facundo Puchulu (Hospital Justo José de Urquiza, Entre Ríos), Marianela B. Canil (Clínica Zabala, CABA), Josefina Argañaraz (Hospital Centro de Salud Zenón Santillán, Tucumán), Deborah Gelabert (Clínica 25 de Mayo, Buenos Aires), Jorge González Nizzo (Sanatorio Adventista del Plata, CABA), José A. Grucci (Hospital Madre Catalina, San Luis), Verónica Lozano (Hospital Público Autogestión SAMIC el Dorado, Misiones), Ivonne Kunzi (Hospital de Rehabilitación Respiratoria María Ferrer, CABA), Matías Brizuela (Sanatorio Privado del Intenterio SRL, Tucumán), Gustavo Werber (Instituto de Trasplante y Alta Complejidad – ITAC, CABA), Patricia A. Domenichini (Hospital Italiano Regional del Sur, Buenos Aires), Fernando Ríos (Sanatorio Las Lomas, Buenos Aires), Melina Esteves (Hospital Privado Dr. Raúl Matera, Buenos Aires), Damián Fernandez (Clínica Pasteleros, CABA), Ignacio Ledesma (Hospital Privado de Córdoba, Córdoba), Janina Lebus (Hospital Central Reconquista, Santa Fe y Sanatorio Norte SRL, Santa Fe), Nahuel Dargains (HIEA y C San Juan de Dios, Buenos Aires), Ayelén García (Hospital Regional V. Sanguinetti, Chubut), Florencia Pugliese (Hospital Dr. D. Vélez Sarsfield, CABA), María V. Migueles (Centro Integral de Salud Banda, Sgo. del Estero), Federico J. Giménez (Centro de Cuidados Intensivos, San Juan), Daniela Inchaurrondo (Clínica Argentina, La Pampa), Sabrina E. Díaz (Sanatorio Español, Santa Fe), Daniela Coria (Hospital Público Descentralizado Dr. Marcial V. Quiroga, San Juan), Luis Corsiglia (IPENSA, Buenos Aires), Carlos A. Morales (Sanatorio Lavalle, Jujuy), Agustina Quijano (Hospital Público de Autogestión Dr. Arturo Oñativia, Salta), Fernanda Ignisci (Hospital Municipal de Agudos Dr. Pedro Ecay, Buenos Aires), Natalio O Grazziani (Clínica Santa Clara de la Provincia de San Juan, San Juan), María S. Fernandez Altamirano (Clínica Santa María, Córdoba), Agustina Jarque (Hospital Ramón Santamarina, Buenos Aires), Santiago Burgos (Hospital Regional de Ushuaia, Tierra del Fuego), Luis Sepúlveda (Clínica Adventista Belgrano, CABA), Nicolas Lezzi (Instituto de Investigaciones Médicas Alfredo Lanari, CABA), Marta Verdúguez (Hospital Simplemente Evita, Buenos Aires), Melisa Diiorio (Hospital Sirio Libanes, CABA).
Please cite this article as: Plotnikow GA, Gogniat E, Accoce M, Navarro E, Dorado JH, en representación de Grupo de Estudio EpVAr. Epidemiología de la ventilación mecánica en Argentina. Estudio observacional multicéntrico EpVAr. Med Intensiva. 2022;46:372–382.
This study was presented by G.A. Plotnikow at the 30th Argentinean and International Congress of Intensive Care, 4 November 2020, Argentina (2nd Prize as best study of the Congress).